2.1. H/D Isotopic Exchange on Ring-A Protons at Positions C(6) and C(8) of Flavanols
Although the solubility in water of simple flavanols, such as catechin (
1) or epicatechin (
2) is low (≈10 mM), aqueous homogeneous solutions can be prepared and investigated through NMR without any apparent phase separation along several days. When they are dissolved in deuterated water (D
2O), all phenolic O-H protons are quickly exchanged by deuterium ions leading to
4a or
5a O-D labelled substrates (
Scheme 2). At the concentrations of the homogenous solutions of
1 and
2 achievable in D
2O (≈10 mM) the pH was measured to be 6.9 (pD = 7.3) using
1H-NMR signals of imidazole as pH indicator [
23]. We found that catechin
4a undergoes a slow process of deuterium incorporation leading to the fully deuterated ring A species
4d through the partially deuterated intermediates
4b-4c. Similarly, the O-D labelled epicatechin species
5a in D
2O is slowly converted into
5d, through the intermediates
5b-
5c.
Although the standard Gibbs free energy difference (∆G
0) of the overall equilibrium process (1):
is expected to be about zero due to self-compensating enthalpic and entropic effects, the equilibrium position is completely shifted towards the products since D
2O is present in the reacting system in a much larger molar excess than
4a, thus leading to complete irreversible conversion of
4a into
4d. Since the free energy of activation (∆G
‡) of the process (1) is not so high as it would be expected for any C-H/C-D exchange, its rate can be evaluated at r.t. (or within a small T range around it) by following the time (t) and temperature (T) dependence of the
1H-NMR signals of ring A aromatic protons.
In fact, at T = 310 K,
4a was observed to undergo in a few hours a significant D-labelling at C(6) and C(8) as highlighted in
Figure 1a (blue dotted boxes) by the significant decrease of the
1H-NMR signals H-C(6) (δ
H6 = 6.13) and H-C(8) (δ
H8 = 6.05). The same behaviour was shown (
Figure 1b, blue dotted box) by the corresponding signals of epicatechin (
5a) (δ
H6 = 6.15 and δ
H8 = 6.13).
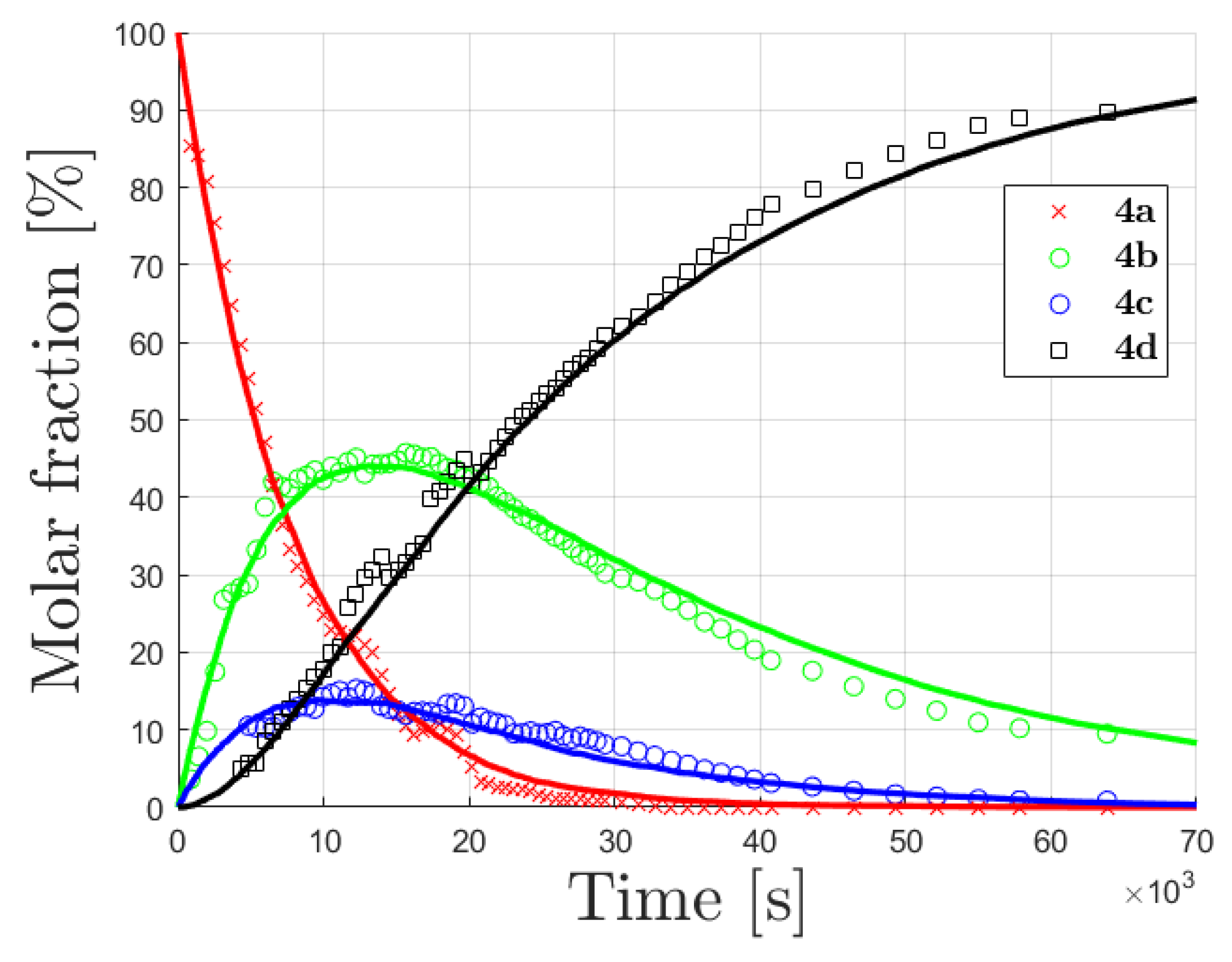
For the overall process
4a →
4d (
Figure 2a) the time-varying amount of (
4a +
4b) and (
4a +
4c) were obtained from the NMR area integration of the signals H-C(6) and H-C(8), respectively, and were normalized to the unperturbed area of the ring B protons.
