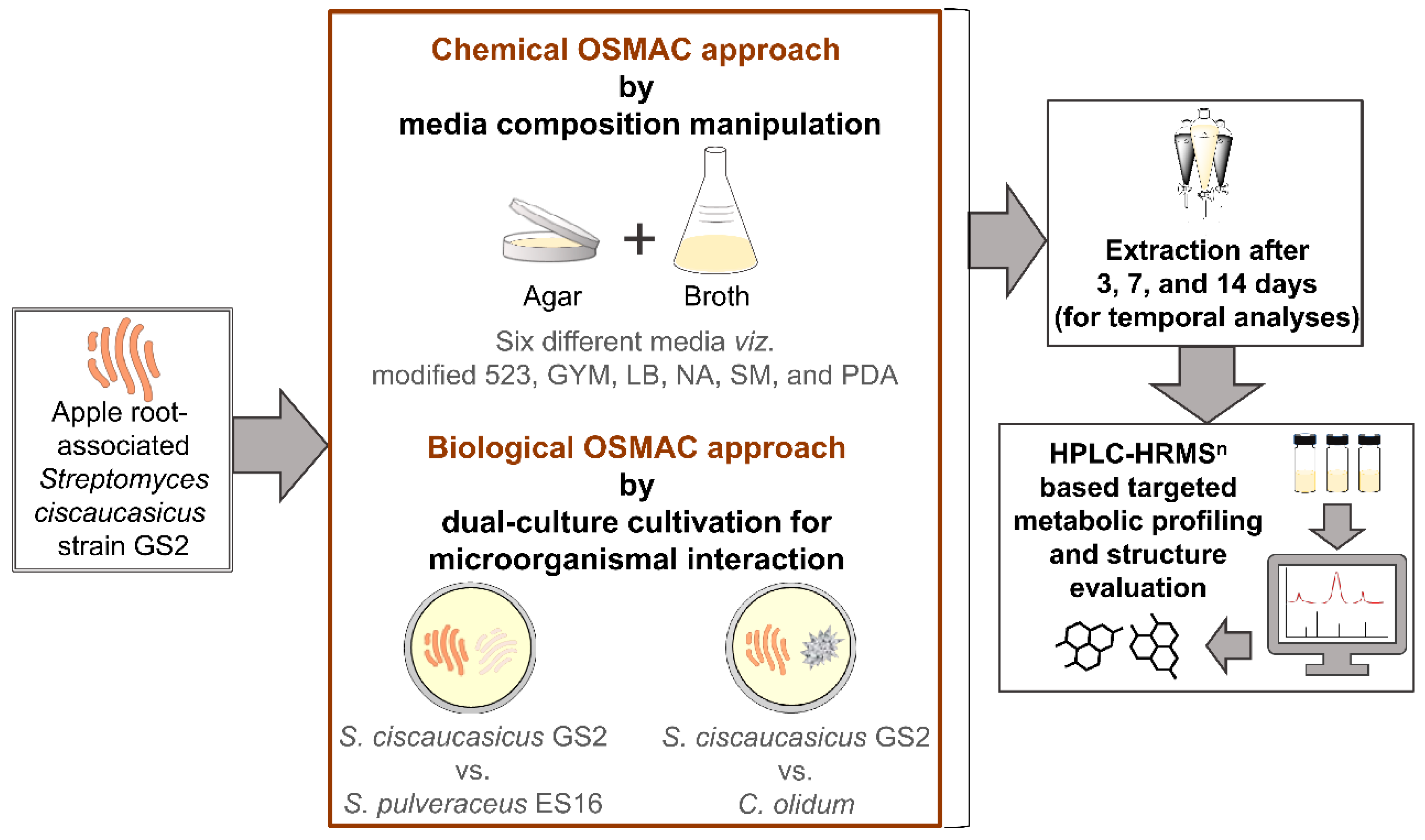
Production of Siderophores by an Apple Root-Associated Streptomyces ciscaucasicus Strain GS2 Using Chemical and Biological OSMAC Approaches
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Characterization of S. ciscaucasicus GS2 under the Influence of Chemical OSMAC Approach by Alteration of Media Composition
2.2. Phenotypic Characterization of S. ciscaucasicus GS2 under the Influence of Biological OSMAC Approach by Dual-Culture Cultivation for Microorganismal Interaction
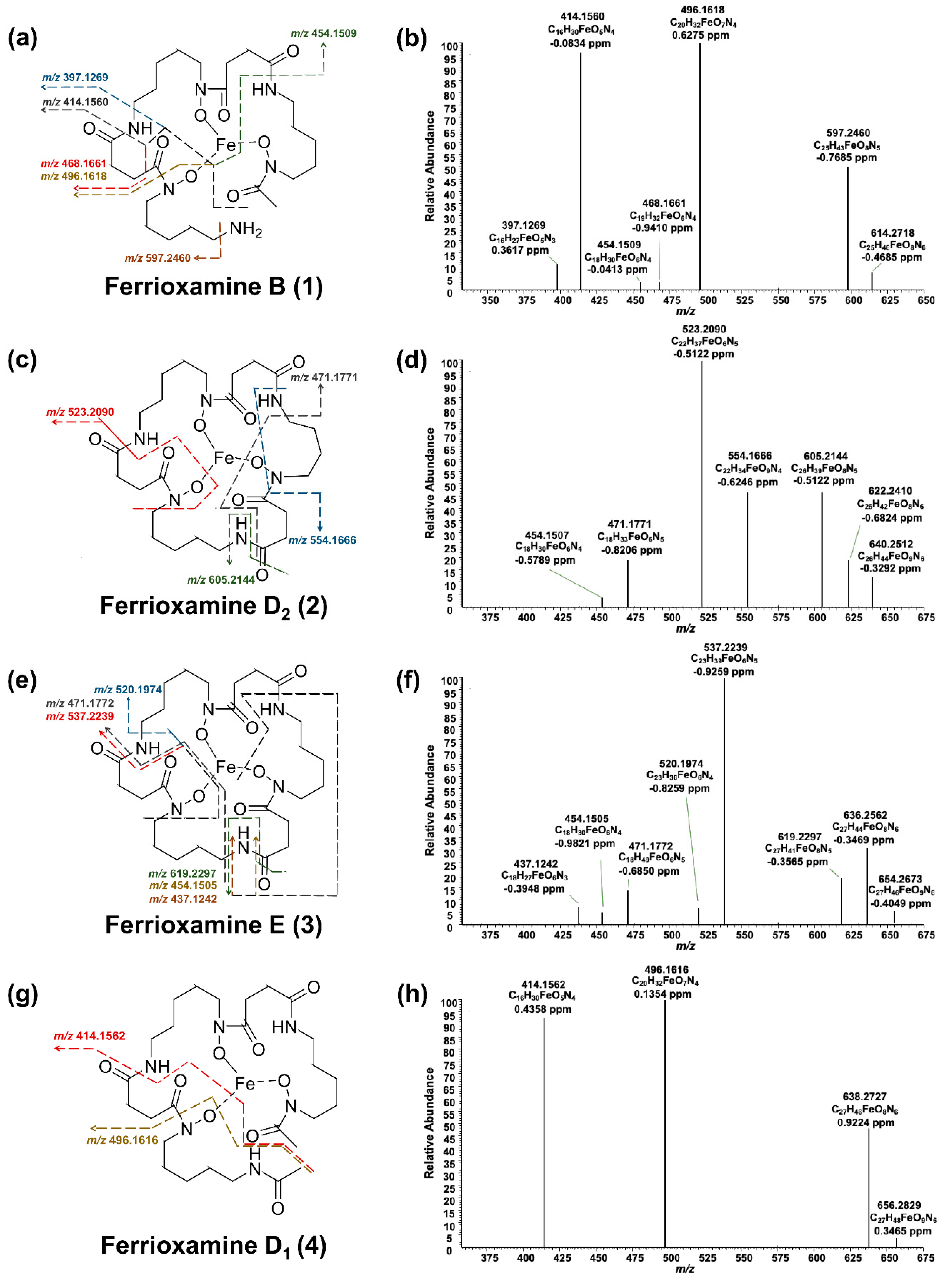
2.3. Production of Siderophores by S. ciscaucasicus GS2 in Axenic Culture and Structural Dereplication Using HPLC-HRMSn
2.4. Production of Siderophores by S. ciscaucasicus GS2 during Dual-Culture Microorganismal Interaction and Structural Dereplication Using HPLC-HRMSn
2.5. Trend and Dynamics of Siderophore Production by S. ciscaucasicus GS2 under the Influence of Chemical and Biological OSMAC Triggers
3. Discussion
3.1. Siderophore-Mediated Communication between Different Microbial Species Belonging to the Same Genus: Mutualistic Cooperation or Piracy?
