Cerium/Ascorbic Acid/Iodine Active Species for Redox Flow Energy Storage Battery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the Composite Electrodes and the Modification of the Nafion 117 Membrane
2.2. Electrocatalytic Activity Test of Composite Electrodes
2.3. Measurement of Electrochemical Characteristics of Active Species on Composite Electrodes
2.3.1. Cerium Salt Active Species
2.3.2. Iodine/Ascorbic Acid Active Species
2.4. Charge/Discharge Test
2.4.1. Preparation of Positive and Negative Electrolytes
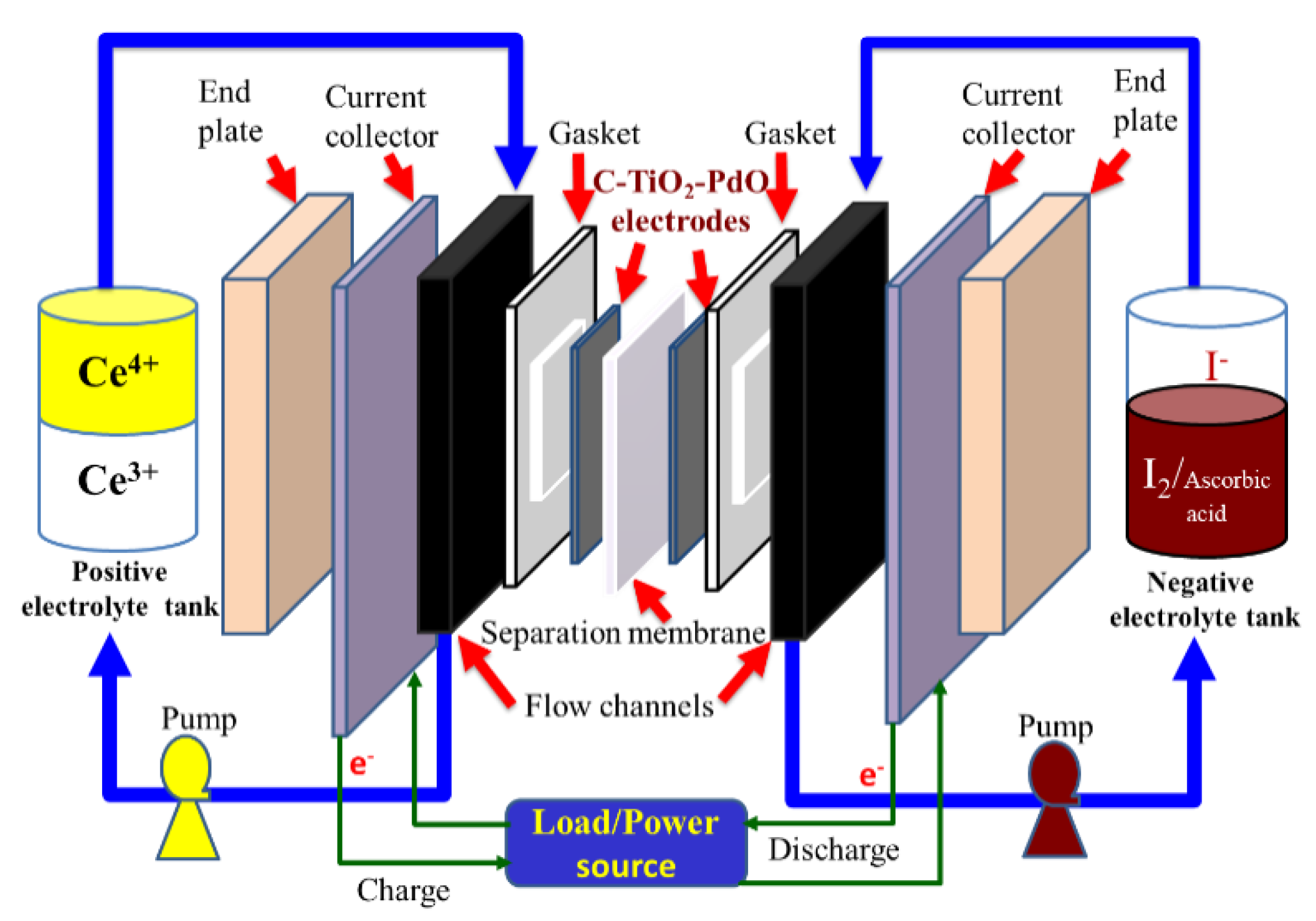
2.4.2. Cell Performance of a Single Ce/Ascorbic Acid/I RFB System by Modifying Electrode and Separation Membrane
3. Results and Discussion
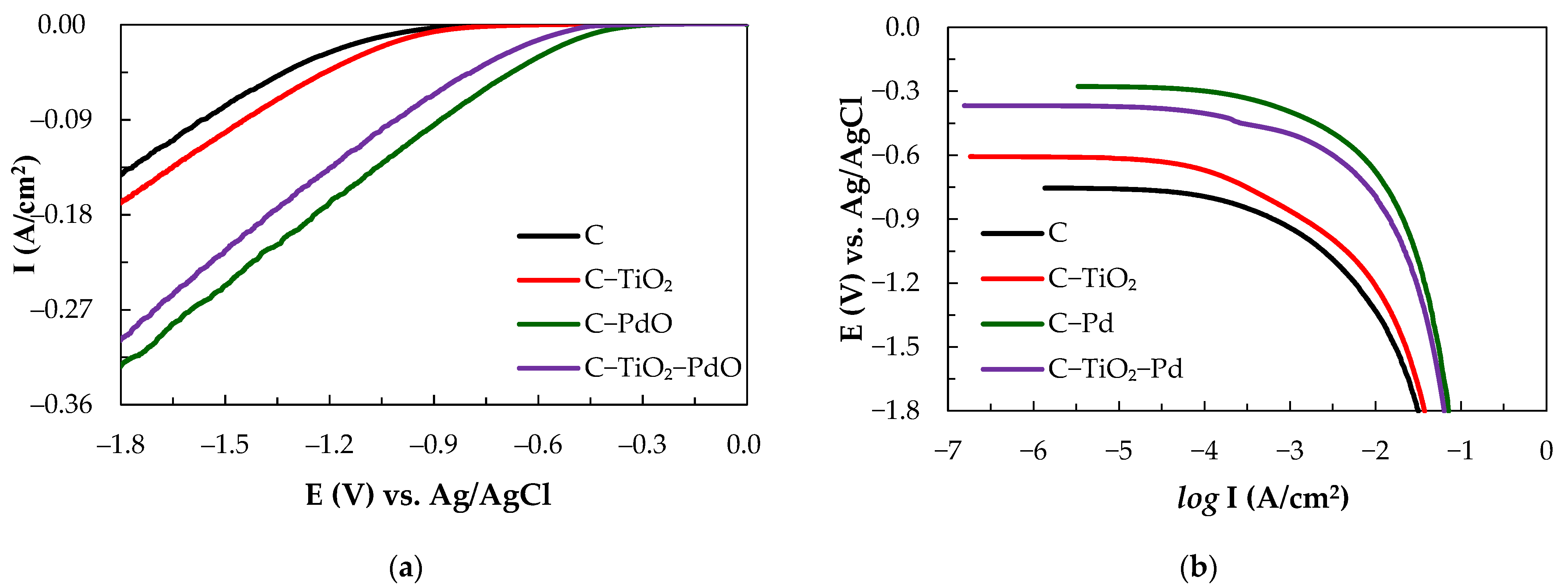
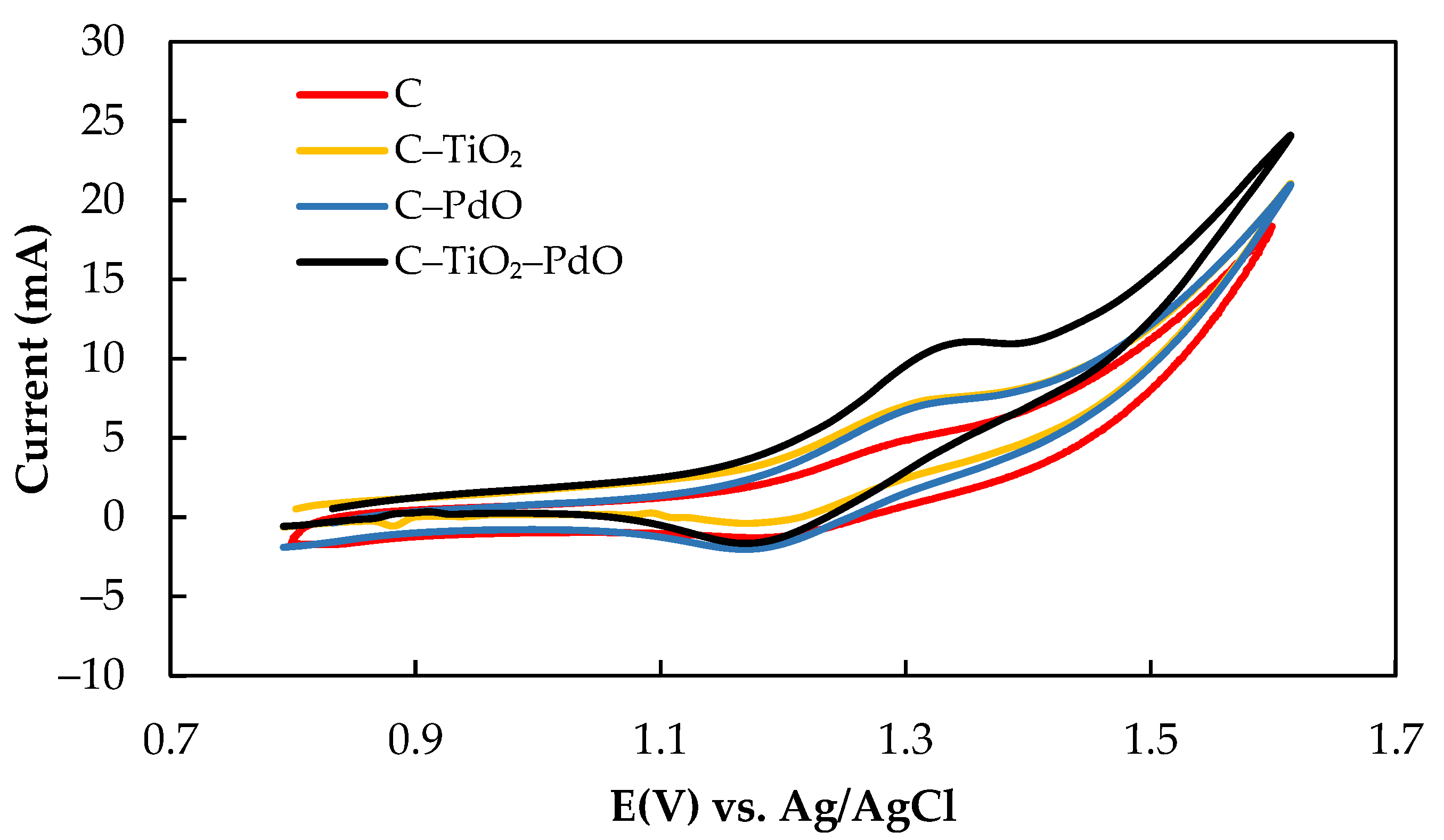
3.1. Electrocatalytic Characteristics of Composite Electrodes
3.2. Electrochemical Characteristics of Active Electrolytes on Composite Electrodes
3.2.1. Cerium Salt Active Electrolyte
3.2.2. Iodine/Ascorbic Acid Active Species
3.3. Cell Performances
3.3.1. Cell Performance of a Single Ce/Ascorbic Acid/I RFB System with Different Supporting Electrolyte
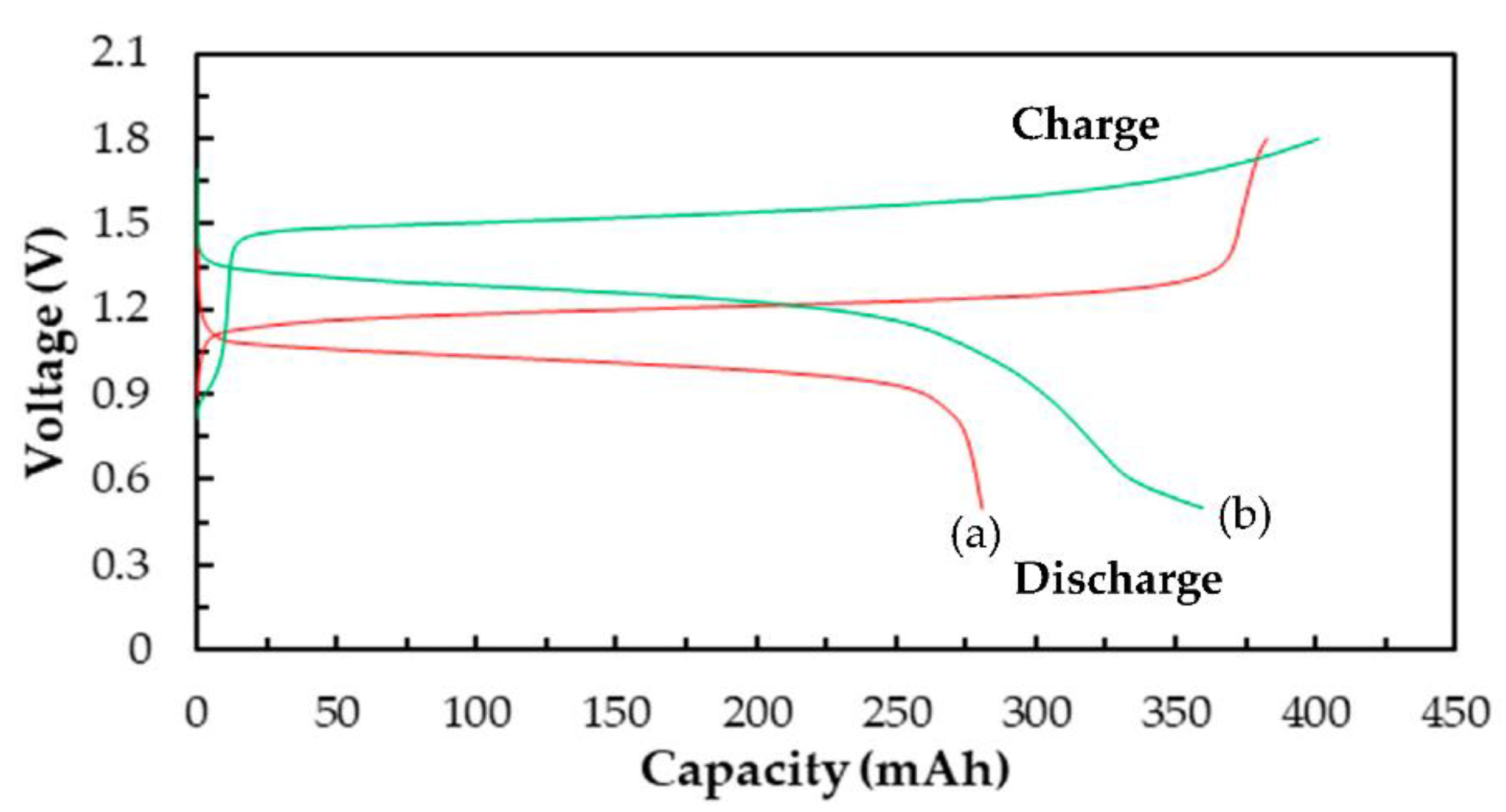
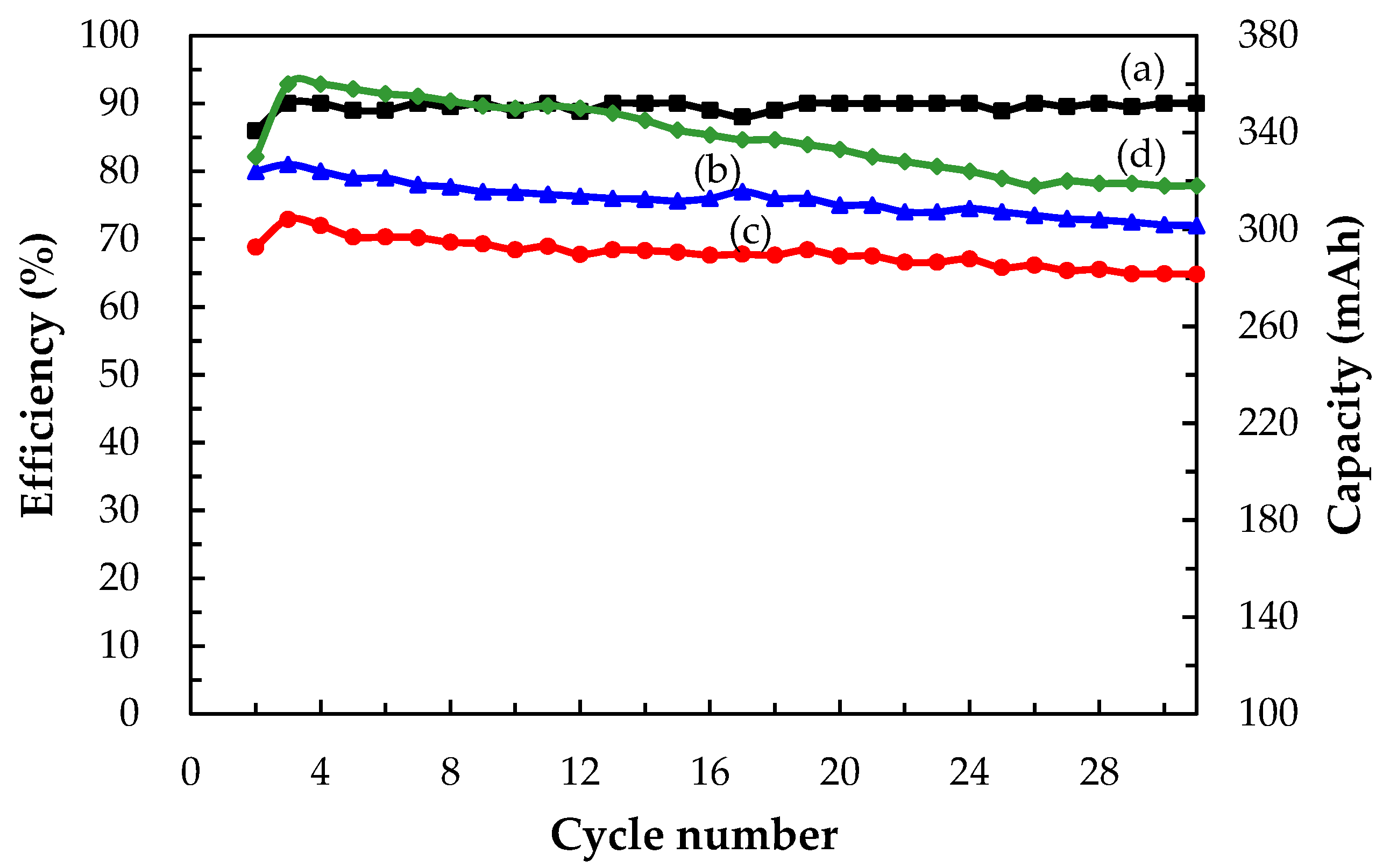
3.3.2. Cell Performances of a Single Ce/Ascorbic Acid/I RFB System by Modifying Electrode and Separation Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sánchez-Díez, E.; Ventosa, E.; Guarnieri, M.; Trovò, A.; Flox, C.; Marcilla, R.; Soavi, F.; Mazur, P.; Aranzabe, E.; Ferret, R. Redox