Methyl-Cyclohexane Methanol (MCHM) Isomer-Dependent Binding on Amorphous Carbon Surfaces
Abstract
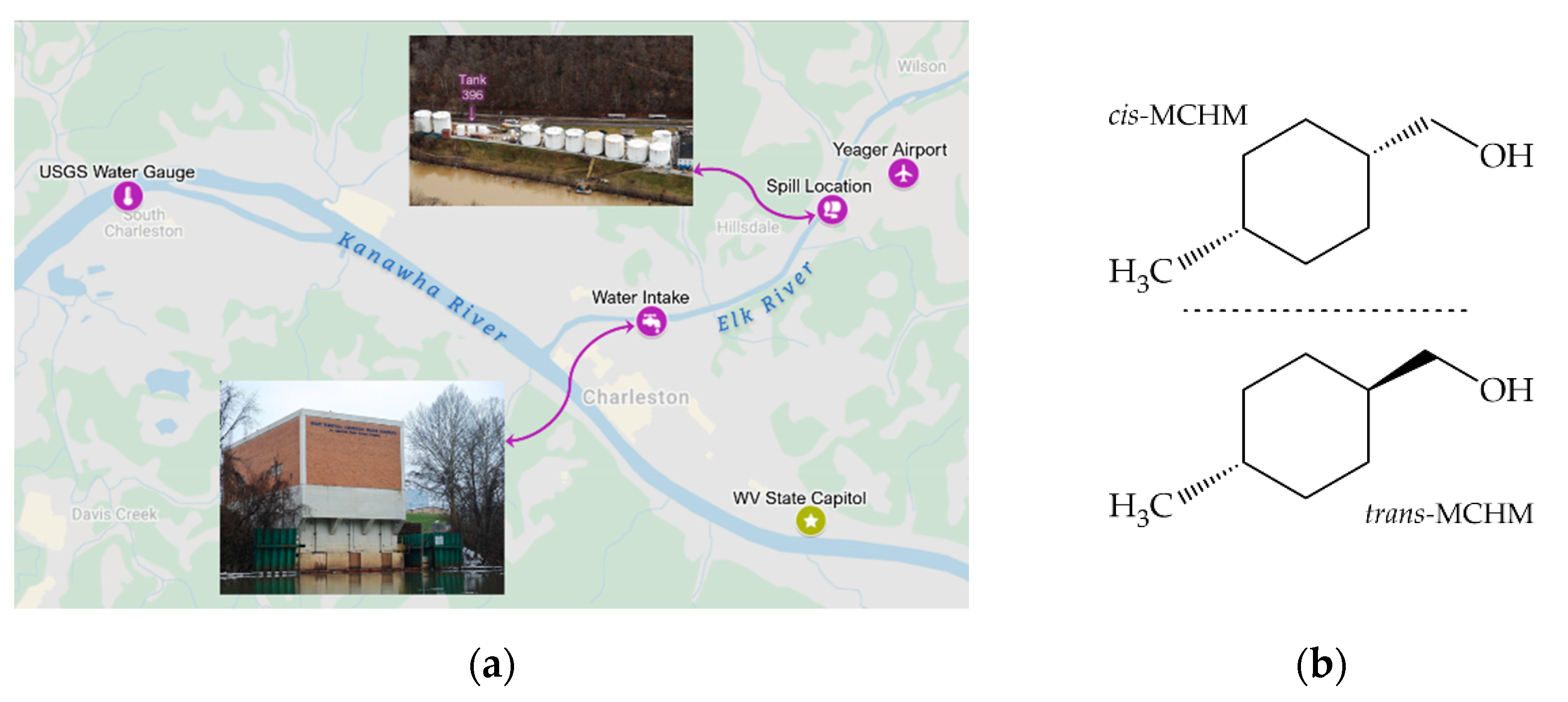
1. Introduction
2. Results
2.1. Computed MCHM Physisorption Potentials with Carbon Surfaces
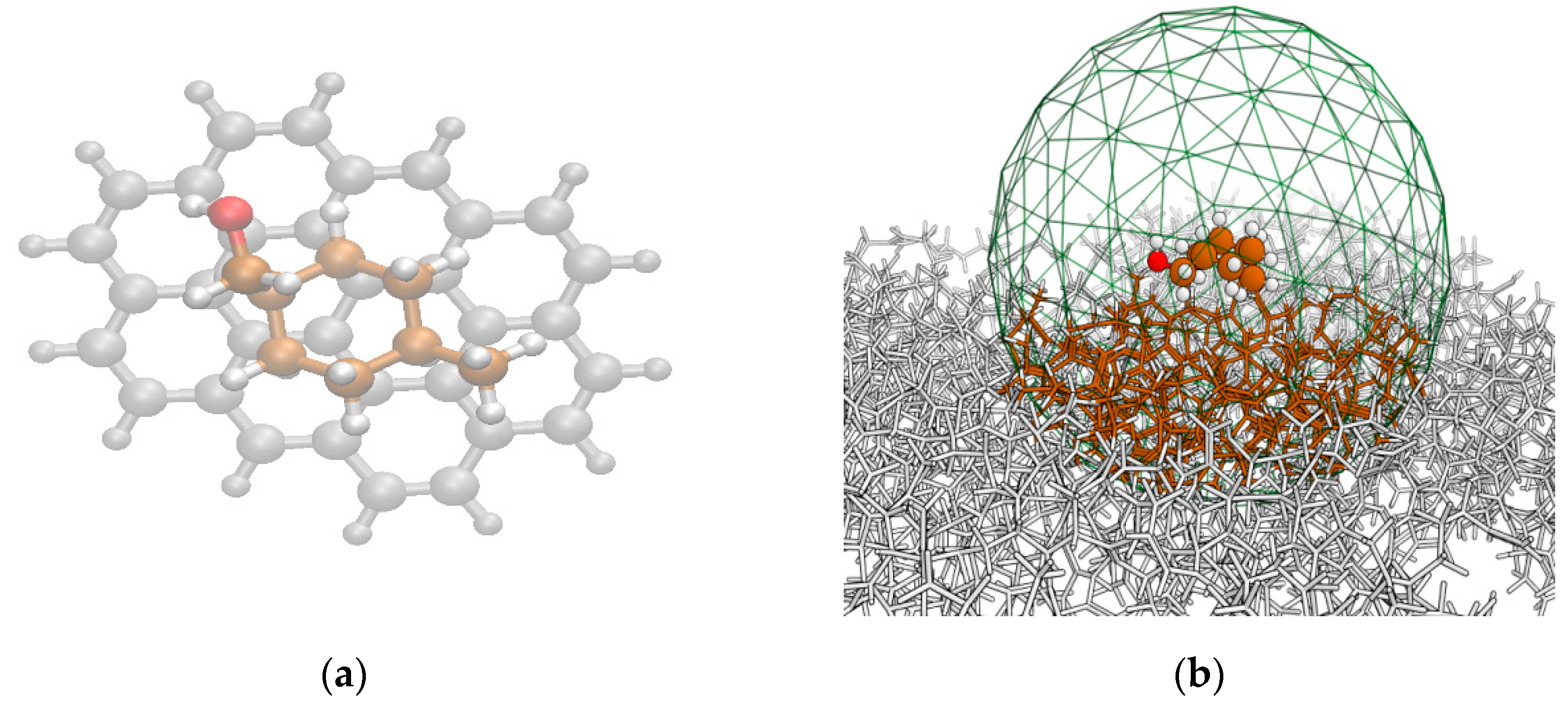
2.1.1. cis- and trans-MCHM Plus Ovalene PAH Structures
2.1.2. cis- and trans-MCHM Plus AC Structures
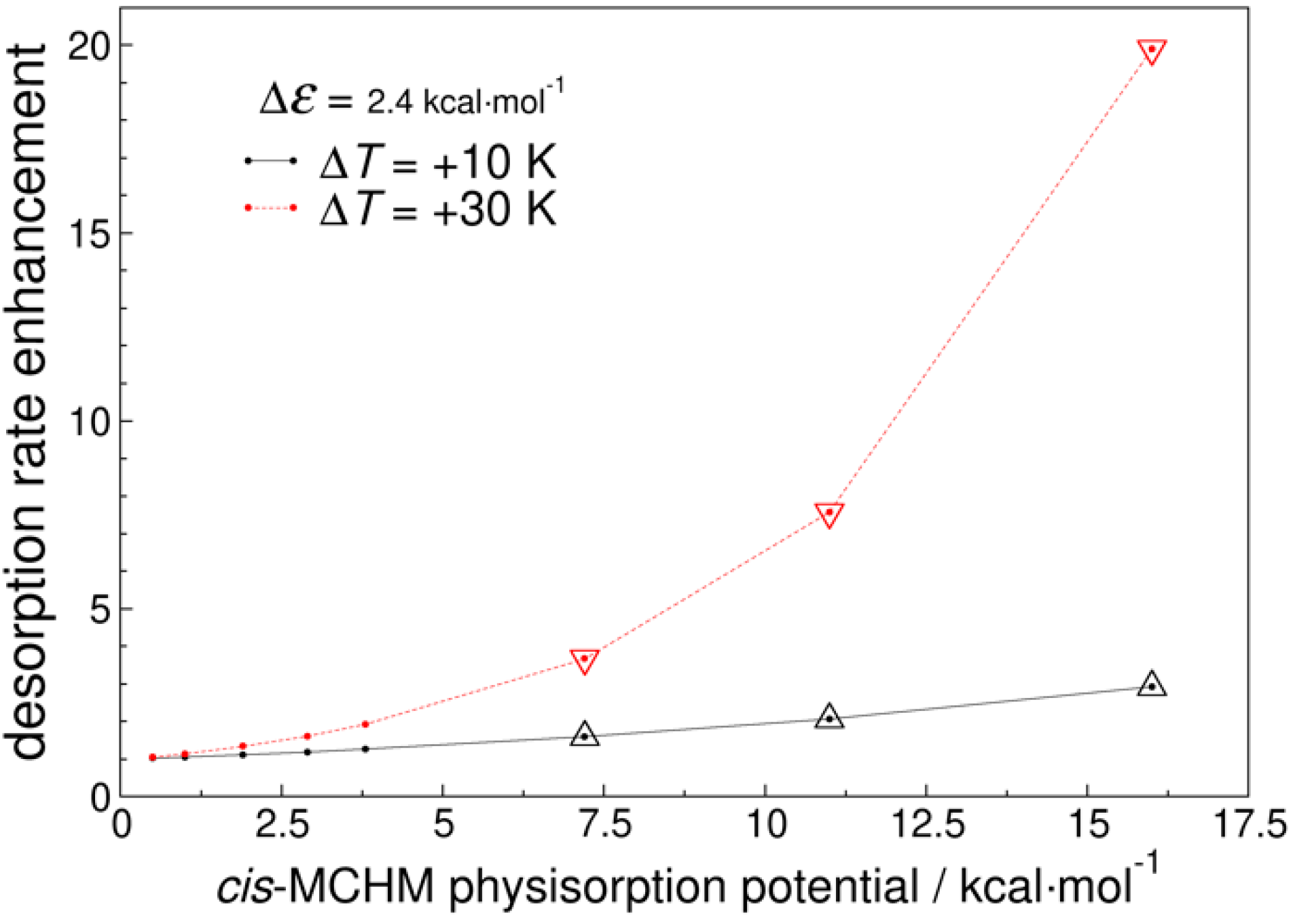
2.2. Kinetic Analysis of MCHM Isomer Desorption from Carbon Surfaces
3. Discussion
3.1. Isomeric Differences in MCHM Properties
3.2. MCHM Sorption and Desorption
3.3. Kinetic Analysis of MCHM Isomer Desorption from Carbon Surfaces
3.4. Odor Implications in Drinking Water
4. Materials and Methods
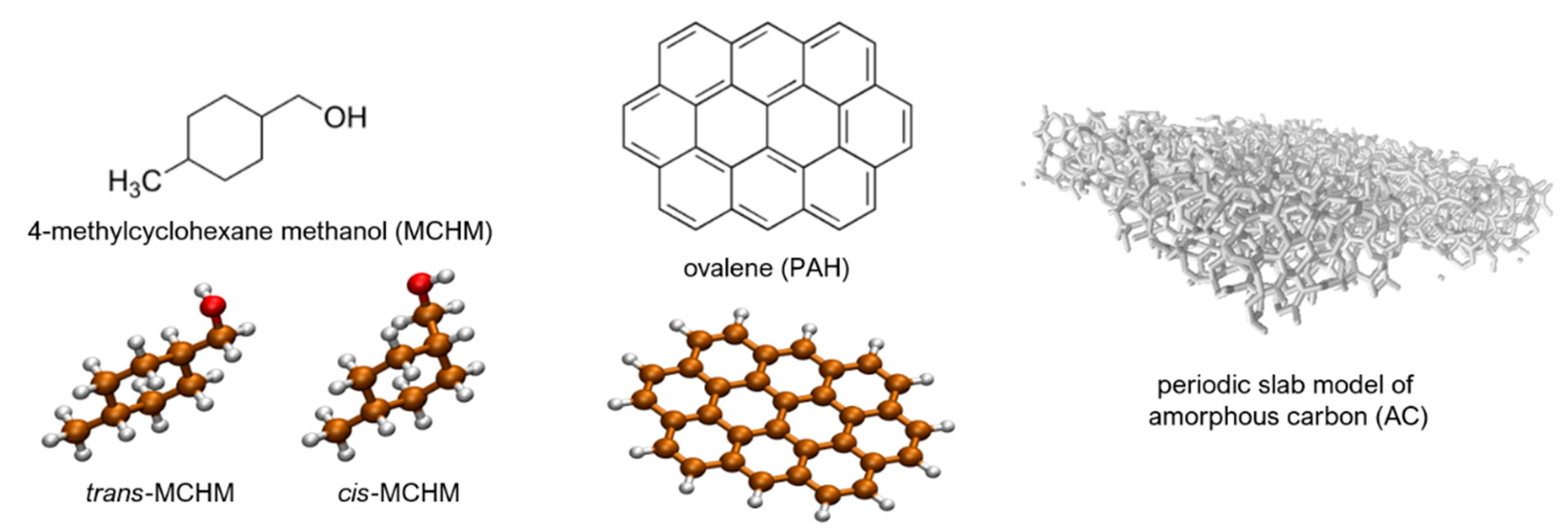
4.1. Molecular Modelling
4.1.1. cis- and trans-MCHM Plus Ovalene PAH Structures
4.1.2. Amorphous Carbon (AC) Model
4.1.3. cis- and trans-MCHM Plus AC Structures
4.2. Surface Water Temperature Estimations
4.2.1. Historical Average Water Temperatures
4.2.2. Accounting for Effects of Ambient Air Temperature (i.e., Weather)
