Monitoring the Reaction of the Body State to Antibiotic Treatment against Helicobacter pylori via Infrared Spectroscopy: A Case Study
Abstract
:1. Introduction
2. Working Hypotheses, Results, and Discussion
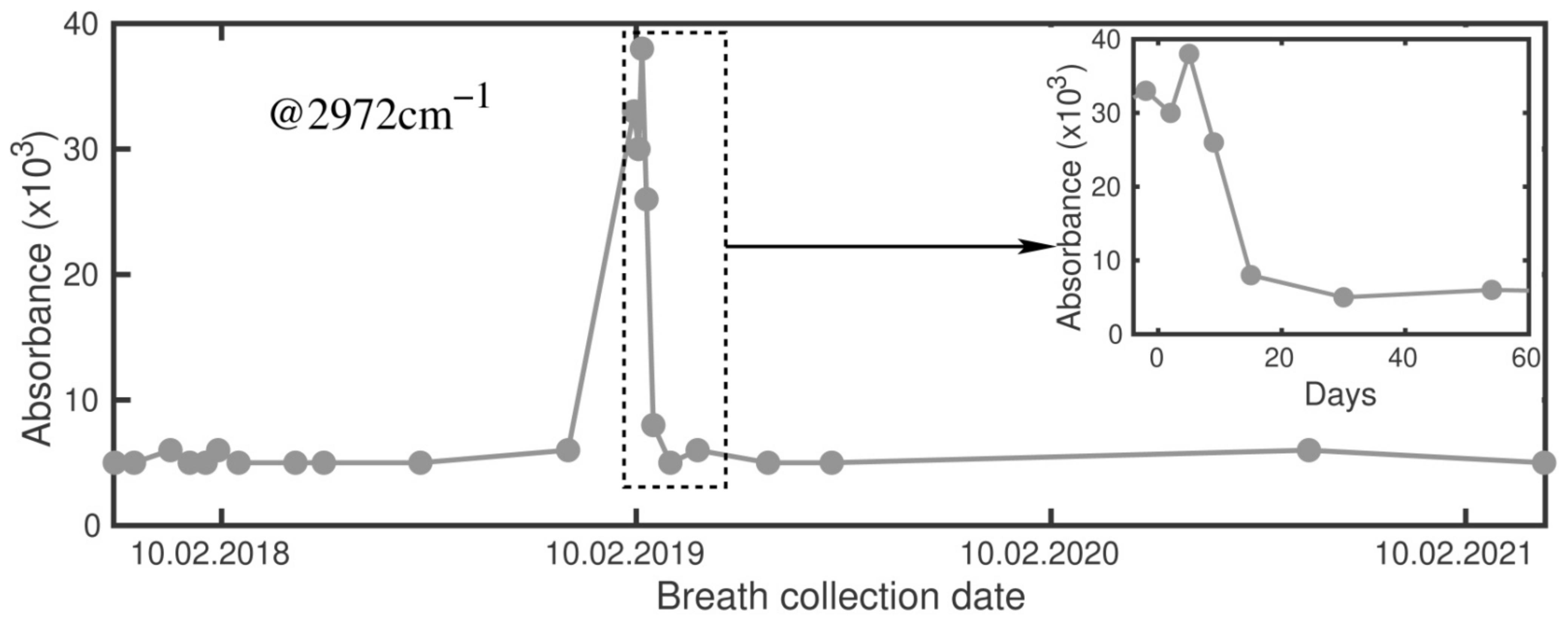
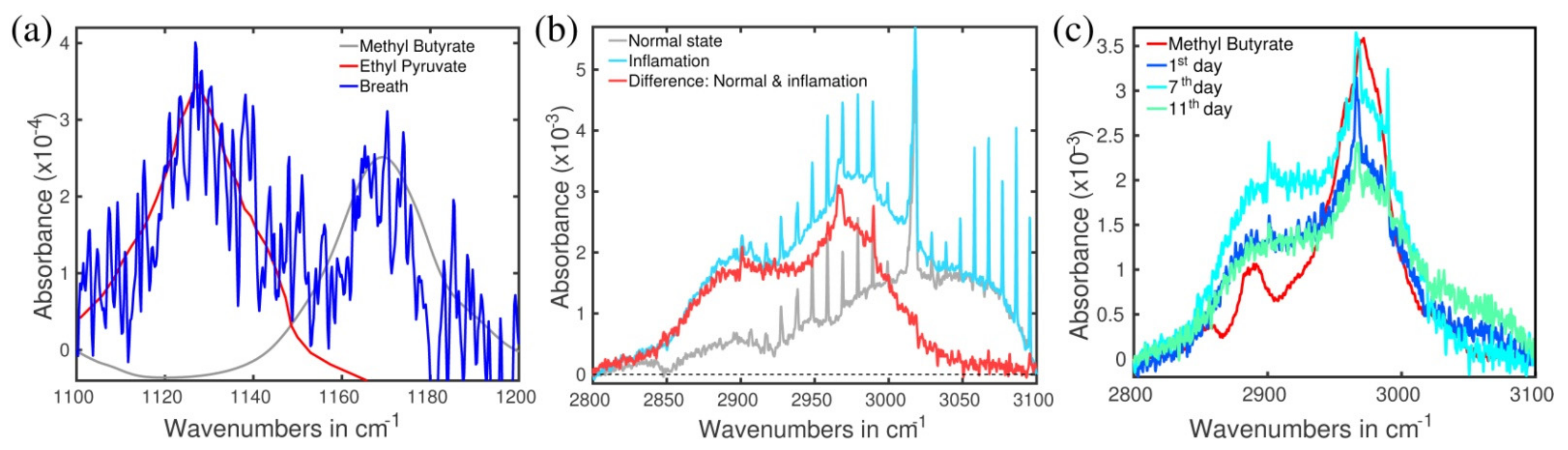
2.1. Signals at 2972 cm−1 and 1170 cm−1
2.2. Signal at 1130 cm−1
2.3. Other Signals
2.4. Identification of the Molecules Responsible for the Spectral Signals
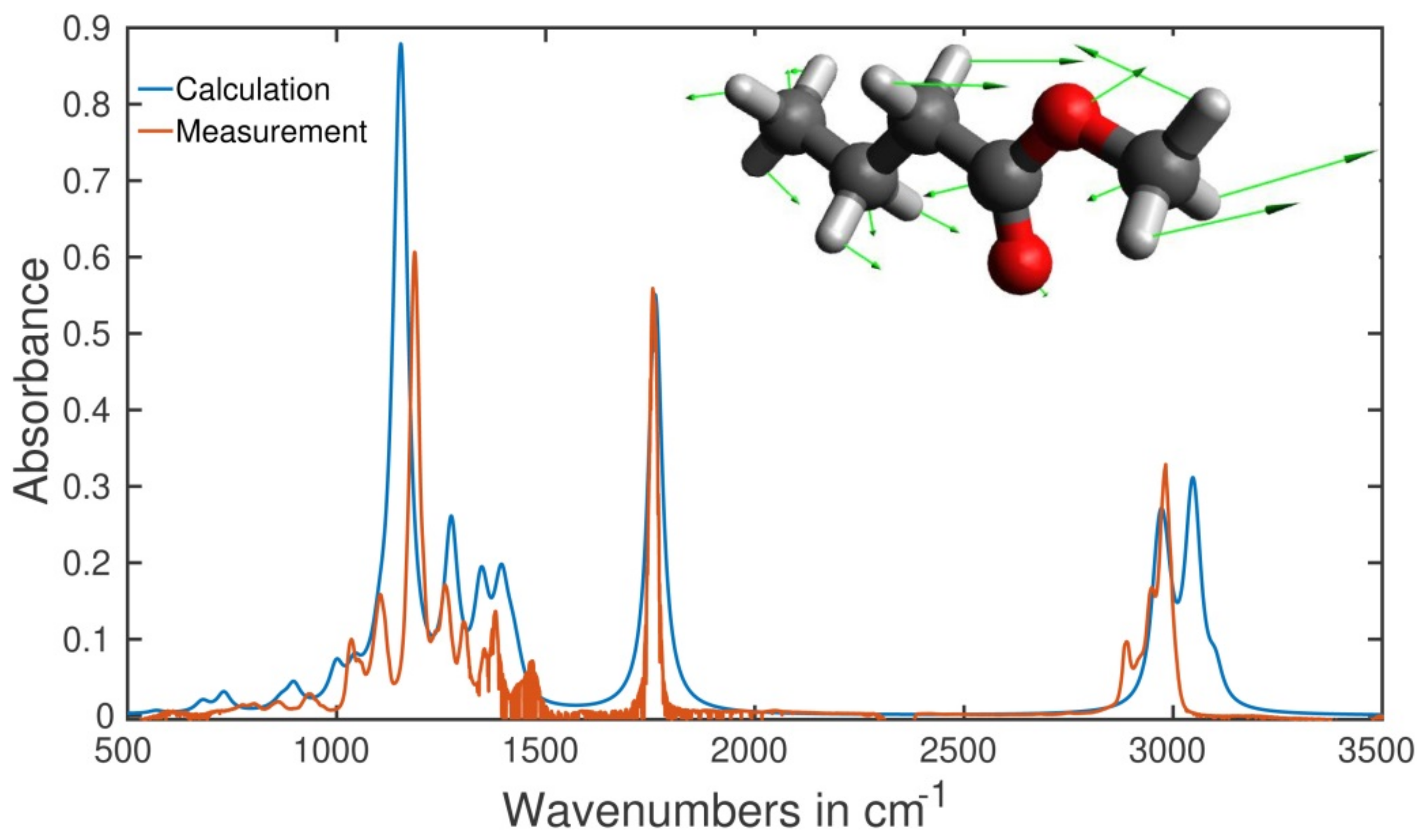
2.5. Type of Vibrations Attributed to the Characteristic Spectral Structures of Ethyl Pyruvate Observed at 1130 cm−1 and Methyl Butyrate at 1170 cm−1
2.6. A Hypothesized Transportation Scheme and Parent Bacteria
3. Materials and Methods
3.1. The Instrument
3.2. Subject of the Study and a Description of the Treatment Antibiotics Course
3.3. Identification Procedures of the Spectral Structures
3.4. Numerical Simulation
4. Conclusions and Future Work
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Goderska, K.; Pena, S.A.; Alarcon, T. Helicobacter pylori treatment: Antibiotics or probiotics. Appl. Microbiol. Biotechnol. 2018, 102, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, E.J. Helicobacter pylori and the risk and management of associated diseases: Gastritis, ulcer disease, atrophic gastritis and gastric cancer. Aliment. Pharmacol. Ther. 1997, 11, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Debraekeleer, A.; Remaut, H. Future perspectives for potential Helicobacter pylori eradication therapies. Future Microbiol. 