3.2. Siderophore-Mediated Microbial Communication at the Root-Soil Interface
3.3. Siderophore-Mediated Microbial Contribution to Plant Immunity via Induced Systemic Resistance
4. Materials and Methods
4.1. Microorganisms Used in the Present Study
4.2. OSMAC-Assisted Cultivation and Fermentation
4.3. Extraction of Agar Plates
4.4. Extraction of Fermented Cultures (Broths)
4.5. High-Performance Liquid Chromatography-High Resolution Tandem Mass Spectrometry (HPLC-HRMSn)
4.6. Disk Diffusion Assays to Test Siderophore-Mediated Antagonism of S. pulveraceus Strain ES16
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhu, Y.; Fazio, G.; Mazzola, M. Elucidating the Molecular Responses of Apple Rootstock Resistant to ARD Pathogens: Challenges and Opportunities for Development of Genomics-Assisted Breeding Tools. Hortic. Res. 2014, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Manici, L.M.; Kelderer, M.; Franke-Whittle, I.; Rühmer, T.; Baab, G.; Nicoletti, F.; Caputo, F.; Topp, A.; Insam, H.; Naef, A. Relationship between Root-Endophytic Microbial Communities and Replant Disease in Specialized Apple Growing Areas in Europe. Appl. Soil Ecol. 2013, 72, 207–214. [Google Scholar] [CrossRef]
- Wang, G.; Yin, C.; Pan, F.; Wang, X.; Xiang, L.; Wang, Y.; Wang, J.; Tian, C.; Chen, J.; Mao, Z. Analysis of the Fungal Community in Apple Replanted Soil Around Bohai Gulf. Hortic. Plant J. 2018, 4, 175–181. [Google Scholar] [CrossRef]
- Isutsa, D.K.; Merwin, I.A. Malus Germplasm Varies in Resistance or Tolerance to Apple Replant Disease in a Mixture of New York Orchard Soils. HortScience 2000, 35, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Winkelmann, T.; Smalla, K.; Amelung, W.; Baab, G.; Grunewaldt-Stöcker, G.; Kanfra, X.; Meyhöfer, R.; Reim, S.; Schmitz, M.; Vetterlein, D.; et al. Apple Replant Disease: Causes and Mitigation Strategies. Curr. Issues Mol. Biol. 2019, 30, 89–106. [Google Scholar] [CrossRef] [Green Version]
- Reim, S.; Siewert, C.; Winkelmann, T.; Wöhner, T.; Hanke, M.-V.; Flachowsky, H. Evaluation of Malus Genetic Resources for Tolerance to Apple Replant Disease (ARD). Sci. Hortic. 2019, 256, 108517. [Google Scholar] [CrossRef]
- Mazzola, M.; Manici, L.M. Apple Replant Disease: Role of Microbial Ecology in Cause and Control. Annu. Rev. Phytopathol. 2012, 50, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Yim, B.; Winkelmann, T.; Ding, G.C.; Smalla, K. Different Bacterial Communities in Heat and Gamma Irradiation Treated Replant Disease Soils Revealed by 16S RRNA Gene Analysis—Contribution to Improved Aboveground Apple Plant Growth? Front. Microbiol. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunewaldt-Stöcker, G.; Mahnkopp-Dirks, F.; Popp, C.; Maiss, E.; Winkelmann, T. Diagnosis of Apple Replant Disease (ARD): Microscopic Evidence of Early Symptoms in Fine Roots of Different Apple Rootstock Genotypes. Sci. Hortic. 2019, 243, 583–594. [Google Scholar] [CrossRef]
- Yim, B.; Smalla, K.; Winkelmann, T. Evaluation of Apple Replant Problems Based on Different Soil Disinfection Treatments—Links to Soil Microbial Community Structure? Plant Soil 2013, 366, 617–631. [Google Scholar] [CrossRef]
- Weiß, S.; Bartsch, M.; Winkelmann, T. Transcriptomic Analysis of Molecular Responses in Malus domestica “M26” Roots Affected by Apple Replant Disease. Plant Mol. Biol. 2017, 94, 303–318. [Google Scholar] [CrossRef]
