Preparation and Electrical Properties of Polyacrylonitrile Based Porous Carbon by Different Activation Methods
Abstract
1. Introduction
2. Results and Discussion
2.1. Two-Step Method of Carbonization and Activation Gradually
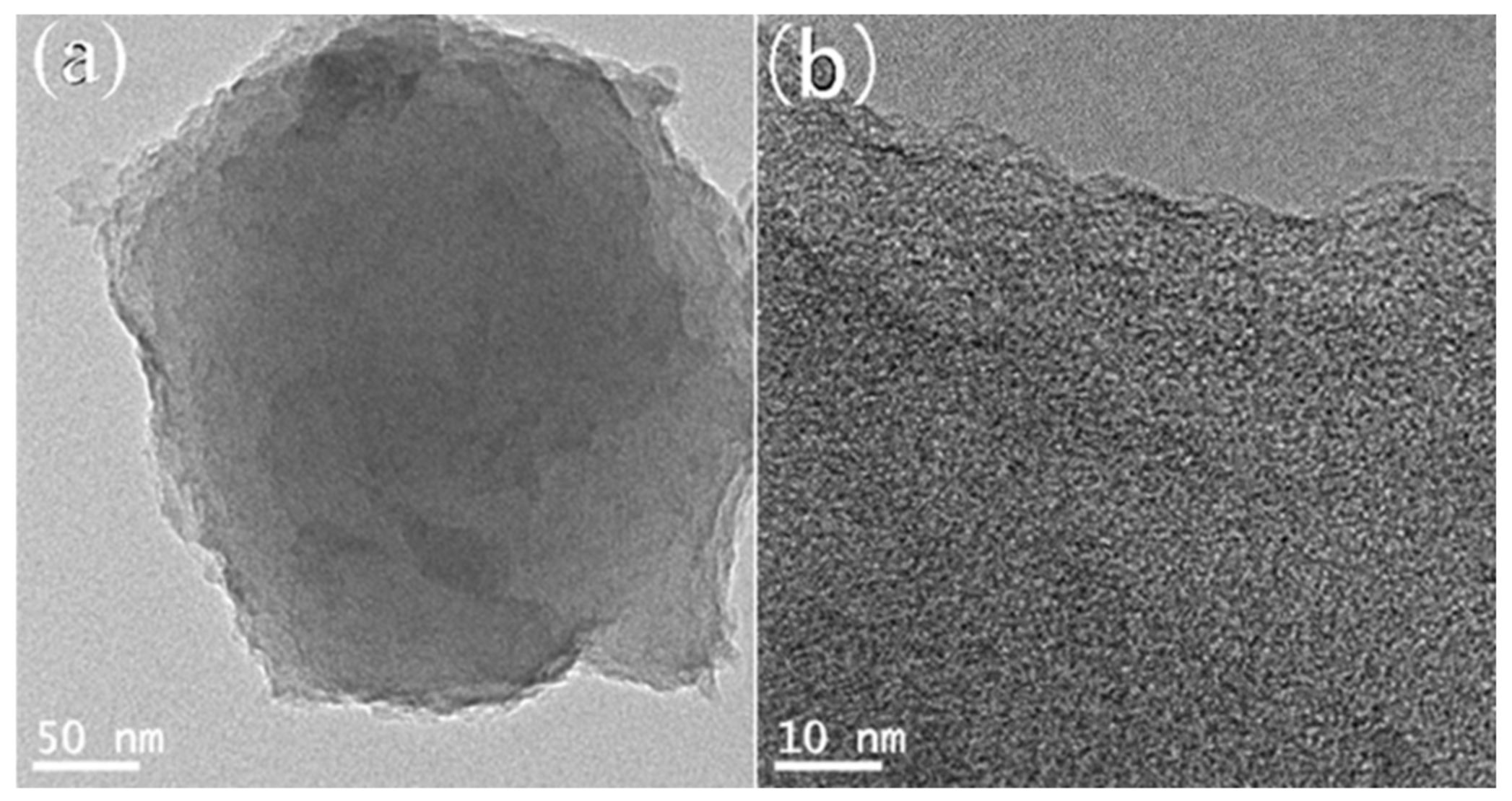
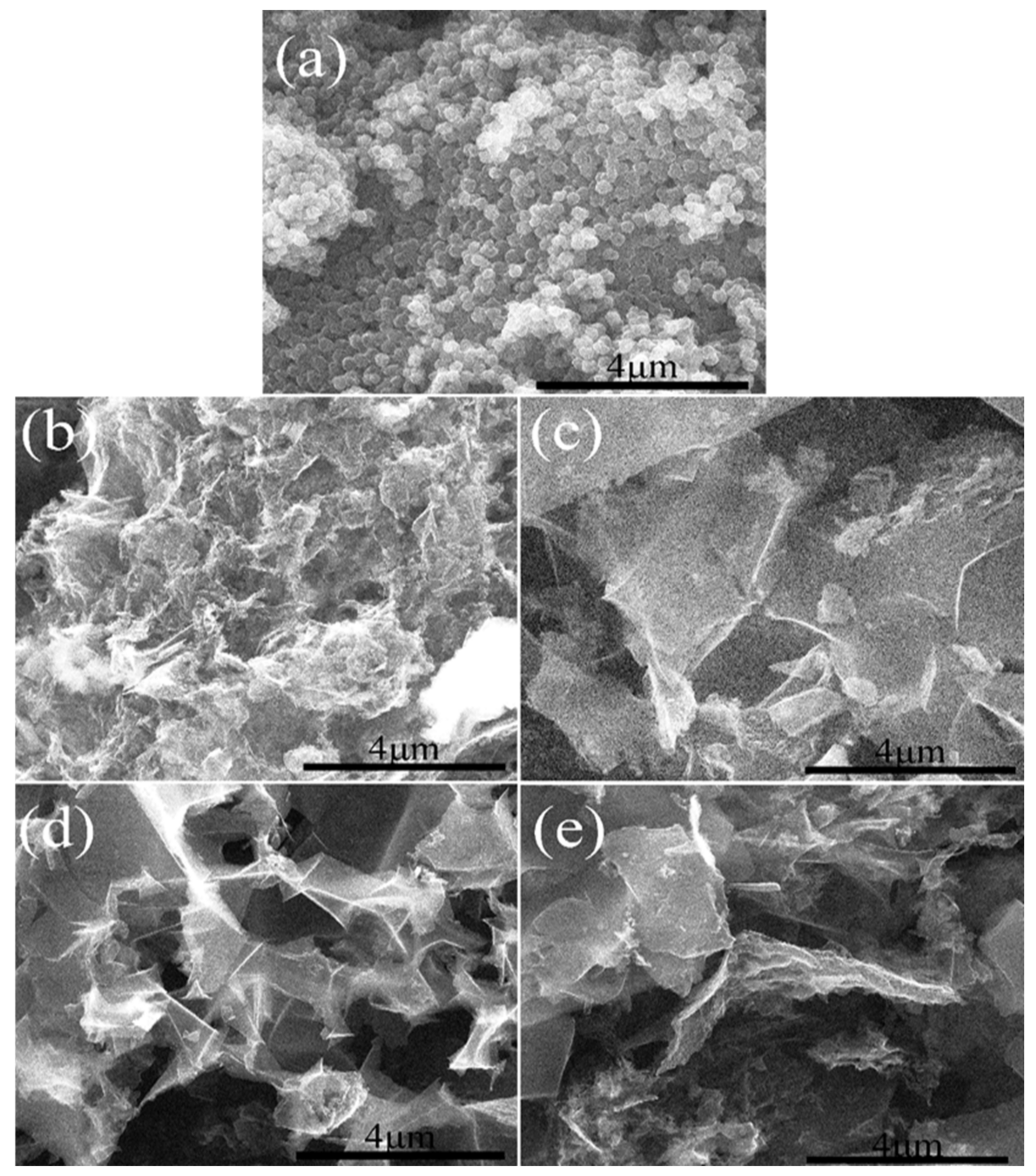
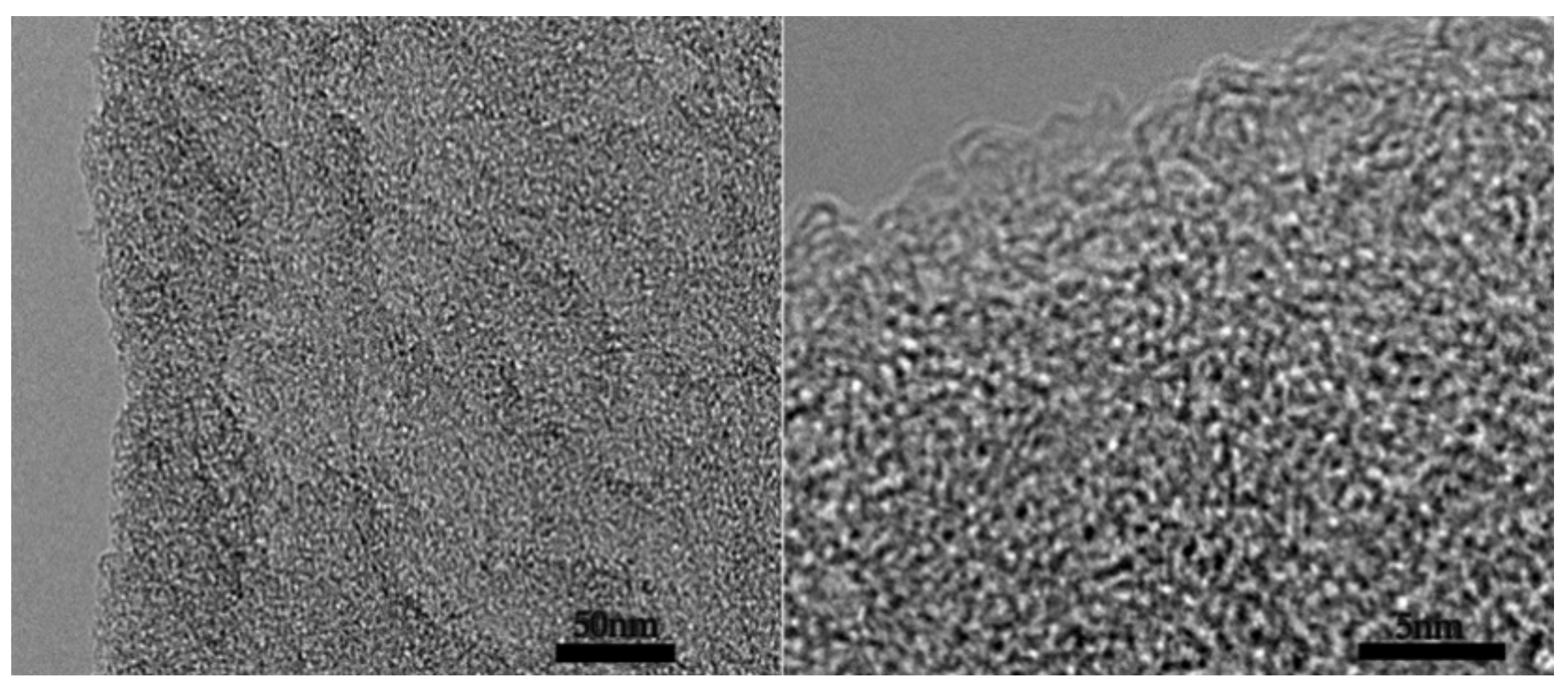
2.1.1. Morphology Analysis
2.1.2. Elemental Analysis
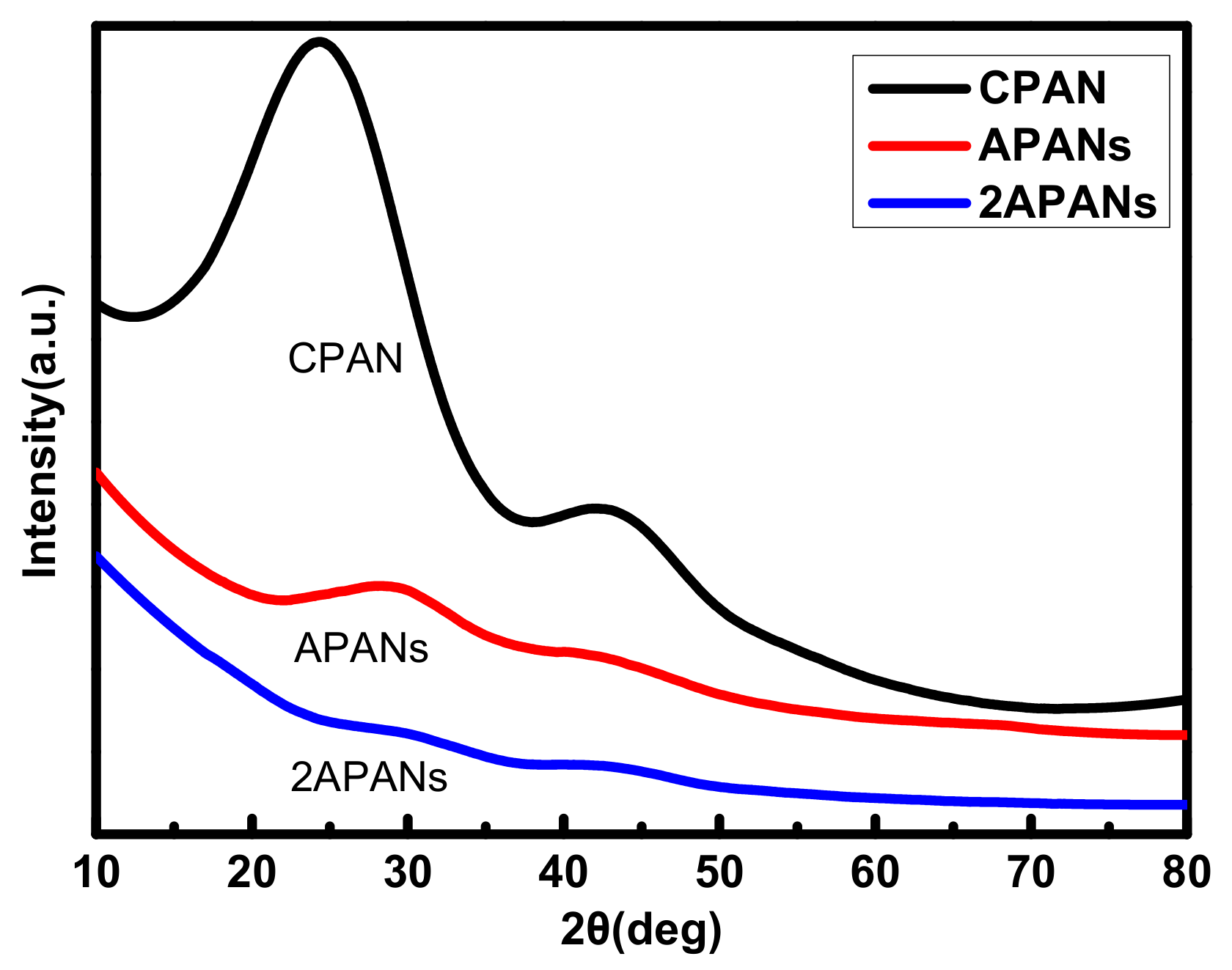
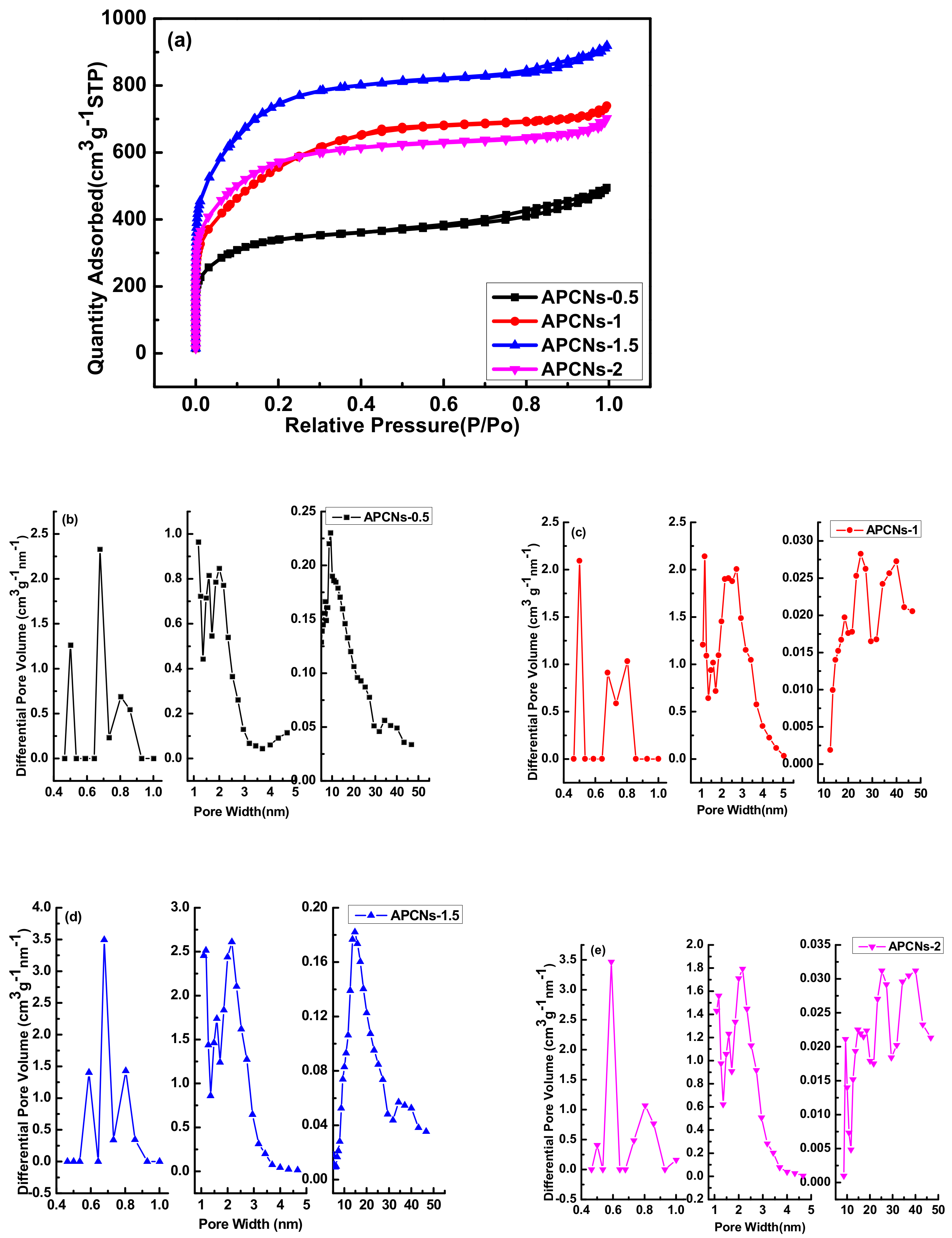
2.1.3. Pore Structure Analysis
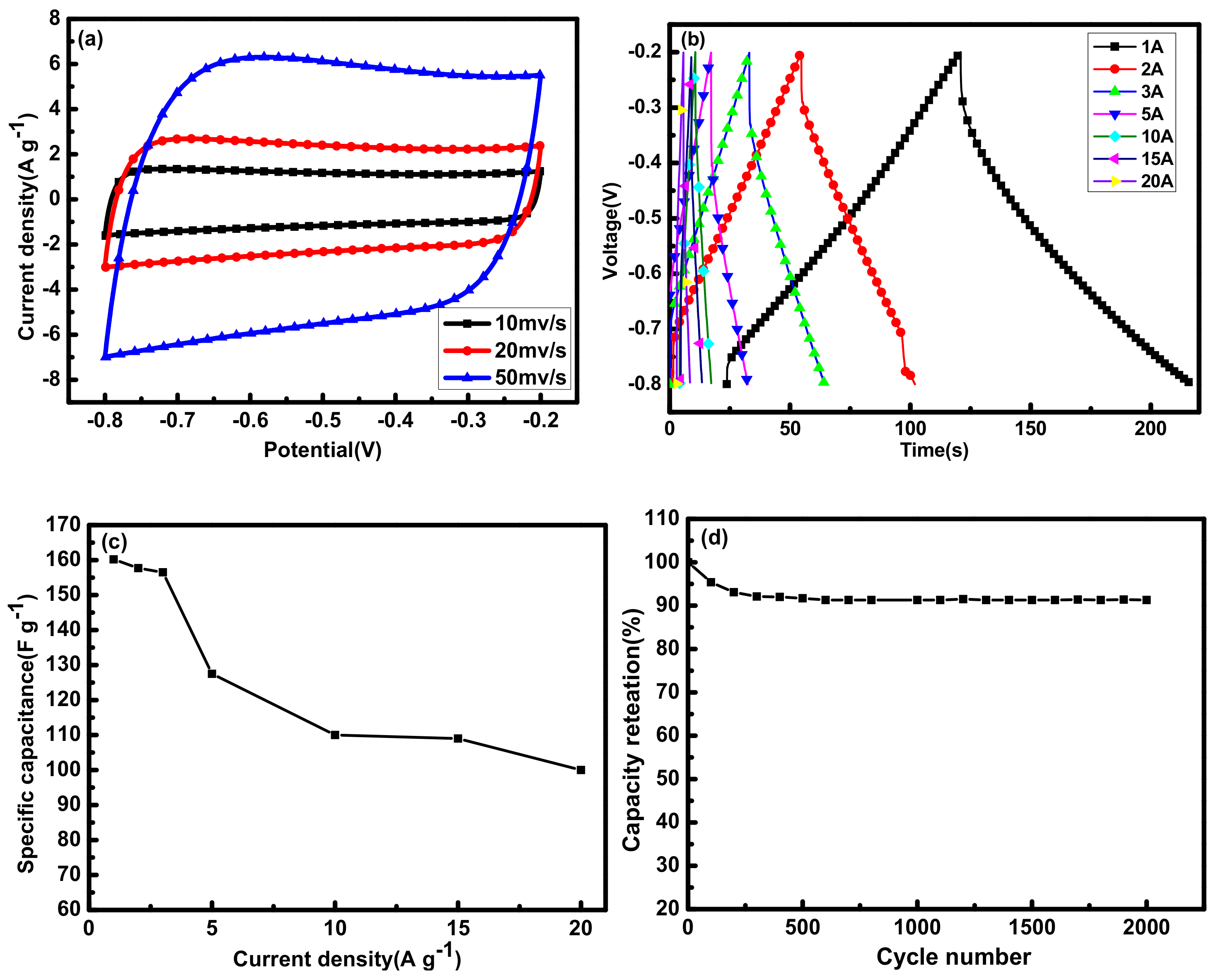
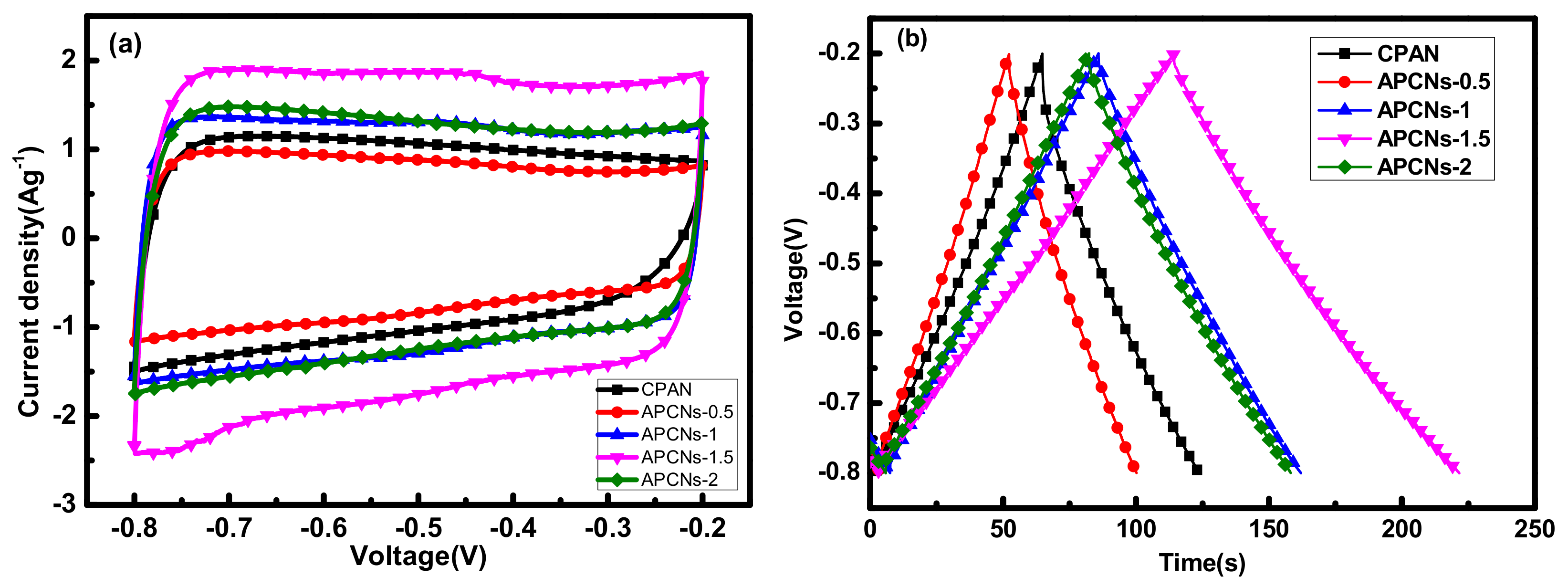
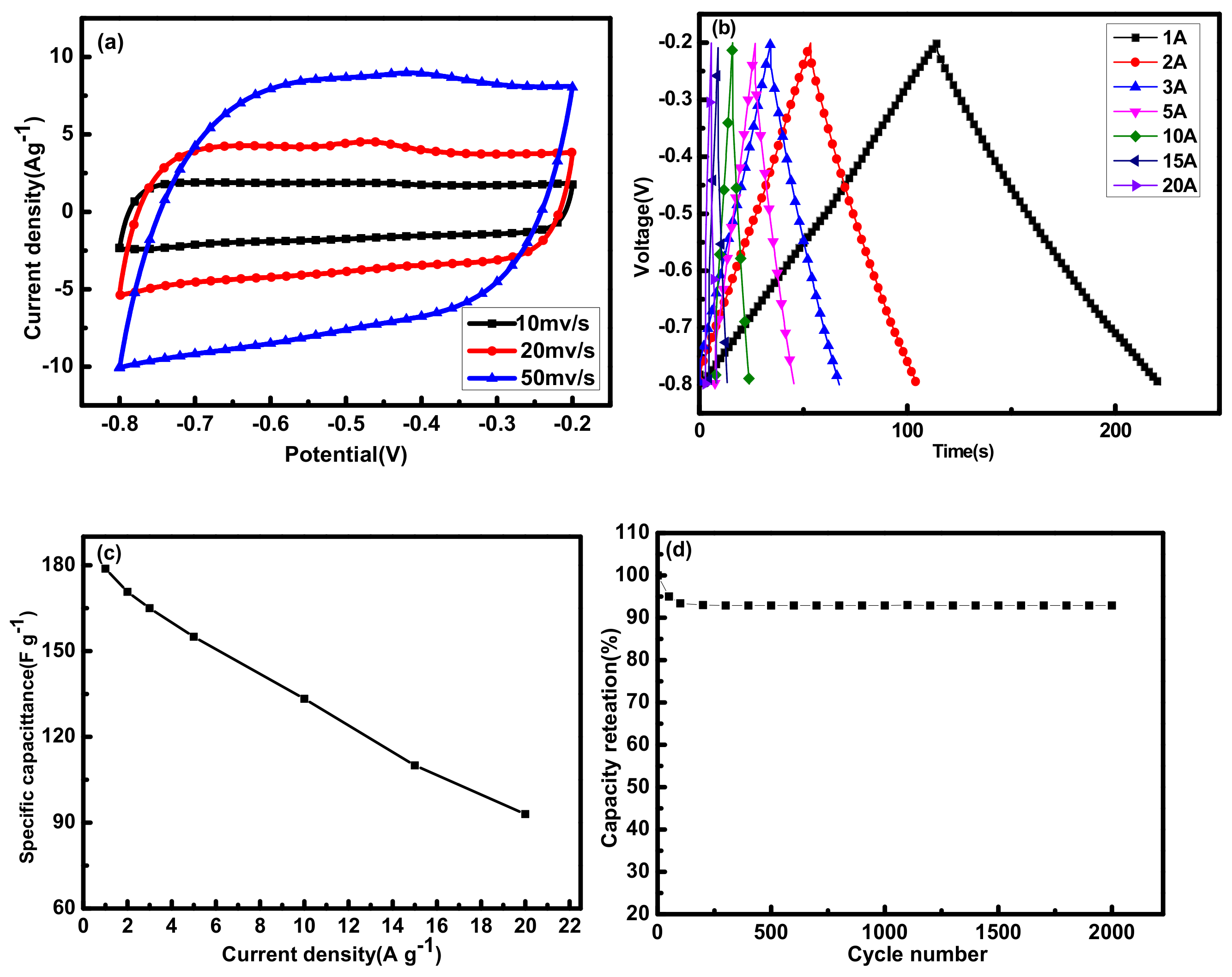
2.1.4. Electrochemical Performance Analysis
2.2. One-Step Method of Carbonization and Activation Simultaneously
2.2.1. Morphology Analysis
2.2.2. Elemental Analysis
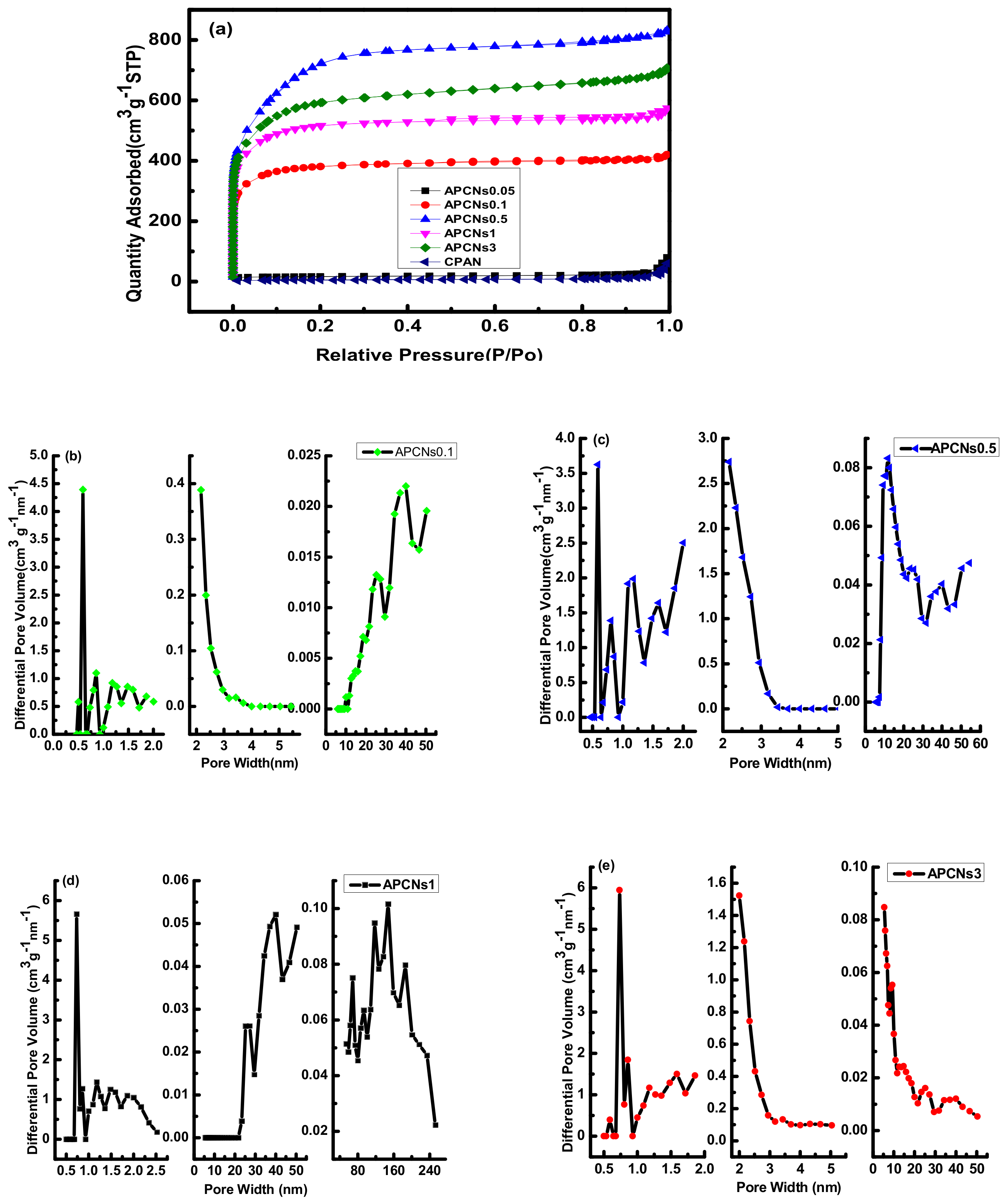
2.2.3. Structure Analysis
2.2.4. Electrochemical Performance Analysis
3. Materials and Methods
3.1. Preparation of PAN Nanospheres
3.2. Preparation of PAN-Based Porous Carbon Materials
3.3. Electrode Preparation and Electrochemical Testing