2018, 13, 671–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Váradi, L.; Luo, J.L.; Hibbs, D.E.; Perry, J.D.; Anderson, R.J.; Orenga, S.; Groundwater, P.W. Methods for the detection and identification of pathogenic bacteria: Past, present, and future. Chem. Soc. Rev. 2017, 46, 4818–4832. [Google Scholar] [CrossRef]
- Saito, M.K.M.; Fukuda, S.; Kato, C.; Ohara, S.; Hamada, S.; Nagashima, R.; Obara, K.; Suzuki, M.; Honda, H.; Asaka, M.; et al. 13C-urea breath test, using a new compact nondispersive isotope-selective infrared spectrophotometer: Comparison with mass spectrometry. J. Gastroenterol. 2004, 39, 629–634. [Google Scholar]
- Gisbert, J.P. Comparison between two 13C-urea breath tests for the diagnosis of Helicobacter pylori infection: Isotope ratio mass spectrometer versus infrared spectrometer. Gastroenterol. Hepatol. 2003, 26, 141–146. [Google Scholar] [CrossRef]
- Ferwana, M.; Abdulmajeed, I.; Alhajiahmed, A.; Madani, W.; Firwana, B.; Hasan, R.; Altayar, O.; Limburg, P.J.; Murad, M.H.; Knawy, B. Accuracy of urea breath test in Helicobacter pylori infection: Meta-analysis. World J. Gastroenterol. 2015, 21, 1305–1314. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep16S rRNA sequencing. PLoS Biol. 2008, 6, e280. [Google Scholar] [CrossRef]
- Cully, M. Antibiotics alter the gut microbiome and host health. Nat. Milest. 2019, 6, S19. [Google Scholar]