5. Environmental Implications and Concluding Remarks
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
References
- Alexander, W.A. Particle Beam Scattering from the Vacuum-Liquid Interface. In Physical Chemistry of Gas-Liquid Interfaces; Faust, J.A., House, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Nathanson, G.M.; Davidovits, P.; Worsnop, D.R.; Kolb, C.E. Dynamics and Kinetics at the Gas−Liquid Interface. J. Phys. Chem. 1996, 100, 13007–13020. [Google Scholar] [CrossRef]
- Alexander, W.A.; Zhang, J.; Murray, V.J.; Nathanson, G.M.; Minton, T.K. Kinematics and dynamics of atomic-beam scattering on liquid and self-assembled monolayer surfaces. Faraday Discuss. 2012, 157, 355–374. [Google Scholar] [CrossRef]
- Davidovits, P.; Kolb, C.E.; Williams, L.R.; Jayne, J.T.; Worsnop, D.R. Mass Accommodation and Chemical Reactions at Gas−Liquid Interfaces. Chem. Rev. 2006, 106, 1323–1354. [Google Scholar] [CrossRef]
- Goldstein, A.H.; Galbally, I.E. Known and Unexplored Organic Constituents in the Earth’s Atmosphere. Environ. Sci. Technol. 2007, 41, 1514–1521. [Google Scholar] [CrossRef]
