Neurodegeneration in Multiple Sclerosis: Symptoms of Silent Progression, Biomarkers and Neuroprotective Therapy—Kynurenines Are Important Players
Abstract
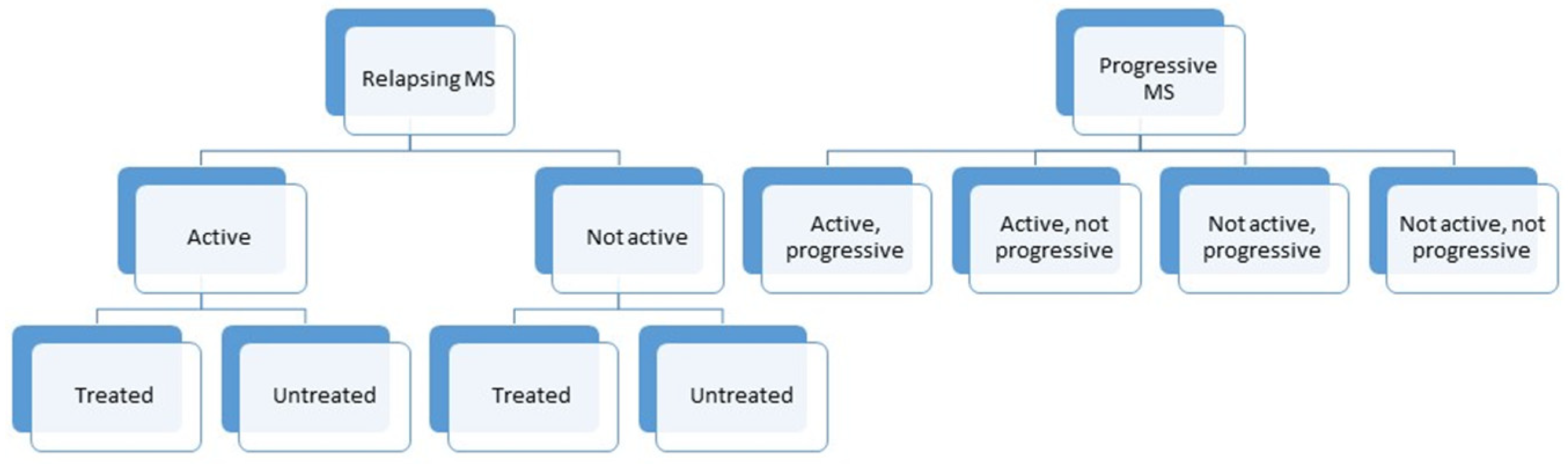
:1. Introduction
2. The Pathogenic Mechanism behind Multiple Sclerosis
3. Progression Independent of Relapse (and MRI) Activity (PIRA and PIRMA)
3.1. Cognitive Impairment
3.2. Other Pathophysiological Symptoms: Fatigue and Depression
3.3. The Problem of “Benign” MS
4. Biomarkers
4.1. Imaging Biomarkers
4.1.1. MRI
4.1.2. Optical Coherence Tomography
4.2. Molecular Biomarkers
4.2.1. Neurofilaments
4.2.2. Other Possible Molecular Biomarkers
5. Kynurenines as Biomarkers for Progression and as Possible Therapeutic Targets
5.1. Kynurenines and the Kynurenine Pathway: Neuroactive Metabolites
5.1.1. Kynurenic Acid
5.1.2. Quinolinic Acid
5.2. The Role of the Kynurenine Pathway in Neurodegenerative Conditions
5.2.1. Kynurenines in “Classical” Neurodegenerative Diseases
Huntington’s Disease
Alzheimer’s Disease
Parkinson’s Disease
5.2.2. Kynurenines in the Retina and Its Diseases
5.2.3. Mood Disorders and Suicide
5.3. The Role of the Kynurenine Pathway in the Neurodegenerative Processes of Multiple Sclerosis
5.3.1. In Vitro Results and Animal Model Studies
5.3.2. The Kynurenine Pathway Metabolites in Multiple Sclerosis
5.4. Therapeutic Capabilities of Kynurenines
5.4.1. Preclinical Studies
5.4.2. Clinical Studies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-HAA | 3-hydroxyanthranilic acid |
| 3-HAO | 3-hydroxyanthranilate oxidase |
| 3-HK | 3-hydroxy-l-kynurenine |
| 9HP | 9-hole peg test |
| AA | anthranilic acid |
| AD | Alzheimer’s disease |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| Aβ | β-amyloid |
| BBB | blood–brain barrier |
| BICAMS | brief international cognitive assessment for multiple sclerosis |
| BVMT-R | brief visuospatial memory test |
| CDA | confirmed disability accumulation |
| CGRP | calcitonin gene-related peptide |
| CI | cognitive impairment |
| CIS | clinically isolated syndrome |
| CNS | central nervous system |
| CVLT-II | California verbal learning test |
| CSF | cerebrospinal fluid |
| DMT | disease modifying therapy |
| EAE | experimental autoimmune encephalomyelitis |
| ECL | electrochemiluminscent assay |
| EDSS | expanded disability status scale |
| ELISA | enzyme-linked immunosorbent assay |
| GPR35 | orphan G-protein coupled receptor |
| HTT | huntingtin gene |
| IDO | indolamine 2,3-dioxygenase |
| IFN-γ | interferon-γ |
| JCV | John Cunningham virus |
| KAT | kynurenine aminotransferase |
| KMO | kynurenine 3-monooxygenase |
| KP | kynurenine pathway |
| KYNA | kynurenic acid |
| L-KYN | L-kynurenine |
| LP | lumbar puncture |
| mCGL | macular ganglion cell layer |
| MHC | major histocompatibility complex |
| MRI | magnetic resonance imaging |
| MS | multiple sclerosis |
| MSFC | multiple sclerosis functional composite |
| NAD+ | nicotinamide adenine dinucleotide |
| NADP+ | nicotinamide adenine dinucleotide phosphate |
| NEDA | no evidence of disease activity |
| NfL | neurofilament |
| NMDA | N-methyl-d-aspartate |
| OCT | optical coherence tomography |
| ON | optic neuritis |
| OPN | osteopontin |
| PASAT | paced auditory serial adding test |
| PD | Parkinson’s disease |
| PetCO2 | end-tidal partial pressure of CO2 |
| PIRA | progression independent from relapse activity |
| PIRMA | progression independent from relapse and MRI activity |
| PNS | peripheral nervous system |
| PPAR-γ | peroxisome proliferator-activated receptor-γ |
| PPMS | primary progressive multiple sclerosis |
| QoL | quality of life |
| QUIN | quinolinic acid |
| RA | radial artery |
| RGC | retinal ganglion cell |
| RIS | radiologically isolated syndrome |
| RNFL | retinal nerve fiber layer |
| ROS | reactive oxygen species |
| RRMS | relapsing-remitting multiple sclerosis |
| SDMT | symbol digit modalities test |
| SiMoA | single molecular array |
| SPMS | secondary progressive multiple sclerosis |
| STA | superficial temporal artery |
| T25W | timed 25-feet walk test |
| TDO | tryptophan 2,3-dioxygenase |
| TNF-α | tumor necrosis factor-α |
| Trp | tryptophan |
| VMCA | blood flow velocity of the middle cerebral artery |
| WBA | whole brain atrophy |
| WHO | World Health Organization |
References
- Lublin, F.D. New multiple sclerosis phenotypic classification. Eur. Neurol. 2014, 72 (Suppl. S1), 1–5. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Confavreux, C.; Vukusic, S.; Moreau, T.; Adeleine, P. Relapses and progression of disability in multiple sclerosis. N. Engl. J. Med. 2000, 343, 1430–1438. [Google Scholar] [CrossRef]
- Leray, E.; Yaouanq, J.; Le Page, E.; Coustans, M.; Laplaud, D.; Oger, J.; Edan, G. Evidence for a two-stage disability progression in multiple sclerosis. Brain 2010, 133, 1900–1913. [Google Scholar] [CrossRef] [Green Version]
- Abdelhak, A.; Weber, M.S.; Tumani, H. Primary progressive multiple sclerosis: Putting together the puzzle. Front. Neurol. 2017, 8, 234. [Google Scholar] [CrossRef] [Green Version]
- Friese, M.A.; Schattling, B.; Fugger, L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat. Rev. Neurol. 2014, 10, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front. Immunol. 2018, 9, 3116. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H.; Bradl, M. Multiple sclerosis: Experimental models and reality. Acta Neuropathol. 2017, 133, 223–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolders, J.; Heutinck, K.M.; Fransen, N.L.; Remmerswaal, E.B.M.; Hombrink, P.; Ten Berge, I.J.M.; van Lier, R.A.W.; Huitinga, I.; Hamann, J. Tissue-resident memory T cells populate the human brain. Nat. Commun. 2018, 9, 4593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado-Santos, J.; Saji, E.; Troscher, A.R.; Paunovic, M.; Liblau, R.; Gabriely, G.; Bien, C.G.; Bauer, J.; Lassmann, H. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 2018, 141, 2066–2082. [Google Scholar] [CrossRef] [PubMed]
- Van Nierop, G.P.; van Luijn, M.M.; Michels, S.S.; Melief, M.J.; Janssen, M.; Langerak, A.W.; Ouwendijk, W.J.D.; Hintzen, R.Q.; Verjans, G. Phenotypic and functional characterization of T cells in white matter lesions of multiple sclerosis patients. Acta Neuropathol. 2017, 134, 383–401. [Google Scholar] [CrossRef]
- Li, R.; Patterson, K.R.; Bar-Or, A. Reassessing B cell contributions in multiple sclerosis. Nat. Immunol. 2018, 19, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Lucchinetti, C.; Bruck, W.; Parisi, J.; Scheithauer, B.; Rodriguez, M.; Lassmann, H. Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann. Neurol. 2000, 47, 707–717. [Google Scholar] [CrossRef]
- Aloisi, F.; Pujol-Borrell, R. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 2006, 6, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Esiri, M.M.; Gay, D. Immunological and neuropathological significance of the Virchow-Robin space. J. Neurol. Sci. 1990, 100, 3–8. [Google Scholar] [CrossRef]
- Lassmann, H.; van Horssen, J.; Mahad, D. Progressive multiple sclerosis: Pathology and pathogenesis. Nat. Rev. Neurol. 2012, 8, 647–656. [Google Scholar] [CrossRef]
- Frischer, J.M.; Weigand, S.D.; Guo, Y.; Kale, N.; Parisi, J.E.; Pirko, I.; Mandrekar, J.; Bramow, S.; Metz, I.; Bruck, W.; et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann. Neurol. 2015, 78, 710–721. [Google Scholar] [CrossRef]