- Shaw, L.P.; Bassam, H.; Barnes, C.P.; Walker, A.S.; Klein, N.; Balloux, F. Modelling microbiome recovery after antibiotics using a stability landscape framework. ISME J. 2019, 13, 1845–1856. [Google Scholar] [CrossRef] [Green Version]
- Logan, J.; Edwards, K.; Saunders, N. (Eds.) Real-Time PCR: Current Technology and Applications; Caister Academic Press: Norfolk, UK, 2009; ISBN 978-1-904455-39-4. [Google Scholar]
- Poretsky, R.; Rodriguez-R, L.M.; Luo, C.; Tsementzi, D.; Konstantinidis, K.T. Strengths and limitations of 16S rRNA gene amplicon sequencing in revealing temporal microbial community dynamics. PLoS ONE 2014, 9, e93827. [Google Scholar] [CrossRef] [Green Version]
- Hiergeist, A.; Reischl, U.; Gessner, A. Multicenter quality assessment of 16S ribosomal DNA-sequencing for microbiome analyses reveals high inter-center variability. Int. J. Med Microbiol. 2016, 306, 334–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepherd, L.T.W.A.J.; Doherty, C.P.; McColl, K.E.L.; Williams, C.L. Helicobacter pylori in the faeces? QJM Int. J. Med. 1999, 92, 361–364. [Google Scholar]
- Kahn, S.H.S.M.; Jiang, W.; Green, P.H.; Neu, H.C.; Chin, N.; Morotomi, M.; LoGerfo, P.; Weinstein, I.B. Direct detection and amplification of Helicobacter pylori ribosomal 16S gene segments from gastric endoscopic analysis. Diagn. Microbiol. Infect. Dis. 1990, 13, 473–479. [Google Scholar]
- Costello, B.d.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef]
- Chan, D.K.; Leggett, C.L.; Wang, K.K. Diagnosing gastrointestinal illnesses using fecal headspace volatile organic compounds. World J. Gastroenterol. 2016, 22, 1639–1649. [Google Scholar] [CrossRef]