- Mohr, C.; Huffman, J.A.; Cubison, M.J.; Aiken, A.C.; Docherty, K.S.; Kimmel, J.R.; Ulbrich, I.M.; Hannigan, M.; Jimenez, J.L. Characterization of Primary Organic Aerosol Emissions from Meat Cooking, Trash Burning, and Motor Vehicles with High-Resolution Aerosol Mass Spectrometry and Comparison with Ambient and Chamber Observations. Environ. Sci. Technol. 2009, 43, 2443–2449. [Google Scholar] [CrossRef]
- Donaldson, D.J.; Vaida, V. The Influence of Organic Films at the Air−Aqueous Boundary on Atmospheric Processes. Chem. Rev. 2006, 106, 1445–1461. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.W.; Flores, J.M.; Lavi, A.; Abo-Riziq, A.; Rudich, Y. Changes in the Optical Properties of Benzo [a] pyrene-Coated Aerosols upon Heterogeneous Reactions with NO2 and NO3. Phys. Chem. Chem. Phys. 2011, 13, 6484–6492. [Google Scholar] [CrossRef] [PubMed]
- Freidlander, S.K. Smoke, Dust, and Haze: Fundamentals of Aerosol Dynamics, 2nd ed.; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Hatch, C.D.; Grassian, V.H. 10th Anniversary Review: Applications of analytical techniques in laboratory studies of the chemical and climatic impacts of mineral dust aerosol in the Earth’s atmosphere. J. Environ. Monit. 2008, 10, 919–934. [Google Scholar] [CrossRef]
- Prather, K.A.; Hatch, C.D.; Grassian, V.H. Analysis of atmospheric aerosols. Annu. Rev. Anal. Chem. 2008, 1, 485–514. [Google Scholar] [CrossRef] [PubMed]
- Ertl, G.; Knözinger, H.; Weitkamp, J. Handbook of Heterogeneous Catalysis; Wiley-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Ertl, G. Reactions at Surfaces: From Atoms to Complexity (Nobel Lecture). Angew. Chem. Int. Ed. 2008, 47, 3524–3535. [Google Scholar] [CrossRef]
- NSF News Release 14-018. NSF Awards Rapid Response Grants to Study West Virginia Chemical Spill. National Science Foundtion. [Online]. 30 January 2014. Available online: https://www.nsf.gov/news/news_summ.jsp?cntn_id=130304 (accessed on 30 April 2021).
- Charleston WV Weather History (6 January 2014), Yeager Airport Station. Available online: https://www.wunderground.com/history/daily/us/wv/charleston/KCRW/date/2014-1-6 (accessed on 29 April 2021).
- West Virginia Department of Health and Human Resources. 2014 Winter Storm Information for West Virginia. 7 January 2014. Available online: https://dhhr.wv.gov/healthprep/eventsandannouncements/Pages/2014-Winter-Storm-Information----WV.aspx (accessed on 4 May 2021).
- U.S. Chemical Safety and Hazard Investigation Board. Investigation Report No. 2014-01-I-WV. “Chemical Spill Contaminates Public Water Supply in Charleston, West Virginia”. Available online: https://www.csb.gov/assets/1/20/final_freedom_industries_investigation_report_(5-11-2017).pdf?15829 (accessed on 28 April 2021).
- Markham, P.; Gianato, J.; Hoyer, J. “After Action Review Emergency Response To 9 January 2014 Freedom Industries Chemical Leak” Charleston, WV, USA. 2014. Available online: https://emd.wv.gov/West%20Virginia%20Public%20Water%20Supply%20Study%20Commission/Documents/After%20Action%20Review.PDF (accessed on 4 May 2021).