flow batteries: Status and perspective towards sustainable stationary energy storage. J. Power Sources 2021, 481, 228804. [Google Scholar] [CrossRef]
- Clemente, A.; Costa-Castelló, R. Redox flow batteries: A literature review oriented to automatic control. Energies 2020, 13, 4514. [Google Scholar] [CrossRef]
- Sanz, L.; Lloyd, D.; Magdalena, E.; Palma, J.; Kontturi, K. Description and performance of a novel aqueous all-copper redox flow battery. J. Power Sources 2014, 268, 121–128. [Google Scholar] [CrossRef]
- Pan, F.; Wang, Q. Redox species of redox flow batteries: A review. Molecules 2015, 20, 20499–20517. [Google Scholar] [CrossRef] [PubMed]
- Navarro Garcia, S.; Yang, X.; Bereczki, L.; Kónya, D. Aqueous solubility of organic compounds for flow battery applications: Symmetry and counter ion design to avoid low-solubility polymorphs. Molecules 2021, 26, 1203. [Google Scholar] [CrossRef]
- Rychcik, M.; Skyllas-kazacos, M. Characteristics of a new all-vanadium redox flow battery. J. Power Sources 1988, 22, 59–67. [Google Scholar] [CrossRef]
- Pugach, M.; Vyshinsky, V.; Bischi, A. Energy efficiency analysis for a kilo-watt class vanadium redox flow battery system. Appl. Energy 2019, 253, 113533. [Google Scholar] [CrossRef]