- Wang, Q.; Teng, G.; Qiao, X.; Zhao, Y.; Kong, J.; Dong, L.; Cui, X. Importance evaluation of spectral lines in laser-induced breakdown spectroscopy for classification of pathogenic bacteria. Biomed. Opt. Express 2018, 9, 5837–5850. [Google Scholar] [CrossRef]
- Leja, M.; Amal, H.; Lasina, I.; Skapars, R.; Sivins, A.; Ancans, G.; Tolmanis, I.; Vanags, A.; Kupcinskas, J.; Ramonaite, R.; et al. Analysis of the effects of microbiome-related confounding factors on the reproducibility of the volatolomic test. J. Breath Res. 2016, 10, 037101. [Google Scholar] [CrossRef]
- Maiti, K.S.; Lewton, M.; Fill, E.; Apolonski, A. Human beings as islands of stability: Monitoring body states using breath profiles. Sci. Reports 2019, 9, 16167. [Google Scholar] [CrossRef] [Green Version]
- Nahar, S.; Mukhopadhyay, A.K.; Khan, R.; Ahmad, M.M.; Datta, S.; Chattopadhyay, S.; Dhar, S.C.; Sarker, S.A.; Engstrand, L.; Berg, D.E.; et al. Antimicrobial Susceptibility of Helicobacter pylori Strains Isolated in Bangladesh. J. Clin. Microbiol. 2004, 42, 4856–4858. [Google Scholar] [CrossRef] [Green Version]
- Theriot, C.M.; Koenigsknecht, M.J., Jr.; Hatton, E.G.; Nelson, A.M.; Li, B.; Huffnagle, G.B.; Li, J.Z.; Young, V.B. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat. Commun. 2014, 5, 3114. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.omnibioticlife.com/omnibiotic/ab-10/ (accessed on 6 June 2021).