- Luchetti, S.; Fransen, N.L.; van Eden, C.G.; Ramaglia, V.; Mason, M.; Huitinga, I. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: A retrospective autopsy cohort analysis. Acta Neuropathol. 2018, 135, 511–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnett, M.H.; Prineas, J.W. Relapsing and remitting multiple sclerosis: Pathology of the newly forming lesion. Ann. Neurol. 2004, 55, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Baxi, E.G.; DeBruin, J.; Tosi, D.M.; Grishkan, I.V.; Smith, M.D.; Kirby, L.A.; Strasburger, H.J.; Fairchild, A.N.; Calabresi, P.A.; Gocke, A.R. Transfer of myelin-reactive th17 cells impairs endogenous remyelination in the central nervous system of cuprizone-fed mice. J. Neurosci. 2015, 35, 8626–8639. [Google Scholar] [CrossRef] [PubMed]
- Scheld, M.; Ruther, B.J.; Grosse-Veldmann, R.; Ohl, K.; Tenbrock, K.; Dreymuller, D.; Fallier-Becker, P.; Zendedel, A.; Beyer, C.; Clarner, T.; et al. Neurodegeneration triggers peripheral immune cell recruitment into the forebrain. J. Neurosci. 2016, 36, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Veto, S.; Acs, P.; Bauer, J.; Lassmann, H.; Berente, Z.; Setalo, G., Jr.; Borgulya, G.; Sumegi, B.; Komoly, S.; Gallyas, F., Jr.; et al. Inhibiting poly(ADP-ribose) polymerase: A potential therapy against oligodendrocyte death. Brain 2010, 133, 822–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziabreva, I.; Campbell, G.; Rist, J.; Zambonin, J.; Rorbach, J.; Wydro, M.M.; Lassmann, H.; Franklin, R.J.; Mahad, D. Injury and differentiation following inhibition of mitochondrial respiratory chain complex IV in rat oligodendrocytes. Glia 2010, 58, 1827–1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahad, D.J.; Ziabreva, I.; Campbell, G.; Lax, N.; White, K.; Hanson, P.S.; Lassmann, H.; Turnbull, D.M. Mitochondrial changes within axons in multiple sclerosis. Brain 2009, 132, 1161–1174. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Fischer, M.T.; Bauer, J.; Felts, P.A.; Smith, K.J.; Misu, T.; Fujihara, K.; Bradl, M.; Lassmann, H. Inflammation induced by innate immunity in the central nervous system leads to primary astrocyte dysfunction followed by demyelination. Acta Neuropathol. 2010, 120, 223–236. [Google Scholar] [CrossRef] [Green Version]
- Kappos, L.; Wolinsky, J.S.; Giovannoni, G.; Arnold, D.L.; Wang, Q.; Bernasconi, C.; Model, F.; Koendgen, H.; Manfrini, M.; Belachew, S.; et al. Contribution of relapse-independent progression vs. relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020, 77, 1132–1140. [Google Scholar] [CrossRef]
- Kappos, L.; Butzkueven, H.; Wiendl, H.; Spelman, T.; Pellegrini, F.; Chen, Y.; Dong, Q.; Koendgen, H.; Belachew, S.; Trojano, M.; et al. Greater sensitivity to multiple sclerosis disability worsening and progression events using a roving versus a fixed reference value in a prospective cohort study. Mult. Scler. 2018, 24, 963–973. [Google Scholar] [CrossRef] [Green Version]
- University of California, San Francisco MS-EPIC Team; Cree, B.A.C.; Hollenbach, J.A.; Bove, R.; Kirkish, G.; Sacco, S.; Caverzasi, E.; Bischof, A.; Gundel, T.; Zhu, A.H.; et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann. Neurol. 2019, 85, 653–666. [Google Scholar] [CrossRef] [PubMed]
- University of California; Cree, B.A.; Gourraud, P.A.; Oksenberg, J.R.; Bevan, C.; Crabtree-Hartman, E.; Gelfand, J.M.; Goodin, D.S.; Graves, J.; Green, A.J.; et al. Long-term evolution of multiple sclerosis disability in the treatment era. Ann. Neurol. 2016, 80, 499–510. [Google Scholar] [CrossRef]
- Giovannoni, G.; Turner, B.; Gnanapavan, S.; Offiah, C.; Schmierer, K.; Marta, M. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult. Scler. Relat. Disord. 2015, 4, 329–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef]
- Chiaravalloti, N.D.; DeLuca, J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008, 7, 1139–1151. [Google Scholar] [CrossRef]
- Amato, M.P.; Hakiki, B.; Goretti, B.; Rossi, F.; Stromillo, M.L.; Giorgio, A.; Roscio, M.; Ghezzi, A.; Guidi, L.; Bartolozzi, M.L.; et al. Association of MRI metrics and cognitive impairment in radiologically isolated syndromes. Neurology 2012, 78, 309–314. [Google Scholar] [CrossRef]
- Cortese, M.; Riise, T.; Bjornevik, K.; Bhan, A.; Farbu, E.; Grytten, N.; Hogenesch, I.; Midgard, R.; Smith Simonsen, C.; Telstad, W.; et al. Preclinical disease activity in multiple sclerosis: A prospective study of cognitive performance prior to first symptom. Ann. Neurol. 2016, 80, 616–624. [Google Scholar] [CrossRef]
- Sandi, D.; Biernacki, T.; Szekeres, D.; Fuvesi, J.; Kincses, Z.T.; Rozsa, C.; Matyas, K.; Kasa, K.; Matolcsi, J.; Zboznovits, D.; et al. Prevalence of cognitive impairment among Hungarian patients with relapsing-remitting multiple sclerosis and clinically isolated syndrome. Mult. Scler. Relat. Disord. 2017, 17, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Hojsgaard Chow, H.; Schreiber, K.; Magyari, M.; Ammitzboll, C.; Bornsen, L.; Romme Christensen, J.; Ratzer, R.; Soelberg Sorensen, P.; Sellebjerg, F. Progressive multiple sclerosis, cognitive function, and quality of life. Brain Behav. 2018, 8, e00875. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.M.; Leo, G.J.; Bernardin, L.; Unverzagt, F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 1991, 41, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Glanz, B.I.; Holland, C.M.; Gauthier, S.A.; Amunwa, E.L.; Liptak, Z.; Houtchens, M.K.; Sperling, R.A.; Khoury, S.J.; Guttmann, C.R.; Weiner, H.L. Cognitive dysfunction in patients with clinically isolated syndromes or newly diagnosed multiple sclerosis. Mult. Scler. 2007, 13, 1004–1010. [Google Scholar] [CrossRef]
- Langdon, D.W. Cognition in multiple sclerosis. Curr. Opin. Neurol. 2011, 24, 244–249. [Google Scholar] [CrossRef]
- Langdon, D.W.; Amato, M.P.; Boringa, J.; Brochet, B.; Foley, F.; Fredrikson, S.; Hamalainen, P.; Hartung, H.P.; Krupp, L.; Penner, I.K.; et al. Recommendations for a brief international cognitive assessment for multiple sclerosis (BICAMS). Mult. Scler. 2012, 18, 891–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandi, D.; Rudisch, T.; Fuvesi, J.; Fricska-Nagy, Z.; Huszka, H.; Biernacki, T.; Langdon, D.W.; Langane, E.; Vecsei, L.; Bencsik, K. The Hungarian validation of the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) battery and the correlation of cognitive impairment with fatigue and quality of life. Mult. Scler. Relat. Disord. 2015, 4, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Benedict, R.H.; Morrow, S.; Rodgers, J.; Hojnacki, D.; Bucello, M.A.; Zivadinov, R.; Weinstock-Guttman, B. Characterizing cognitive function during relapse in multiple sclerosis. Mult. Scler. 2014, 20, 1745–1752. [Google Scholar] [CrossRef]
- Giedraitiene, N.; Kaubrys, G.; Kizlaitiene, R. Cognition during and after multiple sclerosis relapse as assessed with the brief international cognitive assessment for multiple sclerosis. Sci. Rep. 2018, 8, 8169. [Google Scholar] [CrossRef]
- Benedict, R.H.; Pol, J.; Yasin, F.; Hojnacki, D.; Kolb, C.; Eckert, S.; Tacca, B.; Drake, A.; Wojcik, C.; Morrow, S.A.; et al. Recovery of cognitive function after relapse in multiple sclerosis. Mult. Scler. 2021, 27, 71–78. [Google Scholar] [CrossRef]
- Mahad, D.H.; Trapp, B.D.; Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015, 14, 183–193. [Google Scholar] [CrossRef]
- DeLuca, G.C.; Yates, R.L.; Beale, H.; Morrow, S.A. Cognitive impairment in multiple sclerosis: Clinical, radiologic and pathologic insights. Brain Pathol. 2015, 25, 79–98. [Google Scholar] [CrossRef]
- Manjaly, Z.M.; Harrison, N.A.; Critchley, H.D.; Do, C.T.; Stefanics, G.; Wenderoth, N.; Lutterotti, A.; Muller, A.; Stephan, K.E. Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Kister, I.; Bacon, T.E.; Chamot, E.; Salter, A.R.; Cutter, G.R.; Kalina, J.T.; Herbert, J. Natural history of multiple sclerosis symptoms. Int. J. MS Care 2013, 15, 146–158. [Google Scholar] [CrossRef] [Green Version]