- Mahnkopp, F.; Simon, M.; Lehndorff, E.; Pätzold, S.; Wrede, A.; Winkelmann, T. Induction and Diagnosis of Apple Replant Disease (ARD): A Matter of Heterogeneous Soil Properties? Sci. Hortic. 2018, 241, 167–177. [Google Scholar] [CrossRef]
- Tewoldemedhin, Y.; Mazzola, M.; Mostert, L.; Mcleod, A. Cylindrocarpon Species Associated with Apple Tree Roots in South Africa and Their Quantification Using Real-Time PCR. Eur. J. Plant Pathol. 2011, 129, 637–651. [Google Scholar] [CrossRef]
- Brayford, D.; Honda, B.M.; Mantiri, F.R.; Samuels, G.J. Neonectria and Cylindrocarpon: The Nectria Mammoidea Group and Species Lacking Microconidia. Mycologia 2004, 96, 572–597. [Google Scholar] [CrossRef]
- Chaverri, P.; Salgado, C.; Hirooka, Y.; Rossman, A.Y.; Samuels, G.J. Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and Related Genera with Cylindrocarpon-like Anamorphs. Stud. Mycol. 2011, 68, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Lombard, L.; van der Merwe, N.; Groenewald, J.Z.; Crous, P. Lineages in Nectriaceae: Re-Evaluating the Generic Status of Ilyonectria and Allied Genera. Phytopathol. Mediterr. 2014, 53, 340–357. [Google Scholar] [CrossRef]
- Salgado-Salazar, C.; Rossman, A.Y.; Chaverri, P. The Genus Thelonectria (Nectriaceae, Hypocreales, Ascomycota) and Closely Related Species with Cylindrocarpon-like Asexual States. Fungal Divers. 2016, 80, 411–455. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Nouri, M.T.; Trouillas, F.P. Taxonomy and Multi-Locus Phylogeny of Cylindrocarpon-like Species Associated with Diseased Roots of Grapevine and Other Fruit and Nut Crops in California. Fungal Syst. Evol. 2019, 4, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Utkhede, R.S.; Vrain, T.C.; Yorston, J.M. Effects of Nematodes, Fungi and Bacteria on the Growth of Young Apple Trees Grown in Apple Replant Disease Soil. Plant Soil 1992, 139, 1–6. [Google Scholar] [CrossRef]
- Manici, L.M.; Kelderer, M.; Caputo, F.; Saccà, M.L.; Nicoletti, F.; Topp, A.R.; Mazzola, M. Involvement of Dactylonectria and Ilyonectria Spp. in Tree Decline Affecting Multi-Generation Apple Orchards. Plant Soil 2018, 425, 217–230. [Google Scholar] [CrossRef]
- Popp, C.; Wamhoff, D.; Winkelmann, T.; Maiss, E.; Grunewaldt-Stöcker, G. Molecular Identification of Nectriaceae in Infections of Apple Replant Disease Affected Roots Collected by Harris Uni-Core Punching or Laser Microdissection. J. Plant Dis. Prot. 2020, 127, 571–582. [Google Scholar] [CrossRef]
- Sánchez, J.; Iturralde, P.; Koch, A.; Tello, C.; Martinez, D.; Proaño, N.; Martínez, A.; Viera, W.; Ayala, L.; Francisco, F. Dactylonectria and Ilyonectria Species Causing Black Foot Disease of Andean Blackberry (Rubus Glaucus Benth) in Ecuador. Diversity 2019, 11, 218. [Google Scholar] [CrossRef] [Green Version]
- Farh, M.E.A.; Kim, Y.J.; Kim, Y.J.; Yang, D.C. Cylindrocarpon destructans/Ilyonectria radicicola-Species Complex: Causative Agent of Ginseng Root-Rot Disease and Rusty Symptoms. J. Ginseng Res. 2018, 42, 9–15. [Google Scholar] [CrossRef]
- Quaghebeur, K.; Coosemans, J.; Toppet, S.; Compernolle, F. Cannabiorci- and 8-Chlorocannabiorcichromenic Acid as Fungal Antagonists from Cylindrocarpon olidum. Phytochemistry 1994, 37, 159–161. [Google Scholar] [CrossRef]