3.4. Material Characterizations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhai, S.L.; Karahan, H.E.; Wang, C.J.; Pei, Z.X.; Wei, L.; Chen, Y. 1D Supercapacitors for Emerging Electronics: Current Status and Future Directions. Adv. Mater. 2020, 32, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, R.; Kannan, A.G.; Ponraj, R.; Thangavel, V.; Kim, D.W.; Lee, Y.S. High-energy green supercapacitor driven by ionic liquid electrolytes as an ultra-high stable next-generation energy storage device. J. Power Sources 2018, 383, 102–109. [Google Scholar] [CrossRef]
- Jost, K.; Dion, G.; Gogotsi, Y. Textile energy storage in perspective. J. Mater. Chem. A 2014, 2, 10776–10787. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhang, L.; Hou, H.Q.; Xu, W.H.; Duan, G.G.; He, S.J.; Liu, K.M.; Jiang, S.H. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Pan, Y.S.; Xu, K.; Wu, C.L. Recent progress in supercapacitors based on the advanced carbon electrodes. Nanotechnol. Rev. 2019, 8, 299–314. [Google Scholar] [CrossRef]
- Korivi, N.S.; Vangari, M.; Jiang, L. Carbon nanotube nanocomposite-modified paper electrodes for supercapacitor applications. Appl. Nanosci. 2017, 7, 41–45. [Google Scholar] [CrossRef]
- Sleptsov, V.; Diteleva, A. Thin-film technology for creating flexible supercapacitor electrodes based on a carbon matrix. High. Temp. Mater. Process. 2020, 24, 167–171. [Google Scholar] [CrossRef]