- Sarkar, T.; Bhattacharjee, A.; Samanta, H.; Bhattacharya, K.; Saha, H. Optimal design and implementation of solar PV-wind-biogas-VRFB storage integrated smart hybrid microgrid for ensuring zero loss of power supply probability. Energy Convers. Manag. 2019, 191, 102–118. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Li, L.; Graff, G.; Liu, J.; Zhang, H.; Yang, Z.; Hu, J.Z. Towards understanding the poor thermal stability of V5+ electrolyte solution in vanadium redox flow batteries. J. Power Sources 2011, 196, 3669–3672. [Google Scholar] [CrossRef]
- Zeng, Y.K.; Zhao, T.S.; An, L.; Zhou, X.L.; Wei, L. A comparative study of all-vanadium and iron-chromium redox flow batteries for large-scale energy storage. J. Power Sources 2015, 300, 438–443. [Google Scholar] [CrossRef]
- Gurieff, N.; Keogh, D.F.; Timchenko, V.; Menictas, C. Enhanced reactant distribution in redox flow cells. Molecules 2019, 24, 3877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.; Kwon, B.W.; Jung, M.; Serhiichuk, D.; Henkensmeier, D.; Kwon, Y. Iron-vanadium redox flow batteries with polybenzimidazole membranes: High coulomb efficiency and low capacity loss. J. Power Sources 2019, 439, 227079. [Google Scholar] [CrossRef]
- Chen, T.S.; Huang, S.L.; Ye, Z.Y.; Lin, Y.S.; Li, C.P. A novel Fe (II/0) redox couple with key components for the V/Fe redox flow battery. J. Electroanal. Chem. 2019, 850, 113396. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Lee, Y.; Jeon, J. 1,2-Dimethylimidazole based bromine complexing agents for vanadium bromine redox flow batteries. Int. J. Hydrog. Energy 2019, 44, 12024–12032. [Google Scholar] [CrossRef]