By integrating the area of these signals, we evaluated not only the time dependence of the disappearance of 4a but also of the appearance/disappearance of the monodeuterated D8 (4b) and the monodeuterated D6 intermediates (4c).
In fact, at a given time of any kinetics run (
Figure 3, t = 500 min, T = 305 K), the peak deconvolution of the signal of H-C(6) at δ
H = 6.07 allows establishing the relative contributions of the residual H-C(6) of
4a (doublet,
J = 2.1 Hz) and a new singlet signal (centred at almost the same frequency as the former) attributable to D-8 monodeuterated species
4b.
By normalizing to the initial concentration of 4a, the % molar fraction of these two species (x4a + x4b) was evaluated to be 60% and peak deconvolution leads to x4a ≈ 2x4b.
Correspondingly, the peak deconvolution of the signal of H-C(8) at δH = 5.98 allows establishing the relative contribution of the residual H-C(8) of 4a (doublet, J = 2.1 Hz) and a new singlet signal attributable to 4c. The % molar fraction of these two species (x4a + x4c = 52%) can be obtained by the relative area integration, whilst the peak deconvolution leads to x4a ≈ 4x4c.
To fulfil the mass balance constrain, the overall normalized % distribution was evaluated to be x
4a:x
4b:x
4c:x
4d = 42:20:1:28, indicating different specific rate constants (i.e., regioselectivity) at the two different sites with a faster H/D exchange at C(8) than at C(6). In principle, the relative amount of
4a–
4d species can be evaluated at any time and any fixed temperature during the kinetic run. Actually, several
1H-NMR spectra taken in kinetic runs carried out at higher temperature do not have sufficient resolution to allow an adequate deconvolution of H(6) and H(8) signals, but this outcome did not hinder our analysis so much. The peak deconvolution of the H-C(6) and H-C(8) signals allowed us to separate the time-dependent contribution of the intermediates
4b and
4c, reaching maximum values of x
4b ≈ 0.20 and x
4c ≈ 0.10 at about the same time (
Figure 4a t
max ≈ 500 min). More importantly, a single exponential fitting (
Figure 4) of time, the varying x
4a (t), was obtained with an observed rate constant (T = 305 K) k
obs (
4a) = 3.90 × 10
−5 s
−1 (t
½ (
4a) = 17,800 s) suggesting that all the processes contributing to the disappearance of
4a are (pseudo) first-order processes, only depending on the initial concentration of
4a (
Figure 4b).