- Rahman, M.; Punja, Z.K. Influence of Iron on Cylindrocarpon Root Rot Development on Ginseng. Phytopathology 2006, 96, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Mahnkopp-Dirks, F.; Radl, V.; Kublik, S.; Gschwendtner, S.; Schloter, M.; Winkelmann, T. Molecular Barcoding Reveals the Genus Streptomyces as Associated Root Endophytes of Apple (Malus domestica) Plants Grown in Soils Affected by Apple Replant Disease. Phytobiomes J. 2021. [Google Scholar] [CrossRef]
- Balbín-Suárez, A.; Lucas, M.; Vetterlein, D.; Sørensen, S.J.; Winkelmann, T.; Smalla, K.; Jacquiod, S. Exploring Microbial Determinants of Apple Replant Disease (ARD): A Microhabitat Approach under Split-Root Design. FEMS Microbiol. Ecol. 2020, 96, fiaa211. [Google Scholar] [CrossRef] [PubMed]
- Grunewaldt-Stöcker, G.; Popp, C.; Wamhoff, D.; Maiss, E.; Winkelmann, T. Microscopic Evidence of Nectriaceae and Other Microbes in Infected Fine Root Tissue of Replant Diseased Apple Plants. Eur. J. Hortic. Sci. 2021, 86, 29–40. [Google Scholar] [CrossRef]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big Effects from Small Changes: Possible Ways to Explore Nature’s Chemical Diversity. Chembiochem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Romano, S.; Jackson, S.A.; Patry, S.; Dobson, A.D.W. Extending the “One Strain Many Compounds” (OSMAC) Principle to Marine Microorganisms. Mar. Drugs 2018, 16, 244. [Google Scholar] [CrossRef] [Green Version]
- Pan, R.; Bai, X.; Chen, J.; Zhang, H.; Wang, H. Exploring Structural Diversity of Microbe Secondary Metabolites Using OSMAC Strategy: A Literature Review. Front. Microbiol. 2019, 10, 294. [Google Scholar] [CrossRef] [Green Version]
- Maghembe, R.; Damian, D.; Makaranga, A.; Nyandoro, S.S.; Lyantagaye, S.L.; Kusari, S.; Hatti-Kaul, R. Omics for Bioprospecting and Drug Discovery from Bacteria and Microalgae. Antibiotics 2020, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Mawji, E.; Gledhill, M.; Worsfold, P.J.; Achterberg, E.P. Collision-Induced Dissociation of Three Groups of Hydroxamate Siderophores: Ferrioxamines, Ferrichromes and Coprogens/Fusigens. Rapid Commun. Mass Spectrom. 2008, 22, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Senges, C.H.R.; Al-Dilaimi, A.; Marchbank, D.H.; Wibberg, D.; Winkler, A.; Haltli, B.; Nowrousian, M.; Kalinowski, J.; Kerr, R.G.; Bandow, J.E. The Secreted Metabolome of Streptomyces chartreusis and Implications for Bacterial Chemistry. Proc. Natl. Acad. Sci. USA 2018, 115, 2490–2495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, E.; Holmström, S.J.M. Siderophores in Environmental Research: Roles and Applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Aznar, A.; Chen, N.W.G.; Rigault, M.; Riache, N.; Joseph, D.; Desmaële, D.; Mouille, G.; Boutet, S.; Soubigou-Taconnat, L.; Renou, J.P.; et al. Scavenging Iron: A Novel Mechanism of Plant Immunity Activation by Microbial Siderophores. Plant Physiol. 2014, 164, 2167–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, A.S.; West, S.A.; Buckling, A. Cooperation and Competition in Pathogenic Bacteria. Nature 2004, 430, 1024–1027. [Google Scholar] [CrossRef]
- Cordero, O.X.; Ventouras, L.-A.; DeLong, E.F.; Polz, M.F. Public Good Dynamics Drive Evolution of Iron Acquisition Strategies in Natural Bacterioplankton Populations. Proc. Natl. Acad. Sci. USA 2012, 109, 20059–20064. [Google Scholar] [CrossRef] [Green Version]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial Siderophores in Community and Host Interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef]
- Krewulak, K.D.; Vogel, H.J. Structural Biology of Bacterial Iron Uptake. Biochim. Biophys. Acta 2008, 1778, 1781–1804. [Google Scholar] [CrossRef] [Green Version]