- Fang, B.; Iwasa, S.; Wei, Y.; Arai, T.; Kumagai, M. A study of the Ce(III)/Ce(IV) redox couple for redox flow battery application. Electrochim. Acta 2002, 47, 3971–3976. [Google Scholar] [CrossRef]
- Chen, T.S.; Huang, S.L.; Chen, M.L.; Tsai, T.J.; Lin, Y.S. Improving electrochemical activity in a semi-V-I redox flow battery by using a C–TiO2–Pd composite electrode. J. Nanomater. 2019, 2019, 7460856. [Google Scholar] [CrossRef] [Green Version]
- Khor, A.; Leung, P.; Mohamed, M.R.; Flox, C.; Xu, Q.; An, L.; Wills, R.G.A.; Morante, J.R.; Shah, A. Review of zinc-based hybrid flow batteries: From fundamentals to applications. Mater. Today Energy 2018, 8, 80–108. [Google Scholar] [CrossRef]
- Chang, S.; Yea, J.; Zhou, W.; Wu, C.; Ding, M.; Long, Y.; Cheng, Y.; Jia, C. A low-cost SPEEK-K type membrane for neutral aqueous zinc-iron redox flow battery. Surf. Coat. Technol. 2019, 358, 190–194. [Google Scholar] [CrossRef]
- Nikiforidis, G.; Berlouis, L.; Hall, D.; Hodgson, D. An electrochemical study on the positive electrode side of the zinc–cerium hybrid redox flow battery. Electrochim. Acta 2014, 115, 621–629. [Google Scholar] [CrossRef]
- Na, Z.; Xu, S.; Yin, D.; Wang, L. A cerium-lead redox flow battery system employing supporting electrolyte of methanesulfonic acid. J. Power Sources 2015, 295, 28–32. [Google Scholar] [CrossRef]