- Iriarte, J.; Subira, M.L.; Castro, P. Modalities of fatigue in multiple sclerosis: Correlation with clinical and biological factors. Mult. Scler. 2000, 6, 124–130. [Google Scholar] [CrossRef]
- Kluger, B.M.; Krupp, L.B.; Enoka, R.M. Fatigue and fatigability in neurologic illnesses: Proposal for a unified taxonomy. Neurology 2013, 80, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Losonczi, E.; Bencsik, K.; Rajda, C.; Lencses, G.; Torok, M.; Vecsei, L. Validation of the Fatigue Impact Scale in Hungarian patients with multiple sclerosis. Qual. Life Res. 2011, 20, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Boeschoten, R.E.; Braamse, A.M.J.; Beekman, A.T.F.; Cuijpers, P.; van Oppen, P.; Dekker, J.; Uitdehaag, B.M.J. Prevalence of depression and anxiety in Multiple Sclerosis: A systematic review and meta-analysis. J. Neurol. Sci. 2017, 372, 331–341. [Google Scholar] [CrossRef]
- Fisk, J.D.; Pontefract, A.; Ritvo, P.G.; Archibald, C.J.; Murray, T.J. The impact of fatigue on patients with multiple sclerosis. Can. J. Neurol. Sci. 1994, 21, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biernacki, T.; Sandi, D.; Kincses, Z.T.; Fuvesi, J.; Rozsa, C.; Matyas, K.; Vecsei, L.; Bencsik, K. Contributing factors to health-related quality of life in multiple sclerosis. Brain Behav. 2019, 9, e01466. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.M.; Poettgen, J.; Fischer, A.; Gold, S.; Stellmann, J.P.; Heesen, C. Impairment and restrictions in possibly benign multiple sclerosis. Brain Behav. 2019, 9, e01259. [Google Scholar] [CrossRef] [PubMed]
- Ton, A.M.M.; Vasconcelos, C.C.F.; Alvarenga, R.M.P. Benign multiple sclerosis: Aspects of cognition and neuroimaging. Arq. Neuropsiquiatr. 2017, 75, 394–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. International Programme on Chemical Safety—Biomarkers in Risk Assessment: Validity and Validation. 2001. Available online: http://www.inchem.org/documents/ehc/ehc/ehc222.htm (accessed on 29 April 2021).
- Tintore, M.; Rovira, A.; Rio, J.; Otero-Romero, S.; Arrambide, G.; Tur, C.; Comabella, M.; Nos, C.; Arevalo, M.J.; Negrotto, L.; et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain 2015, 138, 1863–1874. [Google Scholar] [CrossRef] [Green Version]
- Comi, G.; Radaelli, M.; Soelberg Sorensen, P. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet 2017, 389, 1347–1356. [Google Scholar] [CrossRef]
- Kapica-Topczewska, K.; Collin, F.; Tarasiuk, J.; Czarnowska, A.; Chorazy, M.; Mironczuk, A.; Kochanowicz, J.; Kulakowska, A. Assessment of disability progression independent of relapse and brain MRI activity in patients with multiple sclerosis in poland. J. Clin. Med. 2021, 10, 868. [Google Scholar] [CrossRef]
- Giovannoni, G.; Butzkueven, H.; Dhib-Jalbut, S.; Hobart, J.; Kobelt, G.; Pepper, G.; Sormani, M.P.; Thalheim, C.; Traboulsee, A.; Vollmer, T. Brain health: Time matters in multiple sclerosis. Mult. Scler. Relat. Disord. 2016, 9 (Suppl. S1), S5–S48. [Google Scholar] [CrossRef] [Green Version]
- Battaglini, M.; Gentile, G.; Luchetti, L.; Giorgio, A.; Vrenken, H.; Barkhof, F.; Cover, K.S.; Bakshi, R.; Chu, R.; Sormani, M.P.; et al. Lifespan normative data on rates of brain volume changes. Neurobiol. Aging 2019, 81, 30–37. [Google Scholar] [CrossRef]
- Zivadinov, R.; Uher, T.; Hagemeier, J.; Vaneckova, M.; Ramasamy, D.P.; Tyblova, M.; Bergsland, N.; Seidl, Z.; Dwyer, M.G.; Krasensky, J.; et al. A serial 10-year follow-up study of brain atrophy and disability progression in RRMS patients. Mult. Scler. 2016, 22, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Roosendaal, S.D.; Bendfeldt, K.; Vrenken, H.; Polman, C.H.; Borgwardt, S.; Radue, E.W.; Kappos, L.; Pelletier, D.; Hauser, S.L.; Matthews, P.M.; et al. Grey matter volume in a large cohort of MS patients: Relation to MRI parameters and disability. Mult. Scler. 2011, 17, 1098–1106. [Google Scholar] [CrossRef]
- De Stefano, N.; Stromillo, M.L.; Giorgio, A.; Bartolozzi, M.L.; Battaglini, M.; Baldini, M.; Portaccio, E.; Amato, M.P.; Sormani, M.P. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jehna, M.; Pirpamer, L.; Khalil, M.; Fuchs, S.; Ropele, S.; Langkammer, C.; Pichler, A.; Stulnig, F.; Deutschmann, H.; Fazekas, F.; et al. Periventricular lesions correlate with cortical thinning in multiple sclerosis. Ann. Neurol. 2015, 78, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Charil, A.; Dagher, A.; Lerch, J.P.; Zijdenbos, A.P.; Worsley, K.J.; Evans, A.C. Focal cortical atrophy in multiple sclerosis: Relation to lesion load and disability. Neuroimage 2007, 34, 509–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andorra, M.; Nakamura, K.; Lampert, E.J.; Pulido-Valdeolivas, I.; Zubizarreta, I.; Llufriu, S.; Martinez-Heras, E.; Sola-Valls, N.; Sepulveda, M.; Tercero-Uribe, A.; et al. Assessing biological and methodological aspects of brain volume loss in multiple sclerosis. JAMA Neurol. 2018, 75, 1246–1255. [Google Scholar] [CrossRef]
- Toth, E.; Farago, P.; Kiraly, A.; Szabo, N.; Vereb, D.; Kocsis, K.; Kincses, B.; Sandi, D.; Bencsik, K.; Vecsei, L.; et al. The contribution of various MRI parameters to clinical and cognitive disability in multiple sclerosis. Front. Neurol. 2018, 9, 1172. [Google Scholar] [CrossRef]
- Calabrese, M.; Rinaldi, F.; Mattisi, I.; Grossi, P.; Favaretto, A.; Atzori, M.; Bernardi, V.; Barachino, L.; Romualdi, C.; Rinaldi, L.; et al. Widespread cortical thinning characterizes patients with MS with mild cognitive impairment. Neurology 2010, 74, 321–328. [Google Scholar] [CrossRef]
- Pravata, E.; Rocca, M.A.; Valsasina, P.; Riccitelli, G.C.; Gobbi, C.; Comi, G.; Falini, A.; Filippi, M. Gray matter trophism, cognitive impairment, and depression in patients with multiple sclerosis. Mult. Scler. 2017, 23, 1864–1874. [Google Scholar] [CrossRef]
- Sepulcre, J.; Masdeu, J.C.; Goni, J.; Arrondo, G.; Velez de Mendizabal, N.; Bejarano, B.; Villoslada, P. Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult. Scler. 2009, 15, 337–344. [Google Scholar] [CrossRef]
- Cifelli, A.; Arridge, M.; Jezzard, P.; Esiri, M.M.; Palace, J.; Matthews, P.M. Thalamic neurodegeneration in multiple sclerosis. Ann. Neurol. 2002, 52, 650–653. [Google Scholar] [CrossRef]
- Vercellino, M.; Plano, F.; Votta, B.; Mutani, R.; Giordana, M.T.; Cavalla, P. Grey matter pathology in multiple sclerosis. J. Neuropathol. Exp. Neurol. 2005, 64, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.J.; Cen, S.Y.; Khadka, S.; Liu, S.; Kornak, J.; Shi, Y.; Zheng, L.; Hauser, S.L.; Pelletier, D. Thalamic atrophy in multiple sclerosis: A magnetic resonance imaging marker of neurodegeneration throughout disease. Ann. Neurol. 2018, 83, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Gonzalez-Moron, D.; Garcea, O. Optical coherence tomography as a biomarker of neurodegeneration in multiple sclerosis: A review. Mult. Scler. Relat. Disord. 2018, 22, 77–82. [Google Scholar] [CrossRef]
- Petzold, A.; Balcer, L.J.; Calabresi, P.A.; Costello, F.; Frohman, T.C.; Frohman, E.M.; Martinez-Lapiscina, E.H.; Green, A.J.; Kardon, R.; Outteryck, O.; et al. Retinal layer segmentation in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2017, 16, 797–812. [Google Scholar] [CrossRef] [Green Version]
- Varhaug, K.N.; Torkildsen, O.; Myhr, K.M.; Vedeler, C.A. Neurofilament light chain as a biomarker in multiple sclerosis. Front. Neurol. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cairns, N.J.; Lee, V.M.; Trojanowski, J.Q. The cytoskeleton in neurodegenerative diseases. J. Pathol. 2004, 204, 438–449. [Google Scholar] [CrossRef] [Green Version]