Similar data were also obtained for the
5a →
5d process. However, deuteration of aromatic protons of
5a in D
2O (
Figure 2b) does not show any site selectivity. In fact, the overall rate of disappearance of the H-6 signals (
5a +
5b) was almost the same as that of the H-8 signals (
5a +
5c). In this case, we were not able to evaluate at a given time point the relative contribution of monodeuterated
5b and
5c by peak deconvolution since from one side the difference of chemical shift between H-C(6) and H-C(8) in epicatechin is so small to become comparable to the
J(6,8) coupling constant (leading to non-first order NMR signals) and, from the other side, the signal lineshape was not good enough to allow reliable fittings.
Initially, we thought that the kinetic behaviour showed by deuteration of
4a could be rationalized by assuming two competitive pseudo-first-order conversions starting from the same substrate (
4a →
4b in competition with
4a →
4c) followed by two convergent pseudo-first-order processes leading to the same end product (
4b →
4d in addition to
4c →
4d). To evaluate the four unknown rate constants (k
1, k
2, k
3, k
4), we resorted to the Kinetiscope software using, as the initial guess for the fitting, the values roughly estimated from our raw kinetic data shown in
Figure 2. However, we soon realized that it was almost impossible to find a set of four k
i able to adequately fulfil our experimental x
i(t) data. No set of four k
i was able to fit the general trend of these curves and to be in agreement with several significant constraints imposed by experimental measures, such as the time of the crossing points of the curve x
4d(t) with that of x
4a(t) (26,800 s) and/or the time of the crossing point of the curve of x
4b(t) with those of x
4a(t) (73,000 s), x
4b(t) (42,200 s), and x
4c(t) (≈80,000 s). To find an optimal fitting of our data, another process needed to come into play, i.e., a process able to convert
4a into
4d without any detectable intermediate. Thus, we were somehow forced by experimental findings to suppose the kinetic pathway drawn in
Scheme 3, based on competitive-consecutive pseudo-first-order processes. The time dependence of all the stable species is regulated by a system of linear differential equations (
Scheme 3, top) that, with the appropriate boundary conditions and the mass balance constrain, lead to the integrated solution x
i(t) here reported (
Scheme 3, bottom).
The fitting of these integrated equations with the stochastic simulator Kinetiscope leads to the following best fitting values at T = 305 K: k
1 = 1.7 × 10
−5 s
−1, k
2 = 0.85 × 10
−5 s
−1, k
3 = 1.35 × 10
−5 s
−1, k
4 = 2.5 × 10
−5 s
−1, and k
5 = 2.4 × 10
−5 s
−1, fulfilling the condition k
obs = (k
1 + k
2+ k
3) = 3.90 × 10
−5 s
−1. By introducing these values into integrated equations, we found that the calculated curves were almost superimposable to our experimental kinetic data (
Figure 4b).
Since, in principle, the molar fraction of every species can be evaluated at every time (and temperature), we carried out a detailed kinetic analysis following the time dependence of the NMR-sensitive species
4a/
4b/
4c performing
1H-NMR measurements at five different temperatures (300–320 K). Unfortunately, the peak deconvolution of the H-C(6) and H-C(8) signals was possible only at 300 (
Figure 4a) and 305 K (
Figure 4b) since at higher temperatures the peak resolution of these signals was not sufficient to allow reproducible deconvolutions.