- Yadav, M.S. Metal oxides nanostructure-based electrode materials for supercapacitor application. J. Nanopart. Res. 2020, 22, 1–18. [Google Scholar] [CrossRef]
- Wu, D.; Li, G.N.; Fu, L.J.; Liu, Z.L.; Wang, B.D.; Zhang, X.H.; Qiu, D.T.; Zhang, J.H.; Sudduth, B.; Sun, J.M.; et al. Thermodynamics-structure-performance relations of nickel-aluminum layered double hydroxide as supercapacitor electrode materials. Abstr. Pap. Am. Chem. Soc. 2019, 257, 1. [Google Scholar]
- Ye, J.; Shi, D.J.; Yang, Z.K.; Chen, M.Q. Interpenetrating Network Hydrogels based on Nanostructured Conductive Polymers for Flexible Supercapacitor. Polym. Sci. Ser. A 2018, 60, 647–654. [Google Scholar] [CrossRef]
- Gang, B.J.; Zhang, F.; Li, X.L.; Zhai, B.; Wang, X.Y.; Song, Y. A ulva lactuca-derived porous carbon for high-performance electrode materials in supercapacitor: Synergistic effect of porous structure and graphitization degree. J. Energy Storag. 2021, 33, 1–9. [Google Scholar] [CrossRef]
- Zhang, G.X.; Chen, Y.M.; Chen, Y.G.; Guo, H.B. Activated biomass carbon made from bamboo as electrode material for supercapacitors. Mater. Res. Bull. 2018, 102, 391–398. [Google Scholar] [CrossRef]