- Thebault, S.; Bose, G.; Booth, R.; Freedman, M.S. Serum neurofilament light in MS: The first true blood-based biomarker? Mult. Scler. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kuhle, J.; Barro, C.; Andreasson, U.; Derfuss, T.; Lindberg, R.; Sandelius, A.; Liman, V.; Norgren, N.; Blennow, K.; Zetterberg, H. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin. Chem. Lab. Med. 2016, 54, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Modvig, S.; Degn, M.; Roed, H.; Sorensen, T.L.; Larsson, H.B.; Langkilde, A.R.; Frederiksen, J.L.; Sellebjerg, F. Cerebrospinal fluid levels of chitinase 3-like 1 and neurofilament light chain predict multiple sclerosis development and disability after optic neuritis. Mult. Scler. 2015, 21, 1761–1770. [Google Scholar] [CrossRef]
- Arrambide, G.; Espejo, C.; Eixarch, H.; Villar, L.M.; Alvarez-Cermeno, J.C.; Picon, C.; Kuhle, J.; Disanto, G.; Kappos, L.; Sastre-Garriga, J.; et al. Neurofilament light chain level is a weak risk factor for the development of MS. Neurology 2016, 87, 1076–1084. [Google Scholar] [CrossRef] [Green Version]
- Matute-Blanch, C.; Villar, L.M.; Alvarez-Cermeno, J.C.; Rejdak, K.; Evdoshenko, E.; Makshakov, G.; Nazarov, V.; Lapin, S.; Midaglia, L.; Vidal-Jordana, A.; et al. Neurofilament light chain and oligoclonal bands are prognostic biomarkers in radiologically isolated syndrome. Brain 2018, 141, 1085–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakansson, I.; Tisell, A.; Cassel, P.; Blennow, K.; Zetterberg, H.; Lundberg, P.; Dahle, C.; Vrethem, M.; Ernerudh, J. Neurofilament light chain in cerebrospinal fluid and prediction of disease activity in clinically isolated syndrome and relapsing-remitting multiple sclerosis. Eur. J. Neurol. 2017, 24, 703–712. [Google Scholar] [CrossRef] [Green Version]
- Malmestrom, C.; Haghighi, S.; Rosengren, L.; Andersen, O.; Lycke, J. Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology 2003, 61, 1720–1725. [Google Scholar] [CrossRef]
- Kuhle, J.; Barro, C.; Disanto, G.; Mathias, A.; Soneson, C.; Bonnier, G.; Yaldizli, O.; Regeniter, A.; Derfuss, T.; Canales, M.; et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult. Scler. 2016, 22, 1550–1559. [Google Scholar] [CrossRef]
- Bhan, A.; Jacobsen, C.; Myhr, K.M.; Dalen, I.; Lode, K.; Farbu, E. Neurofilaments and 10-year follow-up in multiple sclerosis. Mult. Scler. 2018, 24, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Salzer, J.; Svenningsson, A.; Sundstrom, P. Neurofilament light as a prognostic marker in multiple sclerosis. Mult. Scler. 2010, 16, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Rajda, C.; Galla, Z.; Polyak, H.; Maroti, Z.; Babarczy, K.; Pukoli, D.; Vecsei, L. Cerebrospinal fluid neurofilament light chain is associated with kynurenine pathway metabolite changes in multiple sclerosis. Int. J. Mol. Sci. 2020, 21, 2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Disanto, G.; Barro, C.; Benkert, P.; Naegelin, Y.; Schadelin, S.; Giardiello, A.; Zecca, C.; Blennow, K.; Zetterberg, H.; Leppert, D.; et al. Serum neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 2017, 81, 857–870. [Google Scholar] [CrossRef]
- Novakova, L.; Zetterberg, H.; Sundstrom, P.; Axelsson, M.; Khademi, M.; Gunnarsson, M.; Malmestrom, C.; Svenningsson, A.; Olsson, T.; Piehl, F.; et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017, 89, 2230–2237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varhaug, K.N.; Barro, C.; Bjornevik, K.; Myhr, K.M.; Torkildsen, O.; Wergeland, S.; Bindoff, L.A.; Kuhle, J.; Vedeler, C. Neurofilament light chain predicts disease activity in relapsing-remitting MS. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siller, N.; Kuhle, J.; Muthuraman, M.; Barro, C.; Uphaus, T.; Groppa, S.; Kappos, L.; Zipp, F.; Bittner, S. Serum neurofilament light chain is a biomarker of acute and chronic neuronal damage in early multiple sclerosis. Mult. Scler. 2019, 25, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Kuhle, J.; Kropshofer, H.; Haering, D.A.; Kundu, U.; Meinert, R.; Barro, C.; Dahlke, F.; Tomic, D.; Leppert, D.; Kappos, L. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019, 92, e1007–e1015. [Google Scholar] [CrossRef]
- Orsi, G.; Cseh, T.; Hayden, Z.; Perlaki, G.; Nagy, S.A.; Giyab, O.; Olsen, D.A.; Madsen, J.S.; Berki, T.; Illes, Z. Microstructural and functional brain abnormalities in multiple sclerosis predicted by osteopontin and neurofilament light. Mult. Scler. Relat. Disord. 2021, 51, 102923. [Google Scholar] [CrossRef] [PubMed]
- Sejbaek, T.; Nielsen, H.H.; Penner, N.; Plavina, T.; Mendoza, J.P.; Martin, N.A.; Elkjaer, M.L.; Ravnborg, M.H.; Illes, Z. Dimethyl fumarate decreases neurofilament light chain in CSF and blood of treatment naive relapsing MS patients. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Jakimovski, D.; Kuhle, J.; Ramanathan, M.; Barro, C.; Tomic, D.; Hagemeier, J.; Kropshofer, H.; Bergsland, N.; Leppert, D.; Dwyer, M.G.; et al. Serum neurofilament light chain levels associations with gray matter pathology: A 5-year longitudinal study. Ann. Clin. Transl. Neurol. 2019, 6, 1757–1770. [Google Scholar] [CrossRef] [Green Version]
- de Flon, P.; Laurell, K.; Sundstrom, P.; Blennow, K.; Soderstrom, L.; Zetterberg, H.; Gunnarsson, M.; Svenningsson, A. Comparison of plasma and cerebrospinal fluid neurofilament light in a multiple sclerosis trial. Acta Neurol. Scand. 2019, 139, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Hauser, S.L.; Bar-Or, A.; Cohen, J.A.; Comi, G.; Correale, J.; Coyle, P.K.; Cross, A.H.; de Seze, J.; Leppert, D.; Montalban, X.; et al. Ofatumumab versus teriflunomide in multiple sclerosis. N. Engl. J. Med. 2020, 383, 546–557. [Google Scholar] [CrossRef]
- Barro, C.; Chitnis, T.; Weiner, H.L. Blood neurofilament light: A critical review of its application to neurologic disease. Ann. Clin. Transl. Neurol. 2020, 7, 2508–2523. [Google Scholar] [CrossRef]
- Joisten, N.; Rademacher, A.; Warnke, C.; Proschinger, S.; Schenk, A.; Walzik, D.; Knoop, A.; Thevis, M.; Steffen, F.; Bittner, S.; et al. Exercise diminishes plasma neurofilament light chain and reroutes the kynurenine pathway in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8. [Google Scholar] [CrossRef]
- Agah, E.; Zardoui, A.; Saghazadeh, A.; Ahmadi, M.; Tafakhori, A.; Rezaei, N. Osteopontin (OPN) as a CSF and blood biomarker for multiple sclerosis: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0190252. [Google Scholar] [CrossRef]
- Szalardy, L.; Zadori, D.; Simu, M.; Bencsik, K.; Vecsei, L.; Klivenyi, P. Evaluating biomarkers of neuronal degeneration and neuroinflammation in CSF of patients with multiple sclerosis-osteopontin as a potential marker of clinical severity. J. Neurol. Sci. 2013, 331, 38–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khademi, M.; Bornsen, L.; Rafatnia, F.; Andersson, M.; Brundin, L.; Piehl, F.; Sellebjerg, F.; Olsson, T. The effects of natalizumab on inflammatory mediators in multiple sclerosis: Prospects for treatment-sensitive biomarkers. Eur. J. Neurol. 2009, 16, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Iaffaldano, P.; Ruggieri, M.; Viterbo, R.G.; Mastrapasqua, M.; Trojano, M. The improvement of cognitive functions is associated with a decrease of plasma osteopontin levels in natalizumab treated relapsing multiple sclerosis. Brain Behav. Immun. 2014, 35, 176–181. [Google Scholar] [CrossRef]
- Daynes, R.A.; Jones, D.C. Emerging roles of PPARs in inflammation and immunity. Nat. Rev. Immunol. 2002, 2, 748–759. [Google Scholar] [CrossRef]