- He, Y.T.; Noble, A.A.; Ziemkiewicz, P. Investigation of MCHM transport mechanisms and fate: Implications for coal beneficiation. Chemosphere 2015, 127, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Noble, A.; He, Y.T.; Ziemkiewicz, P. Partitioning Behavior of 4-Methyl Cyclohexane Methanol in Two Appalachian Coal Preparation Plants. Int. J. Coal Prep. Util. 2016, 36, 69–81. [Google Scholar] [CrossRef]
- West Virginia Attorney General, “Elk River Chemical Spill Incident Report” 8 January 2015. Charleston, WV, USA. Available online: http://ago.wv.gov/Documents/010815-ElkRiverChemicalSpill.PDF (accessed on 4 May 2021).
- Luttrell, W.E. 4-Methylcyclohexane methanol (MCHM). J. Chem. Health Saf. 2015, 22, 39–41. [Google Scholar] [CrossRef]
- McGuire, M.J. Odor Study Technical Memorandum; WVTAP: Santa Monica, CA, USA, 2014. [Google Scholar]
- Ward, K., Jr. Virginia Tech Experts Pinpoint MCHM Odors West Virginia Gazette. [Online]. 26 March 2014. Available online: https://www.wvgazettemail.com/news/special_reports/virginia-tech-experts-pinpoint-mchm-odors/article_f928c3ee-96b6-5ce0-b853-bd93482a837f.html (accessed on 5 May 2021).
- Gallagher, D.L.; Phetxumphou, K.; Smiley, E.; Dietrich, A.M. Tale of two isomers: Complexities of human odor perception for cis- and trans-4-methylcyclohexane methanol from the chemical spill in West Virginia. Environ. Sci. Technol. 2015, 49, 1319–1327. [Google Scholar] [CrossRef]
- Sain, A.; Dietrich, A.M.; Smiley, E.; Gallagher, D.L. Assessing human exposure and odor detection during showering with crude 4-(methylcyclohexyl) methanol (MCHM) contaminated drinking water. Sci. Total Environ. 2015, 538, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Whelton, A.J.; McMillan, L.; Novy, C.L.-R.; White, K.D.; Huang, X. Case study: The crude MCHM chemical spill investigation and recovery in West Virginia USA. Environ. Sci. Water Res. Technol. 2017, 3, 312–332. [Google Scholar] [CrossRef]
- Weidhaas, J.L.; Dietrich, A.M.; DeYonker, N.J.; Dupont, R.R.; Foreman, W.T.; Gallagher, D.; Gallagher, J.E.G.; Whelton, A.J.; Alexander, W.A. Enabling Science Support for Better Decision-Making when Responding to Chemical Spills. J. Environ. Qual. 2016, 45, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.J. Responding to crisis: The West Virginia chemical spill. Environ. Sci. Technol. 2014, 48, 3095. [Google Scholar] [CrossRef]
- Schnoor, J.L. Re-emergence of emerging contaminants. Environ. Sci. Technol. 2014, 48, 11019–11020. [Google Scholar] [CrossRef]
- Weidhaas, J.; Lin, L.-S.; Buzby, K. A case study for orphaned chemicals: 4-methylcyclohexanemethanol (MCHM) and propylene glycol phenyl ether (PPH) in riverine sediment and water treatment processes. Sci. Total Environ. 2017, 574, 1396–1404. [Google Scholar] [CrossRef]
- USEPA. The Use of Structure-Activity Relationships (SAR) in the High Production Volume Chemicals Challenge Program. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P1008MA9.TXT (accessed on 4 May 2021).
- West Virginia, Office of the Governor, “Gov. Tomblin’s Update on State of Emergency” West Virginia Press. [Online]. 10 January 2014. Available online: https://wvpress.org/copydesk/insight/gov-tomblins-update-state-emergency/ (accessed on 5 May 2021).
- West Virginia American Water, American Water Company Customer Advisory ** Alert **. [Online]. 9 January 2014. Available online: https://dhhr.wv.gov/healthprep/eventsandannouncements/Pages/American-Water-Company-Customer-Advisory.aspx (accessed on 5 May 2021).