- Niv, Y. H pylori recurrence after successful eradication. World J. Gastroenterol. 2008, 14, 1477–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gisbert, J.P.; Luna, M.; Gomez, B.; Herrerais, J.M.; Mones, J.; Castro-Fernandez, M.; Sanchez-Porbe, P.; Cosme, A.; Oliveras, D.; Pajares, J.M. Recurrence of Helicobacter pylori infection after several eradication therapies: Long-term follow-up of 1000 patients. Aliment. Pharmacol. Ther. 2006, 23, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Apolonski, A.; Maiti, K.S. Towards a standard operating procedure for revealing hidden volatile organic compounds in breath: The Fourier-transform IR spectroscopy case. Appl. Opt. 2021, 60, 4217–4224. [Google Scholar] [CrossRef]
- Kramida, A.; Ralchenko, Y.; Reader, J.; NIST ASD Team. NIST Atomic Spectra Database (ver. 5.7.1), National Institute of Standards and Technology, Gaithersburg, MD, USA. 2019. Available online: https://physics.nist.gov/asd (accessed on 9 April 2017).
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The Human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Doing, P.; de Jonge, B.L.; Alm, R.A.; Brown, E.D.; Uria-Nickelsen, M.; Noonan, B.; Mills, S.D.; Tummino, P.; Carmel, G.S.; Guild, B.C.; et al. Helicobacter pylori physiology predicted from genomic comparison of two strains. Microbiol. Mol. Biol. Rev. 1999, 63, 675–707. [Google Scholar]
- Maiti, K.S.; Lewton, M.; Fill, E.; Apolonski, A. Sensitive spectroscopic breath analysis by water condensation. J. Breath Res. 2018, 12, 046003. [Google Scholar] [CrossRef]
- Maiti, K.S.; Roy, S.; Lampe, R.; Apolonski, A. Breath indeed carries significant information about a disease. Potential biomarkers of cerebral palsy. J. Biophoton. 2020. [Google Scholar] [CrossRef]
- Gelin, M.F.; Blokhin, A.P.; Ostrozhenkova, E.; Apolonski, A.; Maiti, K.S. Theory helps experiment to reveal VOCs in human breath. Spectrochim. Acta Part A 2021, 258, 119785. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 Citation, Revision, A.02; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Erratum to Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 78, E1396. [Google Scholar] [CrossRef] [Green Version]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lip. Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Berg, R.D. The Indigenous Gastrointestinal Microflora. Trends Microbiol. 1996, 4, 430–454. [Google Scholar] [CrossRef]
- Bäumler, A.J.; Sperandio, V. Interactions Between the Microbiota and Pathogenic Bacteria in the Gut. Nat. Cell Biol. 2016, 535, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Yang, I.; Neil, S.; Suerbaum, S. Survival in Hostile Territory: The Microbiota of the Stomach. FEMS Microbiol. Rev. 2013, 37, 736–761. [Google Scholar] [CrossRef]
- Westhoff, S.; van Wezel, G.P.E.; Rozen, D. Distance-Dependent Danger Responses in Bacteria. Curr. Opin. Microbiol. 2017, 36, 95–101. [Google Scholar] [CrossRef]
- Garcia-Bayona, L.; Comstock, L.E. Bacterial Antagonism in Host-Associated Microbal Communities. Science 2018, 361, 1215. [Google Scholar] [CrossRef] [Green Version]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angers, A.; Kagkli, D.; Patak, A.; Petrillo, M.; Querci, M.; Rüdelsheim, P.; Smets, G.; Van den Eede, G. The Human Gut Microbiota (2018), JRC Technical Report. Available online: http://publications.jrc.ec.europa.eu/repository/Handle/JRC112042 (accessed on 6 June 2021).