- Zeng, Y.K.; Zhao, T.S.; Zhou, X.L.; Wei, L.; Ren, Y.X. A novel iron-lead redox flow battery for large-scale energy storage. J. Power Sources 2017, 346, 97–102. [Google Scholar] [CrossRef]
- Manohar, A.K.; Kim, K.M.; Plichta, E.; Hendrickson, M.; Rawlings, S.; Narayanana, S.R. A high efficiency iron-chloride redox flow battery for large-scale energy storage. J. Electrochem. Soc. 2016, 163, A5118–A5125. [Google Scholar] [CrossRef]
- Collins, J.; Kear, G.; Li, X.; Low, C.T.J.; Pletcher, D.; Tangirala, R.; Stratton-Campbell, D.; Walsh, F.C.; Zhang, C. A novel flow battery: A lead acid battery based on an electrolyte with soluble lead (II) Part VIII. The cycling of a 10 cm × 10 cm flow cell. J. Power Sources 2010, 195, 1731–1738. [Google Scholar] [CrossRef] [Green Version]
- Gong, K.; Xu, F.; Grunewald, J.B.; Ma, X.; Zhao, Y.; Gu, S.; Yan, Y. All-soluble all-iron aqueous redox-flow battery. ACS Energy Lett. 2016, 1, 89–93. [Google Scholar] [CrossRef]
- Leung, P.K.; Ponce de León, C.; Low, C.T.J.; Walsh, F.C. Ce(III)/Ce(IV) in methanesulfonic acid as the positive half cell of a redox flow battery. Electrochim. Acta 2011, 56, 2145–2153. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, Q.; Chang, Z.; Zhang, X. The developments and challenges of cerium half-cell in zinc–cerium redox flow battery for energy storage. Electrochim. Acta 2013, 90, 695–704. [Google Scholar] [CrossRef]