Thus, the kinetic parameters reported in
Table 1 have just an empirical significance, representing the observed time dependence (k’
obs) of the peak area of the H-C(6) (
Figure 5a) and H-C(8) (
Figure 5b) at fixed temperatures and not the true rate constants controlling the time changes of x
4a(t). In other words, k’
obs (and the corresponding activation energies) are not simply related to the true values of the three processes, whereby
4a is converted to the intermediates
4b and
4c and/or to the final product
4d. Anyhow, these empirical data are quite useful since they allow us to estimate how and how much the average site selectivity of deuteration depends on temperature. If we define the ratio (k’
(C6) − k’
(C8))/(k’
(C6) + k’
(C8)) at a given temperature as an empirical descriptor of this selectivity, we can notice that it significantly decreases by increasing the temperature, ranging from ≈0.2 (T = 300 K) until ≈0.1 (T = 320 K). This is largely expected since increasing T, the differential exponential factors regulating the rate constants ΔΔG
‡/RT (ΔΔG
‡ = ΔG
‡(C6) − ΔG
‡(C8)) must decrease.
It is worth noting that the average ΔG‡ ≈ 100 kJmol−1 of the two competitive processes is quite similar and dominated by the contribution of the enthalpic term (75–80%). As already pointed out, however, these values simply represent the time dependence of the deuterium ion attack both to 4a and 4b for the H/D exchange at C(8) and both to 4a and 4c for the H/D exchange at C(6). While in the early part the contributions of 4b and 4c can be neglected, this is not true in the middle or late steps of these kinetic runs.
A deeper insight into the mechanistic details of the pathway proposed in
Scheme 3 can be achieved by considering the kinetic data for all the elementary steps obtained at two different temperatures, 300 and 305 K. For the former, the fitting of the curve x
4a (t) leads to k
obs (
4a, T = 300 K) = 2.71 × 10
−5 s
−1 (t
½ (
4a) = 25,000 s), with a 30% decrease with respect to the value evaluated at T = 305 K. The simulation of all the experimental evaluated x
i(t) affords the following best fitting values at 300 K: k
1 = 1.5 × 10
−5 s
−1, k
2 = 0.75 × 10
−5 s
−1, k
3 = 0.45 × 10
−5 s
−1, k
4 = 2.2 × 10
−5 s
−1, and k
5 = 2.2 × 10
−5 s
−1 (
Figure 4a).
Thus, k3 decreases with the decrease of temperature significantly more than the other four rate constants pointing out that 4a undergoes a different molecular rearrangement before leading the end-product 4d. By inserting these ki values into the Eyring equation we obtained the corresponding ΔG‡, although we cannot estimate the relative contribution of enthalpic and entropic activation terms.
Another interesting feature is the pH-dependence of these rate constants. By lowering the pH (addition of CD
3COOD) in the
4a/D
2O reacting system, we observed as expected that the rate of the deuteration process increases. Thus, whilst the half-lifetime of
4a at 300 K in neutral solution was found at about 25 min, it decreased at about 6 min at pH 3.0. However, we noticed that k
3 became significantly lower than observed in neutral pH conditions. At pH 3.0 (300 K), this effect is largely offset (
Figure 6) by the increase of k
1 and k
2 leading to an observed rate constant significantly larger than in neutral conditions. Another striking feature, related to the pH-induced changes of these rate constants, is represented by the significant increase of the maximum of the curves x
4b(t) and x
4c(t), speaking for a longer lifetime of the key-intermediates
4b and
4c.
To summarize, our explanation relies on the evidence that D2O is strong enough as an acid to promote deuteration by the D+ transfer to C(8) and strong enough as a base to accept the D+ ion from DO-C(7). The effect of acidic catalyst seem to rely on (i) a relevant increase of the rate of the competitive processes 4a → 4b and 4a → 4c leading to higher relative amounts of the monodeuterated intermediates 4b and 4c, (ii) a large decrease of the rate of the process 4a → 4d, and iii) an almost unperturbed site selectivity of deuterium ion attack at C(6) or C(8).