- Kamran, U.; Choi, J.R.; Park, S.J. A Role of Activators for Efficient CO2 Affinity on Polyacrylonitrile-Based Porous Carbon Materials. Front. Chem. 2020, 8, 1–18. [Google Scholar] [CrossRef]
- Zhang, X.L.; Ma, L.; Gan, M.Y.; Fu, G.; Jin, M.; Lei, Y.; Yang, P.S.; Yan, M.F. Fabrication of 3D lawn-shaped N-doped porous carbon matrix/polyaniline nanocomposite as the electrode material for supercapacitors. J. Power Sources 2017, 340, 22–31. [Google Scholar] [CrossRef]
- Wang, C.H.; Hsu, H.C.; Hu, J.H. High-energy asymmetric supercapacitor based on petal-shaped MnO2 nanosheet and carbon nanotube-embedded polyacrylonitrile-based carbon nanofiber working at 2 V in aqueous neutral electrolyte. J. Power Sources 2014, 249, 1–8. [Google Scholar] [CrossRef]
- Zhang, E.G.; Wu, M.J.; Tang, Q.W.; Gong, Q.J.; Sun, S.H.; Qiao, J.L.; Zhang, L. Using aminopyrine as a nitrogen-enriched small molecule precursor to synthesize high-performing nitrogen doped mesoporous carbon for catalyzing oxygen reduction reaction. RSC Adv. 2017, 7, 669–677. [Google Scholar] [CrossRef]
- Heo, Y.J.; Zhang, Y.F.; Rhee, K.Y.; Park, S.J. Synthesis of PAN/PVDF nanofiber composites-based carbon adsorbents for CO2 capture. Composit. Part. B Eng. 2019, 156, 95–99. [Google Scholar] [CrossRef]