- Diab, A.; Deng, C.; Smith, J.D.; Hussain, R.Z.; Phanavanh, B.; Lovett-Racke, A.E.; Drew, P.D.; Racke, M.K. Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis. J. Immunol. 2002, 168, 2508–2515. [Google Scholar] [CrossRef] [Green Version]
- Feinstein, D.L.; Galea, E.; Gavrilyuk, V.; Brosnan, C.F.; Whitacre, C.C.; Dumitrescu-Ozimek, L.; Landreth, G.E.; Pershadsingh, H.A.; Weinberg, G.; Heneka, M.T. Peroxisome proliferator-activated receptor-gamma agonists prevent experimental autoimmune encephalomyelitis. Ann. Neurol. 2002, 51, 694–702. [Google Scholar] [CrossRef]
- Natarajan, C.; Bright, J.J. Peroxisome proliferator-activated receptor-gamma agonists inhibit experimental allergic encephalomyelitis by blocking IL-12 production, IL-12 signaling and Th1 differentiation. Genes Immun. 2002, 3, 59–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raikwar, H.P.; Muthian, G.; Rajasingh, J.; Johnson, C.N.; Bright, J.J. PPARgamma antagonists reverse the inhibition of neural antigen-specific Th1 response and experimental allergic encephalomyelitis by Ciglitazone and 15-deoxy-Delta12,14-prostaglandin J2. J. Neuroimmunol. 2006, 178, 76–86. [Google Scholar] [CrossRef]
- Unoda, K.; Doi, Y.; Nakajima, H.; Yamane, K.; Hosokawa, T.; Ishida, S.; Kimura, F.; Hanafusa, T. Eicosapentaenoic acid (EPA) induces peroxisome proliferator-activated receptors and ameliorates experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2013, 256, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Burgdorf, S.; Dani, I.; Saijo, K.; Flossdorf, J.; Hucke, S.; Alferink, J.; Nowak, N.; Beyer, M.; Mayer, G.; et al. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J. Exp. Med. 2009, 206, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Raikwar, H.P.; Muthian, G.; Rajasingh, J.; Johnson, C.; Bright, J.J. PPARgamma antagonists exacerbate neural antigen-specific Th1 response and experimental allergic encephalomyelitis. J. Neuroimmunol. 2005, 167, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Benedusi, V.; Martorana, F.; Brambilla, L.; Maggi, A.; Rossi, D. The peroxisome proliferator-activated receptor gamma (PPARgamma) controls natural protective mechanisms against lipid peroxidation in amyotrophic lateral sclerosis. J. Biol. Chem. 2012, 287, 35899–35911. [Google Scholar] [CrossRef] [Green Version]
- Drew, P.D.; Xu, J.; Racke, M.K. PPAR-gamma: Therapeutic potential for multiple sclerosis. PPAR Res. 2008, 2008, 627463. [Google Scholar] [CrossRef] [Green Version]
- Luna-Medina, R.; Cortes-Canteli, M.; Alonso, M.; Santos, A.; Martinez, A.; Perez-Castillo, A. Regulation of inflammatory response in neural cells in vitro by thiadiazolidinones derivatives through peroxisome proliferator-activated receptor gamma activation. J. Biol. Chem. 2005, 280, 21453–21462. [Google Scholar] [CrossRef] [Green Version]
- Paintlia, A.S.; Paintlia, M.K.; Singh, I.; Singh, A.K. IL-4-induced peroxisome proliferator-activated receptor gamma activation inhibits NF-kappaB trans activation in central nervous system (CNS) glial cells and protects oligodendrocyte progenitors under neuroinflammatory disease conditions: Implication for CNS-demyelinating diseases. J. Immunol. 2006, 176, 4385–4398. [Google Scholar] [CrossRef] [Green Version]
- Swanson, C.R.; Joers, V.; Bondarenko, V.; Brunner, K.; Simmons, H.A.; Ziegler, T.E.; Kemnitz, J.W.; Johnson, J.A.; Emborg, M.E. The PPAR-gamma agonist pioglitazone modulates inflammation and induces neuroprotection in parkinsonian monkeys. J. Neuroinflamm. 2011, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Szalardy, L.; Zadori, D.; Tanczos, E.; Simu, M.; Bencsik, K.; Vecsei, L.; Klivenyi, P. Elevated levels of PPAR-gamma in the cerebrospinal fluid of patients with multiple sclerosis. Neurosci. Lett. 2013, 554, 131–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakan, B.; Szalardy, L.; Vecsei, L. Exploiting the therapeutic potential of endogenous immunomodulatory systems in multiple sclerosis-special focus on the peroxisome proliferator-activated receptors (PPARs) and the kynurenines. Int. J. Mol. Sci. 2019, 20, 426. [Google Scholar] [CrossRef] [Green Version]
- Oxenkrug, G.F. Genetic and hormonal regulation of tryptophan kynurenine metabolism: Implications for vascular cognitive impairment, major depressive disorder, and aging. Ann. N. Y. Acad. Sci. 2007, 1122, 35–49. [Google Scholar] [CrossRef]
- Oxenkrug, G.F. Interferon-gamma-inducible kynurenines/pteridines inflammation cascade: Implications for aging and aging-associated psychiatric and medical disorders. J. Neural. Transm. 2011, 118, 75–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardridge, W.M. Blood-brain barrier carrier-mediated transport and brain metabolism of amino acids. Neurochem. Res. 1998, 23, 635–644. [Google Scholar] [CrossRef]
- Mellor, A.L.; Munn, D.H. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004, 4, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Biernacki, T.; Sandi, D.; Bencsik, K.; Vecsei, L. Kynurenines in the pathogenesis of multiple sclerosis: Therapeutic perspectives. Cells 2020, 9, 1564. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J.; Smythe, G.; Takikawa, O.; Brew, B.J. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 2005, 49, 15–23. [Google Scholar] [CrossRef]
- Parrott, J.M.; O’Connor, J.C. Kynurenine 3-monooxygenase: An influential mediator of neuropathology. Front. Psychiatry 2015, 6, 116. [Google Scholar] [CrossRef] [Green Version]
- Heyes, M.P.; Chen, C.Y.; Major, E.O.; Saito, K. Different kynurenine pathway enzymes limit quinolinic acid formation by various human cell types. Biochem. J. 1997, 326, 351–356. [Google Scholar] [CrossRef]
- Kiss, C.; Ceresoli-Borroni, G.; Guidetti, P.; Zielke, C.L.; Zielke, H.R.; Schwarcz, R. Kynurenate production by cultured human astrocytes. J. Neural. Transm. 2003, 110, 1–14. [Google Scholar] [CrossRef]
- Guidetti, P.; Hoffman, G.E.; Melendez-Ferro, M.; Albuquerque, E.X.; Schwarcz, R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia 2007, 55, 78–92. [Google Scholar] [CrossRef]
- Zinger, A.; Barcia, C.; Herrero, M.T.; Guillemin, G.J. The involvement of neuroinflammation and kynurenine pathway in Parkinson’s disease. Parkinsons Dis. 2011, 2011, 716859. [Google Scholar] [CrossRef] [Green Version]
- Stone, T.W. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 1993, 45, 309–379. [Google Scholar] [PubMed]
- Boros, F.; Vecsei, L. Progress in the development of kynurenine and quinoline-3-carboxamide-derived drugs. Expert Opin. Investig. Drugs 2020, 29, 1223–1247. [Google Scholar] [CrossRef] [PubMed]
- Prescott, C.; Weeks, A.M.; Staley, K.J.; Partin, K.M. Kynurenic acid has a dual action on AMPA receptor responses. Neurosci. Lett. 2006, 402, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef] [Green Version]
- Rozsa, E.; Robotka, H.; Vecsei, L.; Toldi, J. The Janus-face kynurenic acid. J. Neural. Transm. 2008, 115, 1087–1091. [Google Scholar] [CrossRef]
- Lugo-Huitron, R.; Blanco-Ayala, T.; Ugalde-Muniz, P.; Carrillo-Mora, P.; Pedraza-Chaverri, J.; Silva-Adaya, D.; Maldonado, P.D.; Torres, I.; Pinzon, E.; Ortiz-Islas, E.; et al. On the antioxidant properties of kynurenic acid: Free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Teratol. 2011, 33, 538–547. [Google Scholar] [CrossRef]
- Maddison, D.C.; Giorgini, F. The kynurenine pathway and neurodegenerative disease. Semin. Cell Dev. Biol. 2015, 40, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Perez-De La Cruz, V.; Carrillo-Mora, P.; Santamaria, A. Quinolinic Acid, an endogenous molecule combining excitotoxicity, oxidative stress and other toxic mechanisms. Int. J. Tryptophan Res. 2012, 5, 1–8. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Croitoru-Lamoury, J.; Dormont, D.; Armati, P.J.; Brew, B.J. Quinolinic acid upregulates chemokine production and chemokine receptor expression in astrocytes. Glia 2003, 41, 371–381. [Google Scholar] [CrossRef]