Finally, we carried out kinetic runs at 300 K on 4a using CD3OD in place of D2O as a deuterium ion source for the H/D exchange. The observed rate constant controlling the disappearance of 4a/CD3OD was found much lower than that of 4a/D2O.
2.2. Mechanistic Considerations
In a Frontier Molecular Orbital approach (FMO), the mechanism of the rate-determining steps can be considered as the addition of an electrophilic species (D
3O
+/D
2O) to the highest nucleophilic centres of flavanols, i.e., C(6) or C(8) which are electrons enriched by the multiple
ortho and
para mesomeric effect of the oxygen atoms on ring A. This attack will occur at the nucleophilic site having the largest electron density. According to
1H and
13C-NMR experimental spectra of
4a, no matter the solvent, both H-C(8) and C(8) are slightly shielded with respect to H-C(6) and C(6) confirming their higher nucleophilicity (
Figure 1a). Similar data were also obtained for the
5a →
5d process (
Figure 1b).
The main outcomes of our kinetic data on deuteration of simple flavanols such as 4a and 5a in D2O are here summarized: (i) In a first preliminary step, all O-H bonds are deuterium ion exchanged through a very fast process; (ii) at r.t., flavanols undergo slow H/D exchange at C(6) and C(8) or at both sites; (iii) the first step of deuteration occurs at a slightly higher specific rate at C(8) than at C(6); (iv) the second step of deuteration occurs at both sites with quite similar specific rates (k3 ≈ k4), but slightly faster than the first step; (v) the average ΔG‡ (≈102 kJmol−1) of all these deuteration processes is mainly determined by enthalpic effects; (vi) in other protic, but less polar, solvents (such as CD3OD), these processes occur on a significantly longer time-scale.
With this kinetic information, we can propose a reasonable reaction mechanism (
Scheme 4). It relies on sequential elementary steps, involving a slow, solvent-mediated, C-D bond formation/O-D bond-breaking followed by a fast, solvent-mediated, C-H bond breaking/O-D bond formation where ring-A can regain its aromaticity. Although our observed rate constants (k
i, i = 1, 2, 3, 4, 5) and the corresponding ΔG
‡ values are not related to true elementary reaction steps (i.e., involving single transition states), they can be considered a simple sequence of elementary steps with one of them as the rate-determining step. Assuming the well-known and largely accepted mechanism involving keto-enol tautomerism of 1,3,5 activated aromatic systems [
24], we propose that deuterated keto-analogues (
4ab and
4ac) could be the putative transient intermediates leading to the detectable intermediates
4b (k
1) and
4c (k
2) in the two competitive divergent processes
4a →
4b and
4a →
4c, respectively. Similarly,
4bd and
4cd could be the transient putative intermediates of the two convergent pathways,
4b →
4d regulated by k
4 and
4c →
4d regulated by k
5 leading to the end-product
4d. However, as already discussed, our data suggest a relevant role played by the direct process
4a →
4d, implying the putative intermediate 5,7-diketo derivative
4ad.
Although we can only speculate about the structures of the transition states leading to the above-cited putative intermediates, since the elementary steps dealing with C-D formation/O-D breaking and loss of aromaticity are highly endothermic, they are also expected to be the rate-limiting steps and the structure/energy of the relevant TS similar to the structure/energy of the putative intermediates.
The plausibility of the termolecular elementary process
4a →
4d relies on the need to introduce another step converting
4a into
4d in addition to the bimolecular elementary steps of mono-deuteration. Practically speaking, this termolecular process is feasible due to the interaction between three molecules (
4a + 2D
2O) and could be energetically comparable (if not favourable) over the sequential competing bimolecular mono-deuteration processes [
22], leading to lower enthalpic contribution (ΔH
‡) of the step
4a →
4ad (assuming within the Hammond postulate that the corresponding transition state has a structure similar to
4ad), only partially counterbalanced by a greater entropic term contribution (−TΔS
‡), in agreement with the lower probability to arrange two D
2O molecules in the correct positions for the H/D exchange event.