- Schalk, I.J.; Guillon, L. Fate of Ferrisiderophores after Import across Bacterial Outer Membranes: Different Iron Release Strategies Are Observed in the Cytoplasm or Periplasm Depending on the Siderophore Pathways. Amino Acids 2013, 44, 1267–1277. [Google Scholar] [CrossRef]
- Imbert, M.; Béchet, M.; Blondeau, R. Comparison of the Main Siderophores Produced by Some Species of Streptomyces. Curr. Microbiol. 1995, 31, 129–133. [Google Scholar] [CrossRef]
- Yamanaka, K.; Oikawa, H.; Ogawa, H.O.; Hosono, K.; Shinmachi, F.; Takano, H.; Sakuda, S.; Beppu, T.; Ueda, K. Desferrioxamine E Produced by Streptomyces griseus Stimulates Growth and Development of Streptomyces tanashiensis. Microbiology 2005, 151, 2899–2905. [Google Scholar] [CrossRef]
- Galet, J.; Deveau, A.; Hôtel, L.; Frey-Klett, P.; Leblond, P.; Aigle, B. Pseudomonas fluorescens Pirates Both Ferrioxamine and Ferricoelichelin Siderophores from Streptomyces ambofaciens. Appl. Environ. Microbiol. 2015, 81, 3132–3141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilston, E.L.; Deakin, G.; Bennett, J.; Passey, T.; Harrison, N.; O’Brien, F.; Fernández-Fernández, F.; Xu, X. Candidate Causal Organisms for Apple Replant Disease in the United Kingdom. Phytobiomes J. 2018, 2, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Crowley, D. Microbial Siderophores in the Plant Rhizosphere. In Iron Nutrition in Plants and Rhizospheric Microorganisms; Springer: Dordrecht, The Netherlands, 2006; pp. 169–198. ISBN 978-1-4020-4742-8. [Google Scholar]
- Jurkevitch, E.; Hadar, Y.; Chen, Y. The Remedy of Lime-induced Chlorosis in Peanuts by pseudomonas sp. Siderophores. J. Plant Nutr. 1986, 9, 535–545. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Yang, S.H.; Zhang, L.; Suh, J.W. Effects of Actinobacteria on Plant Disease Suppression and Growth Promotion. Appl. Microbiol. Biotechnol. 2013, 97, 9621–9636. [Google Scholar] [CrossRef]
- Mengel, K. Iron Availability in Plant Tissues-Iron Chlorosis on Calcareous Soils. Plant Soil 1994, 165, 275–283. [Google Scholar] [CrossRef]
- Masalha, J.; Kosegarten, H.; Elmaci, Ö.; Mengel, K. The Central Role of Microbial Activity for Iron Acquisition in Maize and Sunflower. Biol. Fertil. Soils 2000, 30, 433–439. [Google Scholar] [CrossRef]
- Kloepper, J.; Leong, J.; Teintze, M.; Schroth, M. Enhanced Plant Growth by Siderophores Produced by Plant Growth-Promoting Rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Ghazy, N.; El-Nahrawy, S. Siderophore Production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and Their Efficacy in Controlling Cephalosporium maydis in Maize Plant. Arch. Microbiol. 2021, 203, 1195–1209. [Google Scholar] [CrossRef]
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kieu, N.P.; Aznar, A.; Segond, D.; Rigault, M.; Simond-Côte, E.; Kunz, C.; Soulie, M.-C.; Expert, D.; Dellagi, A. Iron Deficiency Affects Plant Defence Responses and Confers Resistance to Dickeya dadantii and Botrytis cinerea. Mol. Plant Pathol. 2012, 13, 816–827. [Google Scholar] [CrossRef] [PubMed]
- López-Berges, M.S.; Turrà, D.; Capilla, J.; Schafferer, L.; Matthijs, S.; Jöchl, C.; Cornelis, P.; Guarro, J.; Haas, H.; Di Pietro, A. Iron Competition in Fungus-Plant Interactions: The Battle Takes Place in the Rhizosphere. Plant Signal. Behav. 2013, 8, e23012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [Green Version]