- Nikiforidis, G.; Daoud, W.A. Effect of mixed acid media on the positive side of the hybrid zinc-cerium redox flow battery. Electrochim. Acta 2014, 141, 255–262. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, B.; Xie, C.; Yang, B.; Jiao, Y.; Cai, D.; Yang, L.; Shu, Q.; Shi, A. Chemically reduced graphene oxide paper as positive electrode for advanced Zn/Ce redox flow battery. Mater. Chem. Phys. 2018, 220, 208–215. [Google Scholar] [CrossRef]
- Na, Z.; Sun, X.; Wang, L. Surface-functionalized graphite felts: Enhanced performance in cerium-based redox flow batteries. Carbon 2018, 138, 363–368. [Google Scholar] [CrossRef]
- Huang, S.L.; Yu, H.F.; Lin, Y.S. Modification of nafion® membrane via a sol-gel route for vanadium redox flow energy storage battery applications. J. Chem. 2017, 2017, 4590952. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, H.; Liu, T.; Li, X.; Liu, Z. Carbon paper coated with supported tungsten trioxide as novel electrode for all-vanadium flow battery. J. Power Sources 2012, 218, 455–461. [Google Scholar] [CrossRef]
- Wang, C.; Podlaha, E.J. Communication—Electrodeposited Co–Mo–P–TiO2 composites electrocatalysts for the hydrogen evolution reaction. J. Electrochem. Soc. 2020, 167, 132502. [Google Scholar] [CrossRef]
- Chaisubanan, N.; Pruksathorn, K.; Vergnes, H.; Senocq, F.; Hunsom, M. Stability of TiO2 promoted PtCo/C catalyst for oxygen reduction reaction. Int. J. Electrochem. Sci. 2016, 11, 1012–1028. [Google Scholar]