The relevant outcome that deuteration processes of
4a occur much more slowly in CD
3OD than in D
2O [
25] can be explained in our view by the proton/deuterium acid-base properties of flavanols in methanol (pK
a ≈ 13–14) with respect to water (pK
a ≈ 9–10) [
26]. The lower acidity of the O-H(D) groups in methanol reflects the reduced thermodynamic ability of methanol to solvate the ions produced by the proton-transfer reaction. However, since the loss of proton/deuterium ions is also involved in all the transition states leading to the putative intermediates, we believe that it can play a relevant role also in the kinetics.
2.4. Results from ab Initio DFT Calculations
To get further molecular details on our kinetic investigations, we resorted to density functional theory (DFT) calculations through codes implemented in the Gaussian 16 software [
27]. To arrive at the energy minima of all the structures of flavanols here reported, a full conformational search with the aid of the GMMX add-on (GaussView program) was undertaken. Conformational searching was carried out, allowing both ring C flipping and single-bond rotations through the use of the default values of the program. The MM-minimized rotamers were used as initial input structures, and the geometry was energy-minimized using the DFT/B3LYP 6–311G level of theory [
28].
Regarding the catechin system, calculations carried out at the B3LYP/6-311G level of theory confirmed the higher Mulliken negative charge at C(8) (−0.20) with respect to that at C(6) (−0.17). Then, ab initio calculations (geometry optimization followed by frequency calculations) were used to estimate the relative energies of all the species participating in this pathway. A set of simulations were also performed to optimize structures and energies of the corresponding transition states. The calculated energy profile is summarized in
Figure 8.
Energies of the mono- and di-deuterated keto forms
4ab,
4ac,
4ad,
4bd, and
4cd were evaluated by QM calculations to be significantly higher than the corresponding mono- and di-deuterated enol forms
4a,
4b,
4c, and
4d. Importantly, the intermediate
4ad was found more stable (by 16 kJmol
−1, even after corrections of their different thermal contributions) than the competitive intermediates
4ab and
4ac, thus giving further support to its existence along the pathway of the overall exchange process, as already guessed by the overall fitting of our kinetic data. The transition states (
4ab‡,
4ac‡,
4ad‡) of the three competitive deuteration processes were found about 130 kJmol
−1 above the energy of reactant
4a. All the structures associated to
4ab‡,
4ac‡,
4ad‡ species showed only one imaginary frequency thus ensuring we were dealing with true transition states. Interestingly, the calculations were also in agreement with the experimental evidence that specific rates of deuteration are lower in deuterated methanol than in deuterated water. In fact, the energy of the
4ab‡ analogue obtained by deuterium ion transfer from CD
3OD was found about 35 kJmol
−1 higher than that of
4ab‡ itself. The intrinsic reaction coordinate (IRC) calculation performed on
4ab† (
Supplementary Figure S1) afforded reactant
4a and product
4b showing that during the process there is a synchronous breaking of the bond D-OC(7) and formation of the bond D-C(8).
Even the bond length O-C(7) is a fine reaction coordinate since, as expected, it undergoes a significant shortening ongoing from the enol form 4a to the keto form 4b.
The calculated rate constants are of course largely lower than the experimental ones (10
−10 s
−1 versus 10
−5 s
−1) since the calculated free energies of activation are about 30 kJ/mol higher than those experimentally measured. However, we are confident that, by putting two and not only one D
2O molecules in the reactive site of these TS, their energy would become significantly lower due to the well-documented assistance [
29] of the 2nd D
2O molecule enhancing the deuterium ion donor and deuterium ion acceptor capability of the first reacting D
2O molecule. Basically, in our approach, the energy profile reported in
Figure 8 is aimed to just provide an approximate agreement (in terms of minimum energy of all species playing a role in the process, such as reactants, products, putative, and isolated intermediates) with our experimental findings. Thus, activation energies of processes
4a →
4b and
4a →
4c were calculated to be almost the same (in fact k
1 ≈ k
2), as well as the processes
4a →
4b and/or
4c →
4d were also calculated to have quite similar activation energies as process
4a →
4d, (in fact k
3 ≈ k
1, k
2, k
4, and k
5).