- Jankiewicz, U.; Kołtonowicz, M. The Involvement of Pseudomonas Bacteria in Induced Systemic Resistance in Plants. Appl. Biochem. Microbiol. 2012, 48, 276–281. [Google Scholar] [CrossRef]
- Buysens, S.; Heungens, K.; Poppe, J.; Hofte, M. Involvement of Pyochelin and Pyoverdin in Suppression of Pythium-Induced Damping-Off of Tomato by Pseudomonas aeruginosa 7NSK2. Appl. Environ. Microbiol. 1996, 62, 865–871. [Google Scholar] [CrossRef] [Green Version]
- Aznar, A.; Dellagi, A. New Insights into the Role of Siderophores as Triggers of Plant Immunity: What Can We Learn from Animals? J. Exp. Bot. 2015, 66, 3001–3010. [Google Scholar] [CrossRef] [Green Version]
- De Vleesschauwer, D.; Djavaheri, M.; Bakker, P.A.H.M.; Höfte, M. Pseudomonas fluorescens WCS374r-Induced Systemic Resistance in Rice against Magnaporthe Oryzae Is Based on Pseudobactin-Mediated Priming for a Salicylic Acid-Repressible Multifaceted Defense Response. Plant Physiol. 2008, 148, 1996–2012. [Google Scholar] [CrossRef] [Green Version]
- Viss, P.R.; Brooks, E.M.; Driver, J.A. A Simplified Method for the Control of Bacterial Contamination in Woody Plant Tissue Culture. Vitr. Cell. Dev. Biol. Plant 1991, 27, 42. [Google Scholar] [CrossRef]
- Quambusch, M.; Pirttilä, A.M.; Tejesvi, M.V.; Winkelmann, T.; Bartsch, M. Endophytic Bacteria in Plant Tissue Culture: Differences between Easy- and Difficult-to-Propagate Prunus avium Genotypes. Tree Physiol. 2014, 34, 524–533. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA Sequencing with Chain-Terminating Inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A Greedy Algorithm for Aligning DNA Sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Eckelmann, D.; Spiteller, M.; Kusari, S. Spatial-Temporal Profiling of Prodiginines and Serratamolides Produced by Endophytic Serratia marcescens Harbored in Maytenus serrata. Sci. Rep. 2018, 8, 5283. [Google Scholar] [CrossRef] [Green Version]
- Armin, R.; Zühlke, S.; Mahnkopp-Dirks, F.; Winkelmann, T.; Kusari, S. Evaluation of Apple Root-Associated Endophytic Streptomyces pulveraceus Strain ES16 by an OSMAC-Assisted Metabolomics Approach. Front. Sustain. Food Syst. 2021, 5, 60. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armin, R.; Zühlke, S.; Grunewaldt-Stöcker, G.; Mahnkopp-Dirks, F.; Kusari, S. Production of Siderophores by an Apple Root-Associated Streptomyces ciscaucasicus Strain GS2 Using Chemical and Biological OSMAC Approaches. Molecules 2021, 26, 3517. https://doi.org/10.3390/molecules26123517
Armin R, Zühlke S, Grunewaldt-Stöcker G, Mahnkopp-Dirks F, Kusari S. Production of Siderophores by an Apple Root-Associated Streptomyces ciscaucasicus Strain GS2 Using Chemical and Biological OSMAC Approaches. Molecules. 2021; 26(12):3517. https://doi.org/10.3390/molecules26123517
Chicago/Turabian StyleArmin, Reyhaneh, Sebastian Zühlke, Gisela Grunewaldt-Stöcker, Felix Mahnkopp-Dirks, and Souvik Kusari. 2021. "Production of Siderophores by an Apple Root-Associated Streptomyces ciscaucasicus Strain GS2 Using Chemical and Biological OSMAC Approaches" Molecules 26, no. 12: 3517. https://doi.org/10.3390/molecules26123517
APA StyleArmin, R., Zühlke, S., Grunewaldt-Stöcker, G., Mahnkopp-Dirks, F., & Kusari, S. (2021). Production of Siderophores by an Apple Root-Associated Streptomyces ciscaucasicus Strain GS2 Using Chemical and Biological OSMAC Approaches. Molecules, 26(12), 3517. https://doi.org/10.3390/molecules26123517