- Guillemin, G.J. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012, 279, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.N.; Stone, T.W. Pharmacology and regional variations of quinolinic acid-evoked excitations in the rat central nervous system. J. Pharmacol. Exp. Ther. 1983, 226, 551–557. [Google Scholar]
- de Carvalho, L.P.; Bochet, P.; Rossier, J. The endogenous agonist quinolinic acid and the non endogenous homoquinolinic acid discriminate between NMDAR2 receptor subunits. Neurochem. Int. 1996, 28, 445–452. [Google Scholar] [CrossRef]
- Scherzer, C.R.; Landwehrmeyer, G.B.; Kerner, J.A.; Standaert, D.G.; Hollingsworth, Z.R.; Daggett, L.P.; Velicelebi, G.; Penney, J.B., Jr.; Young, A.B. Cellular distribution of NMDA glutamate receptor subunit mRNAs in the human cerebellum. Neurobiol. Dis. 1997, 4, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Monaghan, D.T.; Beaton, J.A. Quinolinate differentiates between forebrain and cerebellar NMDA receptors. Eur. J. Pharmacol. 1991, 194, 123–125. [Google Scholar] [CrossRef]
- Perkins, M.N.; Stone, T.W. Quinolinic acid: Regional variations in neuronal sensitivity. Brain Res. 1983, 259, 172–176. [Google Scholar] [CrossRef]
- Munn, D.H.; Zhou, M.; Attwood, J.T.; Bondarev, I.; Conway, S.J.; Marshall, B.; Brown, C.; Mellor, A.L. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998, 281, 1191–1193. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Rohrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, P.; Luthi-Carter, R.E.; Augood, S.J.; Schwarcz, R. Neostriatal and cortical quinolinate levels are increased in early grade Huntington’s disease. Neurobiol. Dis. 2004, 17, 455–461. [Google Scholar] [CrossRef]
- Jauch, D.; Urbanska, E.M.; Guidetti, P.; Bird, E.D.; Vonsattel, J.P.; Whetsell, W.O., Jr.; Schwarcz, R. Dysfunction of brain kynurenic acid metabolism in Huntington’s disease: Focus on kynurenine aminotransferases. J. Neurol. Sci. 1995, 130, 39–47. [Google Scholar] [CrossRef]
- Heyes, M.P.; Saito, K.; Crowley, J.S.; Davis, L.E.; Demitrack, M.A.; Der, M.; Dilling, L.A.; Elia, J.; Kruesi, M.J.; Lackner, A.; et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain 1992, 115, 1249–1273. [Google Scholar] [CrossRef]
- Mazarei, G.; Neal, S.J.; Becanovic, K.; Luthi-Carter, R.; Simpson, E.M.; Leavitt, B.R. Expression analysis of novel striatal-enriched genes in Huntington disease. Hum. Mol. Genet. 2010, 19, 609–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrest, C.M.; Mackay, G.M.; Stoy, N.; Spiden, S.L.; Taylor, R.; Stone, T.W.; Darlington, L.G. Blood levels of kynurenines, interleukin-23 and soluble human leucocyte antigen-G at different stages of Huntington’s disease. J. Neurochem. 2010, 112, 112–122. [Google Scholar] [CrossRef]
- Stoy, N.; Mackay, G.M.; Forrest, C.M.; Christofides, J.; Egerton, M.; Stone, T.W.; Darlington, L.G. Tryptophan metabolism and oxidative stress in patients with Huntington’s disease. J. Neurochem. 2005, 93, 611–623. [Google Scholar] [CrossRef]
- Tanaka, M.; Bohar, Z.; Vecsei, L. Are kynurenines accomplices or principal villains in dementia? Maintenance of kynurenine metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulaj, E.; Pawlak, K.; Bien, B.; Pawlak, D. Kynurenine and its metabolites in Alzheimer’s disease patients. Adv. Med. Sci. 2010, 55, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Bonda, D.J.; Mailankot, M.; Stone, J.G.; Garrett, M.R.; Staniszewska, M.; Castellani, R.J.; Siedlak, S.L.; Zhu, X.; Lee, H.G.; Perry, G.; et al. Indoleamine 2,3-dioxygenase and 3-hydroxykynurenine modifications are found in the neuropathology of Alzheimer’s disease. Redox Rep. 2010, 15, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.; Ting, K.; Cullen, K.M.; Braidy, N.; Brew, B.J.; Guillemin, G.J. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS ONE 2009, 4, e6344. [Google Scholar] [CrossRef]
- Rassoulpour, A.; Wu, H.Q.; Poeggeler, B.; Schwarcz, R. Systemic d-amphetamine administration causes a reduction of kynurenic acid levels in rat brain. Brain Res. 1998, 802, 111–118. [Google Scholar] [CrossRef]
- Venkatesan, D.; Iyer, M.; Narayanasamy, A.; Siva, K.; Vellingiri, B. Kynurenine pathway in Parkinson’s disease—An update. eNeurologicalSci 2020, 21, 100270. [Google Scholar] [CrossRef]
- Brotchie, J.M.; Mitchell, I.J.; Sambrook, M.A.; Crossman, A.R. Alleviation of parkinsonism by antagonism of excitatory amino acid transmission in the medial segment of the globus pallidus in rat and primate. Mov. Disord. 1991, 6, 133–138. [Google Scholar] [CrossRef]
- Havelund, J.F.; Andersen, A.D.; Binzer, M.; Blaabjerg, M.; Heegaard, N.H.H.; Stenager, E.; Faergeman, N.J.; Gramsbergen, J.B. Changes in kynurenine pathway metabolism in Parkinson patients with L-DOPA-induced dyskinesia. J. Neurochem. 2017, 142, 756–766. [Google Scholar] [CrossRef]
- Graham, W.C.; Robertson, R.G.; Sambrook, M.A.; Crossman, A.R. Injection of excitatory amino acid antagonists into the medial pallidal segment of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treated primate reverses motor symptoms of parkinsonism. Life Sci. 1990, 47, PL91–PL97. [Google Scholar] [CrossRef]
- Hartai, Z.; Klivenyi, P.; Janaky, T.; Penke, B.; Dux, L.; Vecsei, L. Kynurenine metabolism in plasma and in red blood cells in Parkinson’s disease. J. Neurol. Sci. 2005, 239, 31–35. [Google Scholar] [CrossRef]
- Rejdak, R.; Zarnowski, T.; Turski, W.A.; Kocki, T.; Zagorski, Z.; Guenther, E.; Kohler, K.; Zrenner, E. Changes of kynurenic acid content in the rat and chicken retina during ontogeny. Graefes Arch. Clin. Exp. Ophthalmol. 2002, 240, 687–691. [Google Scholar] [CrossRef]
- Beal, M.F.; Swartz, K.J.; Isacson, O. Developmental changes in brain kynurenic acid concentrations. Brain Res. Dev. Brain Res. 1992, 68, 136–139. [Google Scholar] [CrossRef]
- Ceresoli-Borroni, G.; Schwarcz, R. Neonatal asphyxia in rats: Acute effects on cerebral kynurenine metabolism. Pediatr. Res. 2001, 50, 231–235. [Google Scholar] [CrossRef] [Green Version]
- Olney, J.W.; Wozniak, D.F.; Jevtovic-Todorovic, V.; Farber, N.B.; Bittigau, P.; Ikonomidou, C. Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathol. 2002, 12, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Csillik, A.E.; Okuno, E.; Csillik, B.; Knyihar, E.; Vecsei, L. Expression of kynurenine aminotransferase in the subplate of the rat and its possible role in the regulation of programmed cell death. Cereb. Cortex 2002, 12, 1193–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otori, Y.; Wei, J.Y.; Barnstable, C.J. Neurotoxic effects of low doses of glutamate on purified rat retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 1998, 39, 972–981. [Google Scholar]
- Schuettauf, F.; Naskar, R.; Vorwerk, C.K.; Zurakowski, D.; Dreyer, E.B. Ganglion cell loss after optic nerve crush mediated through AMPA-kainate and NMDA receptors. Invest. Ophthalmol. Vis. Sci. 2000, 41, 4313–4316. [Google Scholar]
- Sucher, N.J.; Lipton, S.A.; Dreyer, E.B. Molecular basis of glutamate toxicity in retinal ganglion cells. Vis. Res. 1997, 37, 3483–3493. [Google Scholar] [CrossRef] [Green Version]
- Rejdak, R.; Zarnowski, T.; Turski, W.A.; Kocki, T.; Zagorski, Z.; Zrenner, E.; Schuettauf, F. Alterations of kynurenic acid content in the retina in response to retinal ganglion cell damage. Vis. Res. 2003, 43, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Rejdak, R.; Kohler, K.; Kocki, T.; Shenk, Y.; Turski, W.A.; Okuno, E.; Lehaci, C.; Zagorski, Z.; Zrenner, E.; Schuettauf, F. Age-dependent decrease of retinal kynurenate and kynurenine aminotransferases in DBA/2J mice, a model of ocular hypertension. Vis. Res. 2004, 44, 655–660. [Google Scholar] [CrossRef] [Green Version]