- Shu, Y.; Maruyama, J.; Iwasaki, S.; Maruyama, S.; Shen, Y.; Uyama, H. Fabrication of N-doped and shape-controlled porous monolithic carbons from polyacrylonitrile for supercapacitors. RSC Adv. 2017, 7, 43172–43180. [Google Scholar] [CrossRef]
- Lee, B.M.; Choi, B.S.; Lee, J.Y.; Hong, S.K.; Lee, J.S.; Choi, J.H. Fabrication of porous carbon beads from polyacrylonitrile as electrode materials for electric double-layer capacitors. Carbon Lett. 2021, 31, 67–74. [Google Scholar] [CrossRef]
- Yao, L.; Yang, G.Z.; Han, P. Facile self-templating preparation of polyacrylonitrile-derived hierarchical porous carbon nanospheres for high-performance supercapacitors. RSC Adv. 2016, 6, 43748–43754. [Google Scholar] [CrossRef]
- Liu, Y.J.; Cao, J.Y.; Jiang, X.H.; Yang, Y.G.; Yu, L.M.; Yan, X.F. Large scale production of polyacrylonitrile-based porous carbon nanospheres for asymmetric supercapacitors. J. Mater. Chem. A 2018, 6, 6891–6903. [Google Scholar] [CrossRef]
- Chen, G.P.; Zhai, W.L.; Wang, Z.H.; Yu, J.G.; Wang, F.Q.; Zhao, Y.N.; Li, G.D. Fabrication and supercapacitive properties of hierarchical porous carbon from polyacrylonitrile. Mater. Res. Bull. 2015, 72, 204–210. [Google Scholar] [CrossRef]