- Dubouis, N.; Grimaud, A. The hydrogen evolution reaction: From material to interfacial descriptors. Chem. Sci. 2019, 10, 9165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapper, H.; Kang, S.O.; Paul, H.H.; Lohmann, W. The reversibility of the vitamin C redox system: Electrochemical reasons and biological aspects. Z. Naturforsch. C J. Biosci. 1982, 37, 942–946. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Kurokawa, S.; Toyama, T.; Hayashi, T. CMP characteristics of quartz glass substrate by aggregated colloidal ceria slurry. Precis. Eng. 2019, 60, 458–464. [Google Scholar] [CrossRef]




| Electrode | −b | Io | Eeq/Eop | I |
|---|---|---|---|---|
| (mV/dec) | (μA/cm2) | −(mV) at −10 (mA/cm2) | (mA/cm2) at −900 (mV) | |
| C | 120 | 0.25 | 754/1034 | 2.87 |
| C–TiO2 | 169 | 0.85 | 606/947 | 6.5 |
| C–PdO | 79 | 4.2 | 276/458 | 95.38 |
| C–TiO2–PdO | 88 | 2.69 | 367/560 | 66.09 |
| Electrode | Ea (V) | Ec (V) | ΔEp (V) | Ia (mA) | Ic (mA) | Ia/Ic |
|---|---|---|---|---|---|---|
| C | 1.31 | 1.17 | 0.14 | 3.59 | 2.47 | 1.45 |
| C–TiO2 | 1.30 | 0.12 | 0.18 | 3.40 | 2.05 | 1.31 |
| C–PdO | 1.29 | 1.16 | 0.13 | 4.22 | 1.35 | 3.13 |
| C–TiO2–PdO | 1.33 | 1.18 | 0.15 | 4.24 | 3.89 | 1.09 |
| Electrode | Electrolyte I2/Ascorbic Acid/H2SO4Conc. (M) | Ea (V) | Ec (V) | ΔEp (V) | Ia (mA) | Ic (mA) | Ia/Ic |
|---|---|---|---|---|---|---|---|
| C–TiO2–PdO | (a) 1.0/0.00/2.0 M | 0.31 | −0.43 | 0.74 | 27 | 40 | 0.68 |