- Savitz, J.; Drevets, W.C.; Wurfel, B.E.; Ford, B.N.; Bellgowan, P.S.; Victor, T.A.; Bodurka, J.; Teague, T.K.; Dantzer, R. Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain Behav. Immun. 2015, 46, 55–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brundin, L.; Sellgren, C.M.; Lim, C.K.; Grit, J.; Palsson, E.; Landen, M.; Samuelsson, M.; Lundgren, K.; Brundin, P.; Fuchs, D.; et al. An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Transl. Psychiatry 2016, 6, e865. [Google Scholar] [CrossRef] [Green Version]
- Bryleva, E.Y.; Brundin, L. Suicidality and activation of the kynurenine pathway of tryptophan metabolism. Curr. Top. Behav. Neurosci. 2017, 31, 269–284. [Google Scholar] [CrossRef]
- Espey, M.G.; Chernyshev, O.N.; Reinhard, J.F., Jr.; Namboodiri, M.A.; Colton, C.A. Activated human microglia produce the excitotoxin quinolinic acid. Neuroreport 1997, 8, 431–434. [Google Scholar] [CrossRef]
- Heyes, M.P.; Saito, K.; Markey, S.P. Human macrophages convert L-tryptophan into the neurotoxin quinolinic acid. Biochem. J. 1992, 283, 633–635. [Google Scholar] [CrossRef] [Green Version]
- Benveniste, E.N. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J. Mol. Med. 1997, 75, 165–173. [Google Scholar] [CrossRef]
- Cammer, W. Oligodendrocyte killing by quinolinic acid in vitro. Brain Res. 2001, 896, 157–160. [Google Scholar] [CrossRef]
- Schwarcz, R.; Whetsell, W.O., Jr.; Mangano, R.M. Quinolinic acid: An endogenous metabolite that produces axon-sparing lesions in rat brain. Science 1983, 219, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.M.; Erickson, J.B.; Viveros, O.H.; Chang, S.Y.; Reinhard, J.F., Jr. Neurotoxin quinolinic acid is selectively elevated in spinal cords of rats with experimental allergic encephalomyelitis. J. Neurochem. 1995, 64, 1192–1196. [Google Scholar] [CrossRef]
- Pitt, D.; Werner, P.; Raine, C.S. Glutamate excitotoxicity in a model of multiple sclerosis. Nat. Med. 2000, 6, 67–70. [Google Scholar] [CrossRef]
- Salter, M.G.; Fern, R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature 2005, 438, 1167–1171. [Google Scholar] [CrossRef]
- Sundaram, G.; Brew, B.J.; Jones, S.P.; Adams, S.; Lim, C.K.; Guillemin, G.J. Quinolinic acid toxicity on oligodendroglial cells: Relevance for multiple sclerosis and therapeutic strategies. J. Neuroinflamm. 2014, 11, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillemin, G.J.; Wang, L.; Brew, B.J. Quinolinic acid selectively induces apoptosis of human astrocytes: Potential role in AIDS dementia complex. J. Neuroinflamm. 2005, 2, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillemin, G.J.; Kerr, S.J.; Smythe, G.A.; Smith, D.G.; Kapoor, V.; Armati, P.J.; Croitoru, J.; Brew, B.J. Kynurenine pathway metabolism in human astrocytes: A paradox for neuronal protection. J. Neurochem. 2001, 78, 842–853. [Google Scholar] [CrossRef]
- Shear, D.A.; Dong, J.; Haik-Creguer, K.L.; Bazzett, T.J.; Albin, R.L.; Dunbar, G.L. Chronic administration of quinolinic acid in the rat striatum causes spatial learning deficits in a radial arm water maze task. Exp. Neurol. 1998, 150, 305–311. [Google Scholar] [CrossRef]
- Braidy, N.; Grant, R.; Brew, B.J.; Adams, S.; Jayasena, T.; Guillemin, G.J. Effects of kynurenine pathway metabolites on intracellular NAD synthesis and cell death in human primary astrocytes and neurons. Int. J. Tryptophan Res. 2009, 2, 61–69. [Google Scholar] [CrossRef]
- Kerr, S.J.; Armati, P.J.; Guillemin, G.J.; Brew, B.J. Chronic exposure of human neurons to quinolinic acid results in neuronal changes consistent with AIDS dementia complex. AIDS 1998, 12, 355–363. [Google Scholar] [CrossRef]
- Behan, W.M.; McDonald, M.; Darlington, L.G.; Stone, T.W. Oxidative stress as a mechanism for quinolinic acid-induced hippocampal damage: Protection by melatonin and deprenyl. Br. J. Pharmacol. 1999, 128, 1754–1760. [Google Scholar] [CrossRef]
- Santamaria, A.; Jimenez-Capdeville, M.E.; Camacho, A.; Rodriguez-Martinez, E.; Flores, A.; Galvan-Arzate, S. In vivo hydroxyl radical formation after quinolinic acid infusion into rat corpus striatum. Neuroreport 2001, 12, 2693–2696. [Google Scholar] [CrossRef]
- Leipnitz, G.; Schumacher, C.; Scussiato, K.; Dalcin, K.B.; Wannmacher, C.M.; Wyse, A.T.; Dutra-Filho, C.S.; Wajner, M.; Latini, A. Quinolinic acid reduces the antioxidant defenses in cerebral cortex of young rats. Int. J. Dev. Neurosci. 2005, 23, 695–701. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, E.; Camacho, A.; Maldonado, P.D.; Pedraza-Chaverri, J.; Santamaria, D.; Galvan-Arzate, S.; Santamaria, A. Effect of quinolinic acid on endogenous antioxidants in rat corpus striatum. Brain Res. 2000, 858, 436–439. [Google Scholar] [CrossRef]
- Bordelon, Y.M.; Chesselet, M.F.; Nelson, D.; Welsh, F.; Erecinska, M. Energetic dysfunction in quinolinic acid-lesioned rat striatum. J. Neurochem. 1997, 69, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Kwidzinski, E.; Bunse, J.; Aktas, O.; Richter, D.; Mutlu, L.; Zipp, F.; Nitsch, R.; Bechmann, I. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J. 2005, 19, 1347–1349. [Google Scholar] [CrossRef] [PubMed]
- Chiarugi, A.; Cozzi, A.; Ballerini, C.; Massacesi, L.; Moroni, F. Kynurenine 3-mono-oxygenase activity and neurotoxic kynurenine metabolites increase in the spinal cord of rats with experimental allergic encephalomyelitis. Neuroscience 2001, 102, 687–695. [Google Scholar] [CrossRef]
- Sundaram, G.; Lim, C.K.; Brew, B.J.; Guillemin, G.J. Kynurenine pathway modulation reverses the experimental autoimmune encephalomyelitis mouse disease progression. J. Neuroinflamm. 2020, 17, 176. [Google Scholar] [CrossRef] [PubMed]
- Lanz, T.V.; Williams, S.K.; Stojic, A.; Iwantscheff, S.; Sonner, J.K.; Grabitz, C.; Becker, S.; Bohler, L.I.; Mohapatra, S.R.; Sahm, F.; et al. Tryptophan-2,3-Dioxygenase (TDO) deficiency is associated with subclinical neuroprotection in a mouse model of multiple sclerosis. Sci. Rep. 2017, 7, 41271. [Google Scholar] [CrossRef] [PubMed]
- Zwilling, D.; Huang, S.Y.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Guidetti, P.; Wu, H.Q.; Lee, J.; Truong, J.; Andrews-Zwilling, Y.; Hsieh, E.W.; et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell 2011, 145, 863–874. [Google Scholar] [CrossRef] [Green Version]
- Polyak, H.; Cseh, E.K.; Bohar, Z.; Rajda, C.; Zadori, D.; Klivenyi, P.; Toldi, J.; Vecsei, L. Cuprizone markedly decreases kynurenic acid levels in the rodent brain tissue and plasma. Heliyon 2021, 7, e06124. [Google Scholar] [CrossRef]
- Monaco, F.; Fumero, S.; Mondino, A.; Mutani, R. Plasma and cerebrospinal fluid tryptophan in multiple sclerosis and degenerative diseases. J. Neurol. Neurosurg. Psychiatry 1979, 42, 640–641. [Google Scholar] [CrossRef] [PubMed]
- Rudzite, V.; Berzinsh, J.; Grivane, I.; Fuchs, D.; Baier-Bitterlich, G.; Wachter, H. Serum tryptophan, kynurenine, and neopterin in patients with Guillain-Barre-syndrome (GBS) and multiple sclerosis (MS). Adv. Exp. Med. Biol. 1996, 398, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Sandyk, R. Tryptophan availability and the susceptibility to stress in multiple sclerosis: A hypothesis. Int. J. Neurosci. 1996, 86, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Rejdak, K.; Bartosik-Psujek, H.; Dobosz, B.; Kocki, T.; Grieb, P.; Giovannoni, G.; Turski, W.A.; Stelmasiak, Z. Decreased level of kynurenic acid in cerebrospinal fluid of relapsing-onset multiple sclerosis patients. Neurosci. Lett. 2002, 331, 63–65. [Google Scholar] [CrossRef]