- Associated Press. School Closes after Licorice Odor Present. Herald Dispatch. [Online]. 17 February 2014. Available online: https://www.herald-dispatch.com/news/recent_news/school-closes-after-licorice-odor-present/article_5b79ae09-f1bf-5b27-8045-8ab9db7a7baa.html (accessed on 4 May 2021).
- Jenkins, J. Kanawha Closes More Schools over Licorice Odor. West Virginia MetroNews. [Online]. 6 February 2014. Available online: https://wvmetronews.com/2014/02/06/kanawha-closes-more-schools-over-licorice-odor/ (accessed on 4 May 2021).
- Mays, M. Water Response Team Called to Four Schools Monday. Charleston Gazette-Mail. [Online]. 17 February 2014. Available online: https://www.wvgazettemail.com/news/special_reports/water-response-team-called-to-four-schools-monday/article_872ca250-83c7-57a2-9a80-02a55f8ed269.html (accessed on 4 May 2021).
- Ahart, M.; Gallagher, D.L.; Scardina, P.; Dietrich, A.M. Industrial Spills and Water Distribution: Crude MCHM Sorption and Desorption in Polymer Pipes and Linings. J. Environ. Eng. 2016, 142, 04016045. [Google Scholar] [CrossRef]
- Mistich, D. Update: Testing at WVAM Indicates Filtering Process Adds Trace Amounts of MCHM to Water Supply. WVPB. [Online]. 25 March 2014. Available online: https://www.wvpublic.org/news/2014-03-25/update-testing-at-wvam-indicates-filtering-process-adds-trace-amounts-of-mchm-to-water-supply (accessed on 29 April 2021).
- Associated Press. WVAW Changing Carbon Filters at Charleston Plant. WVPB. [Online]. 1 April 2014. Available online: https://www.wvpublic.org/news/2014-04-01/wvaw-changing-carbon-filters-at-charleston-plant (accessed on 5 May 2021).
- Ricolleau, C.; Le Bouar, Y.; Amara, H.; Landon-Cardinal, O.; Alloyeau, D. Random vs. realistic amorphous carbon models for high resolution microscopy and electron diffraction. J. Appl. Phys. 2013, 114, 213504. [Google Scholar] [CrossRef]
- De Yonker, N.J.; Charbonnet, K.A.; Alexander, W.A. Dipole moments of trans- and cis-(4-methylcyclohexyl)methanol (4-MCHM): Obtaining the right conformer for the right reason. Phys. Chem. Chem. Phys. 2016, 18, 17856–17867. [Google Scholar] [CrossRef]
- Dietrich, A.M.; Thomas, A.; Zhao, Y.; Smiley, E.; Shanaiah, N.; Ahart, M.; Charbonnet, K.A.; DeYonker, N.J.; Alexander, W.A.; Gallagher, D.L. Partitioning, Aqueous Solubility, and Dipole Moment Data for cis- and trans-(4-Methylcyclohexyl)methanol, Principal Contaminants of the West Virginia Chemical Spill. Environ. Sci. Technol. Lett. 2015, 2, 123–127. [Google Scholar] [CrossRef]
- US EPA. Estimation Programs Interface Suite™ for Microsoft® Windows, v 4.11; United States Environmental Protection Agency: Washington, DC, USA, 2017.
- SPARC Developed by ARChem, Inc. Danielsville, GA, USA.
- Whelton, A.J.; McMillan, L.; Connell, M.; Kelley, K.M.; Gill, J.P.; White, K.D.; Gupta, R.; Dey, R.; Novy, C. Residential Tap Water Contamination Following the Freedom Industries Chemical Spill: Perceptions, Water Quality, and Health Impacts. Environ. Sci. Technol. 2015, 49, 813–823. [Google Scholar] [CrossRef]
- ACD/Labs Percepta Platform-Phys Chem Module, Advanced Chemistry Development. Available online: http://www.chemspider.com/Chemical-Structure.105625.html (accessed on 2 May 2021).
- ChemAxon Chemicalize Platform. Available online: Chemicalize.com (accessed on 2 May 2021).