- Kubota, M.; Hata, A.; Matsuda, H. Preparation of activated carbon from phenolic resin by KOH chemical activation under microwave heating. Carbon 2009, 47, 2805–2811. [Google Scholar] [CrossRef]
- Hesas, R.H.; Daud, W.; Sahu, J.N.; Arami-Niya, A. The effects of a microwave heating method on the production of activated carbon from agricultural waste: A review. J. Anal. Appl. Pyrolysis. 2013, 100, 1–11. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Ngah CW, Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sust. Energ. Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Li, L.X.; Song, H.H.; Chen, X.H. Pore characteristics and electrochemical performance of ordered mesoporous carbons for electric double-layer capacitors. Electrochim. Acta. 2006, 51, 5715–5720. [Google Scholar] [CrossRef]
- Yu, X.; Kang, Y.; Park, H.S. Sulfur and phosphorus co-doping of hierarchically porous graphene aerogels for enhancing supercapacitor performance. Carbon 2016, 101, 49–56. [Google Scholar] [CrossRef]
- Ma, W.J.; Chen, S.H.; Yang, S.Y.; Chen, W.P.; Weng, W.; Zhu, M.F. Bottom-Up Fabrication of Activated Carbon Fiber for All-Solid-State Supercapacitor with Excellent Electrochemical Performance. ACS Appl. Mater. Int. 2016, 8, 14622–14627. [Google Scholar] [CrossRef]