- Dickson, I. Stability and individuality of adult microbiota. Nat. Milest. 2019, 7, S11. [Google Scholar]
- Quevrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermúdez-Humarán, L.G.; Pigneur, B.; et al. Identification of an Anti-Inflammatory Protein from Faecalibacterium Prausnitzii, a Commensal Bacterium Deficient in Crohn’s Disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The Long-Term Stability of the Human Gut Microbiota. Science 2013, 341, 1237439. [Google Scholar] [CrossRef] [Green Version]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of Antibiotics on Gut Microbiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef]
- Delgado, S.; Flórez, A.B.; Mayo, B. Antibiotic Susceptibility of Lactobacillus and Bifidobacterium Species from the Human Gastrointestinal Tract. Curr. Microbiol. 2005, 50, 202–207. [Google Scholar] [CrossRef]
- Ramsey, M.; Hartke, A.; Huycke, M. The Physiology and Metabolism of Enterococci. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Kristich, C.J.; Rice, L.B.; Arias, C.A. Enterococcal infection—treatment and Antibiotic Resistance. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Hickey, M.W.; Hillier, A.; Jago, G. Metabolism of Pyruvate and Citrate in Lactobacilli. Aust. J. Biol. Sci. 1983, 36, 487. [Google Scholar] [CrossRef] [Green Version]
- Belenguer, A.; Duncan, S.H.; Calder, A.G.; Holtrop, G.; Louis, P.; Lobley, G.E.; Flint, H.J. Two Routes of Metabolic Cross-Feeding Between Bifidobacterium Adolescentis and Butyrate-Producing Anaerobes from the Human Gut. Appl. Envir. Microbiol. 2006, 72, 3593–3599. [Google Scholar] [CrossRef] [Green Version]
- Ferrario, C.; Taverniti, V.; Milani, C.; Fiore, W.; Laureati, M.; De Noni, I.; Stuknyte, M.; Chouaia, B.; Riso, P.; Guglielmetti, S. Modulation of Fecal Clostridiales Bacteria and Butyrate by Probiotic Intervention with Lactobacillus Paracasei DG Varies Among Healthy Adults. J. Nutr. 2014, 144, 1787–1796. [Google Scholar] [CrossRef] [Green Version]
- Venegas, D.P.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Louis, P.; Flint, H.J. Diversity, Metabolism and Microbial Ecology of Butyrate-Producing Bacteria from the Human Large Intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lofgren, J.L.; Whary, M.T.; Ge, Z.; Muthupalani, S.; Taylor, N.S.; Mobley, M.; Potter, A.; Varro, A.; Eibach, D.; Suerbaum, S.; et al. Lack of Commensal Flora in Helicobacter pylori–Infected INS-GAS Mice Reduces Gastritis and Delays Intraepithelial Neoplasia. Gastroenterology 2011, 140, 210–220.e4. [Google Scholar] [CrossRef] [Green Version]
- Boyanova, L.; Kolarov, R.; Mitov, I. Antimicrobial Resistance and the Management of Anaerobic Infections. Expert Rev. Anti Infect. Ther. 2007, 5, 685–701. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiti, K.S.; Apolonski, A. Monitoring the Reaction of the Body State to Antibiotic Treatment against Helicobacter pylori via Infrared Spectroscopy: A Case Study. Molecules 2021, 26, 3474. https://doi.org/10.3390/molecules26113474
Maiti KS, Apolonski A. Monitoring the Reaction of the Body State to Antibiotic Treatment against Helicobacter pylori via Infrared Spectroscopy: A Case Study. Molecules. 2021; 26(11):3474. https://doi.org/10.3390/molecules26113474
Chicago/Turabian StyleMaiti, Kiran Sankar, and Alexander Apolonski. 2021. "Monitoring the Reaction of the Body State to Antibiotic Treatment against Helicobacter pylori via Infrared Spectroscopy: A Case Study" Molecules 26, no. 11: 3474. https://doi.org/10.3390/molecules26113474
APA StyleMaiti, K. S., & Apolonski, A. (2021). Monitoring the Reaction of the Body State to Antibiotic Treatment against Helicobacter pylori via Infrared Spectroscopy: A Case Study. Molecules, 26(11), 3474. https://doi.org/10.3390/molecules26113474