- COSMOS-RS, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands. Available online: https://www.scm.com/COSMO-RS/ (accessed on 2 May 2021).
- Jeter, T.S.; Sarvera, E.A.; McNair, H.M.; Rezaeea, M. 4-MCHM sorption to and desorption from granular activated carbon and raw coal. Chemosphere 2016, 157, 106–165. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Ward, K., Jr. MCHM Odors Show Chemical not Gone, Experts Say. Charleston Gazette-Mail. [Online]. 18 March 2014. Available online: https://www.wvgazettemail.com/news/special_reports/mchm-odors-show-chemical-not-gone-experts-say/article_8f5da982-a634-58b8-8418-872c7c9fbe6d.html (accessed on 4 May 2021).
- Chemical Computing Group. Molecular Operating Environment (MOE); Chemical Computing Group Inc.: Montreal, QC, Canada, 2019. [Google Scholar]
- Labute, P. Low Mode MD-Implicit Low Mode Velocity Filtering Applied to Conformational Search of Macrocycles and Protein Loops. J. Chem. Inf. Model. 2010, 50, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A. The Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comp. Chem. 1996, 17, 490–641. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Phillips, J.C.; Hardy, D.J.; Maia, J.D.C.; Stone, J.E.; Ribeiro, J.V.; Bernardi, R.C.; Buch, R.; Giacomo Fiorin, G.; Henin, J.; Jiang, W.; et al. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J. Chem. Phys. 2020, 153, 044130. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.R.; Müller, K. MAB, a generally applicable molecular force field for structure modelling in medicinal chemistry. J. Comput. Aided Mol. Des. 1995, 9, 251–268. [Google Scholar] [CrossRef] [PubMed]
- US Geological Survey. National Water Information System Data Available on the World Wide Web (USGS Water Data for the Nation). Available online: https://waterdata.usgs.gov/nwis/inventory/?site_no=03198000&agency_cd=USGS (accessed on 10 April 2021).
- Charleston, WV Weather History. Available online: https://www.wunderground.com/history/daily/us/wv/charleston/KCRW (accessed on 30 April 2021).
| Difference in Isomers, Etrans—Ecis 1 | ||||
|---|---|---|---|---|
| System | MMFF94x | Amber14:EHT | PM7 | B3LYP-D3 |
| MCHM:PAH | 0.1 | - | 1.9 | 1.4 2 |
| MCHM:AC | 1.3 | 2.4 | 4.7 3 | - |
| 0 °C | 10 °C | 20 °C | 30 °C | |
|---|---|---|---|---|
| 1.3 | 1.29 × 101 | 1.18 × 101 | 1.08 × 101 | 1.00 × 101 |
| 2.4 | 1.06 × 102 | 9.02 × 101 | 7.74 × 101 | 6.70 × 101 |
| 4.0 | 2.40 × 103 | 1.82 × 103 | 1.41 × 103 | 1.11 × 103 |
| 8.0 | 5.53 × 106 | 3.20 × 106 | 1.92 × 106 | 1.19 × 106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexander, W.A. Methyl-Cyclohexane Methanol (MCHM) Isomer-Dependent Binding on Amorphous Carbon Surfaces. Molecules 2021, 26, 3411. https://doi.org/10.3390/molecules26113411
Alexander WA. Methyl-Cyclohexane Methanol (MCHM) Isomer-Dependent Binding on Amorphous Carbon Surfaces. Molecules. 2021; 26(11):3411. https://doi.org/10.3390/molecules26113411
Chicago/Turabian StyleAlexander, William A. 2021. "Methyl-Cyclohexane Methanol (MCHM) Isomer-Dependent Binding on Amorphous Carbon Surfaces" Molecules 26, no. 11: 3411. https://doi.org/10.3390/molecules26113411
APA StyleAlexander, W. A. (2021). Methyl-Cyclohexane Methanol (MCHM) Isomer-Dependent Binding on Amorphous Carbon Surfaces. Molecules, 26(11), 3411. https://doi.org/10.3390/molecules26113411