| Element | 2APCNs-1 | 2APCNs-1.5 | 2APCNs-2 | 2APCNs-3 |
|---|---|---|---|---|
| C (Atom%) | 82.7 | 88.60 | 89.18 | 88.66 |
| N (Atom%) | 5.56 | 5.21 | 4.05 | 5.12 |
| O (Atom%) | 11.74 | 6.19 | 6.77 | 6.22 |
| O/C (%) | 14.2 | 7.6 | 7.0 | 7.1 |
| Sample | SBET (m2 g−1) | Vtotal (cm3 g−1) | Vmes (cm3 g−1) | Dp (nm) |
|---|---|---|---|---|
| 2APCNs-1 | 1880 | 1.10 | 0.71 | 3.19 |
| 2APCNs-1.5 | 2350 | 1.50 | 1.51 | 3.12 |
| 2APCNs-2 | 2830 | 2.19 | 1.97 | 3.16 |
| 2APCNs-3 | 2670 | 2.11 | 1.88 | 3.19 |
| Element | CPAN | APCNs-0.5 | APCNs-1 | APCNs-1.5 | APCNs-2 |
|---|---|---|---|---|---|
| C (Atom%) | 71.29 | 73.45 | 80.88 | 84.16 | 81.63 |
| N (Atom%) | 19.24 | 4.23 | 6.24 | 6.61 | 4.56 |
| O (Atom%) | 0.53 | 13.29 | 7.36 | 6.39 | 7.31 |
| O/C (%) | 7.43 | 18.09 | 9.09 | 7.59 | 8.95 |
| Sample | SBET (m2 g−1) | Vtotal (cm3 g−1) | Vmes (cm3 g−1) | Dp (nm) |
|---|---|---|---|---|
| CPAN | 20 | 0.09 | -- | -- |
| APCNs-0.5 | 1080 | 0.76 | 0.51 | 4.85 |
| APCNs-1 | 1930 | 1.14 | 1.13 | 2.71 |
| APCNs-1.5 | 2430 | 1.42 | 1.14 | 3.04 |
| APCNs-2 | 1850 | 1.09 | 0.82 | 3.06 |
| Sample | SBET (m2 g−1) | Vtotal (cm3 g−1) | Vmes(cm3 g−1) |
|---|---|---|---|
| CPAN | 20 | 0.09 | -- |
| APCNs0.05 | 19 | 0.10 | 0.08 |