| (b) 1.0/0.18/2.0 M | 0.48 | −0.19 | 0.67 | 44 | 46 | 0.96 | |
| (c) 1.0/0.36/2.0 M | 0.51 | −0.24 | 0.75 | 50 | 48 | 1.04 | |
| (d) 1.0/0.54/2.0 M | 0.54 | −0.20 | 0.74 | 38 | 28 | 1.36 | |
| (e) 1.0/0.72/2.0 M | 0.70 | −0.47 | 1.17 | 44 | 20 | 2.2 | |
| (f) 0.0/1.00/2.0 M | 0.75 | −0.05 | 0.80 | 44 | 19 | 2.31 |
| Key Materials | Types | CE (%) | VE (%) | EE (%) | (V) | Discharge Capacity (mAh) |
|---|---|---|---|---|---|---|
| Supporting electrolyte * | H2SO4 | 18 | 51 | 9 | 1.25 | 36 |
| CH3SO3H | 68 | 65 | 44 | 1.15 | 263 | |
| Electrode * | C–TiO2–PdO | 73 | 79 | 58 | 1.20 | 280 |
| Separation membrane | N-117–SiO2–SO3H | 90 | 81 | 72 | 1.46 | 360 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-C.; Liu, Y.-H.; Chen, M.-L.; Tseng, C.-C.; Lin, Y.-S.; Huang, S.-L. Cerium/Ascorbic Acid/Iodine Active Species for Redox Flow Energy Storage Battery. Molecules 2021, 26, 3443. https://doi.org/10.3390/molecules26113443
Chang T-C, Liu Y-H, Chen M-L, Tseng C-C, Lin Y-S, Huang S-L. Cerium/Ascorbic Acid/Iodine Active Species for Redox Flow Energy Storage Battery. Molecules. 2021; 26(11):3443. https://doi.org/10.3390/molecules26113443
Chicago/Turabian StyleChang, Tzu-Chin, Yu-Hsuan Liu, Mei-Ling Chen, Chen-Chen Tseng, Yung-Sheng Lin, and Shu-Ling Huang. 2021. "Cerium/Ascorbic Acid/Iodine Active Species for Redox Flow Energy Storage Battery" Molecules 26, no. 11: 3443. https://doi.org/10.3390/molecules26113443