- Rejdak, K.; Petzold, A.; Kocki, T.; Kurzepa, J.; Grieb, P.; Turski, W.A.; Stelmasiak, Z. Astrocytic activation in relation to inflammatory markers during clinical exacerbation of relapsing-remitting multiple sclerosis. J. Neural. Transm. 2007, 114, 1011–1015. [Google Scholar] [CrossRef]
- Vecsei, L.; Szalardy, L.; Fulop, F.; Toldi, J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [CrossRef]
- Anderson, J.M.; Hampton, D.W.; Patani, R.; Pryce, G.; Crowther, R.A.; Reynolds, R.; Franklin, R.J.; Giovannoni, G.; Compston, D.A.; Baker, D.; et al. Abnormally phosphorylated tau is associated with neuronal and axonal loss in experimental autoimmune encephalomyelitis and multiple sclerosis. Brain 2008, 131, 1736–1748. [Google Scholar] [CrossRef]
- Hartai, Z.; Klivenyi, P.; Janaky, T.; Penke, B.; Dux, L.; Vecsei, L. Kynurenine metabolism in multiple sclerosis. Acta Neurol. Scand. 2005, 112, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.K.; Brew, B.J.; Sundaram, G.; Guillemin, G.J. Understanding the roles of the kynurenine pathway in multiple sclerosis progression. Int. J. Tryptophan Res. 2010, 3, 157–167. [Google Scholar] [CrossRef]
- Lim, C.K.; Bilgin, A.; Lovejoy, D.B.; Tan, V.; Bustamante, S.; Taylor, B.V.; Bessede, A.; Brew, B.J.; Guillemin, G.J. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci. Rep. 2017, 7, 41473. [Google Scholar] [CrossRef]
- Herman, S.; Akerfeldt, T.; Spjuth, O.; Burman, J.; Kultima, K. Biochemical differences in cerebrospinal fluid between secondary progressive and relapsing(-)remitting multiple sclerosis. Cells 2019, 8, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aeinehband, S.; Brenner, P.; Stahl, S.; Bhat, M.; Fidock, M.D.; Khademi, M.; Olsson, T.; Engberg, G.; Jokinen, J.; Erhardt, S.; et al. Cerebrospinal fluid kynurenines in multiple sclerosis; relation to disease course and neurocognitive symptoms. Brain Behav. Immun. 2016, 51, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G.; Maes, M.; Berk, M. Inflammation-related disorders in the tryptophan catabolite pathway in depression and somatization. Adv. Protein Chem. Struct. Biol. 2012, 88, 27–48. [Google Scholar] [CrossRef]
- Harrison, B.L.; Baron, B.M.; Cousino, D.M.; McDonald, I.A. 4-[(Carboxymethyl)oxy]- and 4-[(carboxymethyl)amino]-5,7-dichloroquinoline-2-carboxylic acid: New antagonists of the strychnine-insensitive glycine binding site on the N-methyl-D-aspartate receptor complex. J. Med. Chem. 1990, 33, 3130–3132. [Google Scholar] [CrossRef]
- Lorinczi, B.; Csampai, A.; Fulop, F.; Szatmari, I. Synthesis of new C-3 substituted kynurenic acid derivatives. Molecules 2020, 25, 937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borza, I.; Kolok, S.; Galgoczy, K.; Gere, A.; Horvath, C.; Farkas, S.; Greiner, I.; Domany, G. Kynurenic acid amides as novel NR2B selective NMDA receptor antagonists. Bioorg. Med. Chem. Lett. 2007, 17, 406–409. [Google Scholar] [CrossRef]
- Mandi, Y.; Endresz, V.; Mosolygo, T.; Burian, K.; Lantos, I.; Fulop, F.; Szatmari, I.; Lorinczi, B.; Balog, A.; Vecsei, L. The opposite effects of kynurenic acid and different kynurenic acid analogs on tumor necrosis factor-alpha (TNF-alpha) production and tumor necrosis factor-stimulated gene-6 (TSG-6) expression. Front. Immunol. 2019, 10, 1406. [Google Scholar] [CrossRef]
- Datki, Z.; Galik-Olah, Z.; Bohar, Z.; Zadori, D.; Fulop, F.; Szatmari, I.; Galik, B.; Kalman, J.; Vecsei, L. Kynurenic acid and its analogs are beneficial physiologic attenuators in bdelloid rotifers. Molecules 2019, 24, 2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirthgen, E.; Leonard, A.K.; Scharf, C.; Domanska, G. The Immunomodulator 1-methyltryptophan drives tryptophan catabolism toward the kynurenic acid branch. Front. Immunol. 2020, 11, 313. [Google Scholar] [CrossRef]
- Molnar, K.; Lorinczi, B.; Fazakas, C.; Szatmari, I.; Fulop, F.; Kmetyko, N.; Berkecz, R.; Ilisz, I.; Krizbai, I.A.; Wilhelm, I.; et al. SZR-104, a Novel kynurenic acid analogue with high permeability through the blood-brain barrier. Pharmaceutics 2021, 13, 61. [Google Scholar] [CrossRef]
- Lajko, N.; Kata, D.; Szabo, M.; Matyas, A.; Dulka, K.; Foldesi, I.; Fulop, F.; Gulya, K.; Vecsei, L.; Mihaly, A. Sensitivity of rodent microglia to kynurenines in models of epilepsy and inflammation in vivo and in vitro: Microglia activation is inhibited by kynurenic acid and the synthetic analogue SZR104. Int. J. Mol. Sci. 2020, 21, 9333. [Google Scholar] [CrossRef] [PubMed]
- Demeter, I.; Nagy, K.; Gellert, L.; Vecsei, L.; Fulop, F.; Toldi, J. A novel kynurenic acid analog (SZR104) inhibits pentylenetetrazole-induced epileptiform seizures. An electrophysiological study: Special issue related to kynurenine. J. Neural. Transm. 2012, 119, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Al-Karagholi, M.A.; Hansen, J.M.; Abou-Kassem, D.; Hansted, A.K.; Ubhayasekera, K.; Bergquist, J.; Vecsei, L.; Jansen-Olesen, I.; Ashina, M. Phase 1 study to access safety, tolerability, pharmacokinetics, and pharmacodynamics of kynurenine in healthy volunteers. Pharmacol. Res. Perspect. 2021, 9, e00741. [Google Scholar] [CrossRef] [PubMed]

| KYNA | QUIN | |
|---|---|---|
| Formation | mainly in astrocytes | mainly in microglia |
| Effect on NMDA receptors | antagonist | agonist |
| Effect on AMPA receptors | antagonist | - |
| Effect on Kainate receptors | antagonist | - |
| Effect on GPR35 | activates | - |
| Effect on glutamate reuptake | - | inhibits |
| Intracellular kation (Ca2+) influx | inhibits | promotes |
| Lipid peroxidation | inhibits | promotes |
| Effect on ROS formation | scavenges ROS | promotes formation |
| QUIN | KMO | IDO-1 |
|---|---|---|
| Induces oligodendrocyte apoptosis | Upregulation leads to elevated QUIN levels | Activation may lead to decrease in inflammation |
| Induces astrocyte apoptosis | Inhibition elevates KYNA levels | Inhibition can cause more severe or less severe disease course based on timing |
| Induces neuronal apoptosis | Inhibition leads to better cognitive performance in animals |
| QUIN | KYNA |
|---|---|
| Elevated levels in the CSF of MS patients | Decreased levels in the CSF of MS patients |
| Elevated levels in the CSF RRMS patients to the point of being one of the best predictors of disease severity | Decreased levels in the CSF SPMS patients |
| Elevated levels in the CSF of PPMS patients | Elevated levels in the CSF of PPMS patients |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandi, D.; Fricska-Nagy, Z.; Bencsik, K.; Vécsei, L. Neurodegeneration in Multiple Sclerosis: Symptoms of Silent Progression, Biomarkers and Neuroprotective Therapy—Kynurenines Are Important Players. Molecules 2021, 26, 3423. https://doi.org/10.3390/molecules26113423
Sandi D, Fricska-Nagy Z, Bencsik K, Vécsei L. Neurodegeneration in Multiple Sclerosis: Symptoms of Silent Progression, Biomarkers and Neuroprotective Therapy—Kynurenines Are Important Players. Molecules. 2021; 26(11):3423. https://doi.org/10.3390/molecules26113423
Chicago/Turabian StyleSandi, Dániel, Zsanett Fricska-Nagy, Krisztina Bencsik, and László Vécsei. 2021. "Neurodegeneration in Multiple Sclerosis: Symptoms of Silent Progression, Biomarkers and Neuroprotective Therapy—Kynurenines Are Important Players" Molecules 26, no. 11: 3423. https://doi.org/10.3390/molecules26113423
APA StyleSandi, D., Fricska-Nagy, Z., Bencsik, K., & Vécsei, L. (2021). Neurodegeneration in Multiple Sclerosis: Symptoms of Silent Progression, Biomarkers and Neuroprotective Therapy—Kynurenines Are Important Players. Molecules, 26(11), 3423. https://doi.org/10.3390/molecules26113423