| APCNs0.1 | 1170 | 0.65 | 0.22 |
| APCNs0.5 | 2340 | 1.29 | 1.04 |
| APCNs1 | 1590 | 0.89 | 0.35 |
| APCNs3 | 1860 | 1.09 | 0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Tan, Y.; Sun, M.; Yu, B.; Yang, J.; Xue, Y.; Yang, G. Preparation and Electrical Properties of Polyacrylonitrile Based Porous Carbon by Different Activation Methods. Molecules 2021, 26, 3499. https://doi.org/10.3390/molecules26123499
Wang X, Tan Y, Sun M, Yu B, Yang J, Xue Y, Yang G. Preparation and Electrical Properties of Polyacrylonitrile Based Porous Carbon by Different Activation Methods. Molecules. 2021; 26(12):3499. https://doi.org/10.3390/molecules26123499
Chicago/Turabian StyleWang, Xiaoqiang, Yifan Tan, Meijiao Sun, Binbin Yu, Junhe Yang, Yuhua Xue, and Guangzhi Yang. 2021. "Preparation and Electrical Properties of Polyacrylonitrile Based Porous Carbon by Different Activation Methods" Molecules 26, no. 12: 3499. https://doi.org/10.3390/molecules26123499
APA StyleWang, X., Tan, Y., Sun, M., Yu, B., Yang, J., Xue, Y., & Yang, G. (2021). Preparation and Electrical Properties of Polyacrylonitrile Based Porous Carbon by Different Activation Methods. Molecules, 26(12), 3499. https://doi.org/10.3390/molecules26123499






