Tailoring the Thermal Conductivity of Rubber Nanocomposites by Inorganic Systems: Opportunities and Challenges for Their Application in Tires Formulation
Abstract
1. Introduction
2. Thermal Transport in Polymer Composites: Basic Principles and Parameters Affecting the Thermal Conductivity
3. Filler Systems for Improving the Thermal Conductivity of Rubber Nanocomposites: The Role of Inorganic Nanomaterials
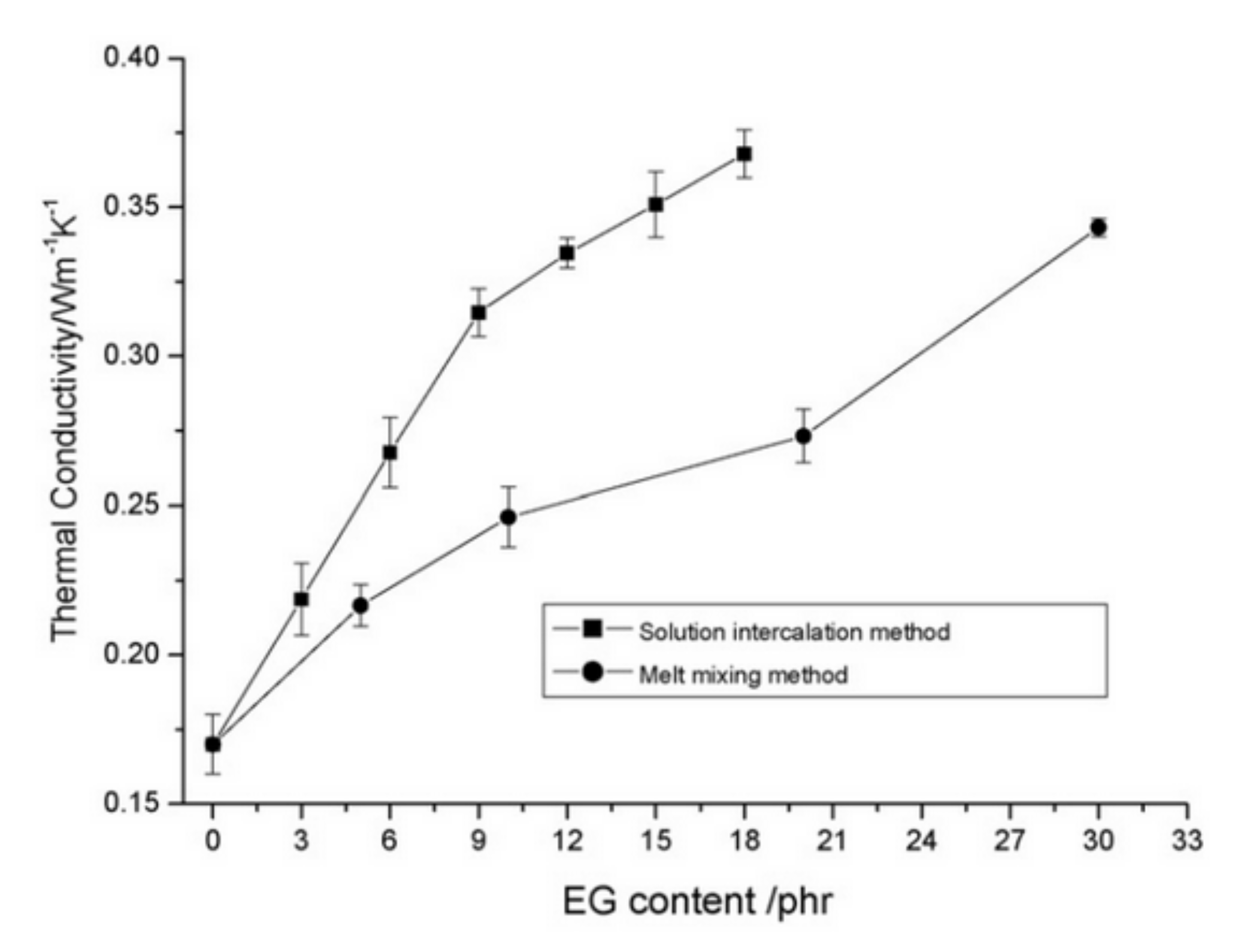
3.1. Carbon-Based Fillers
3.2. Inorganic Filler Systems
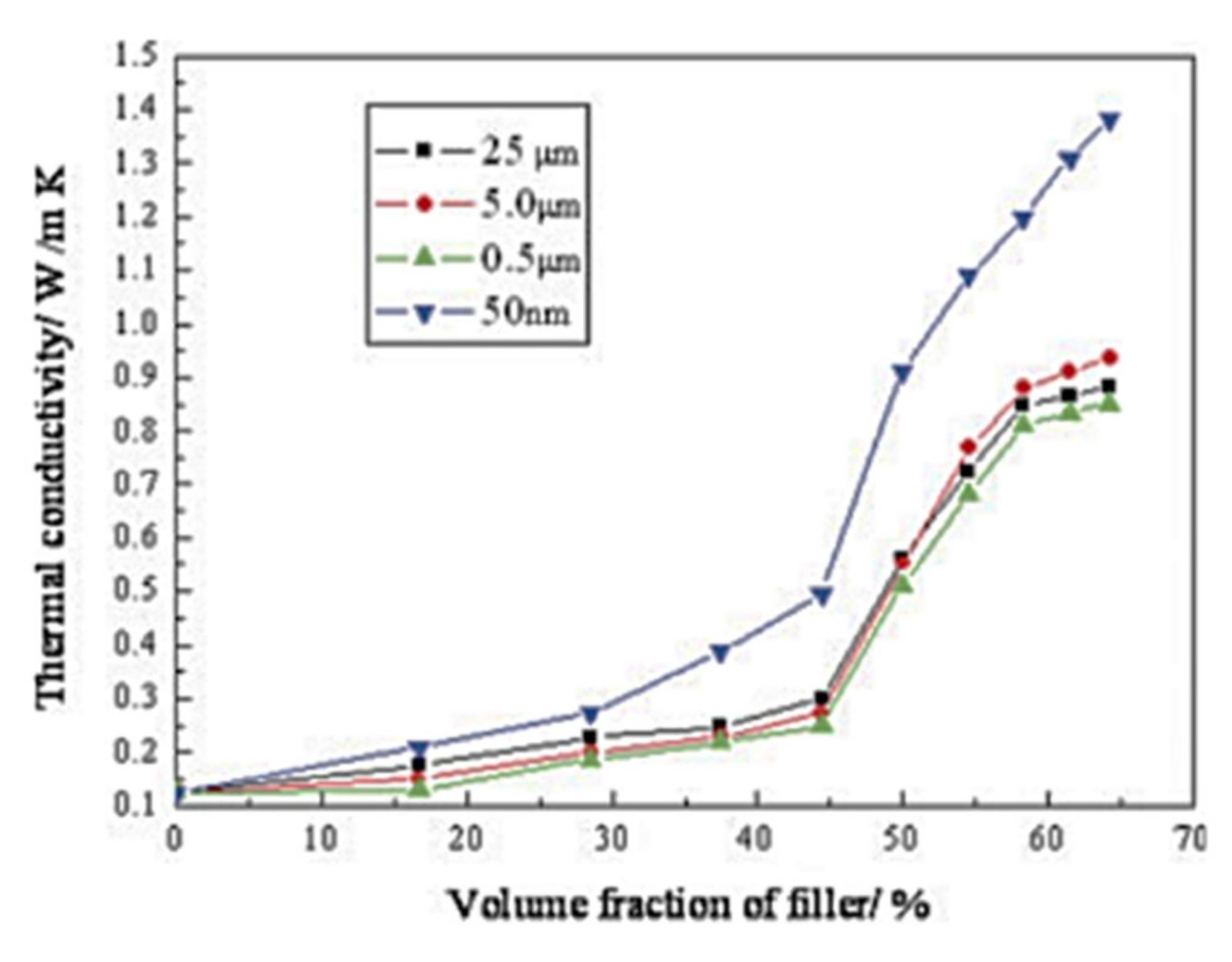
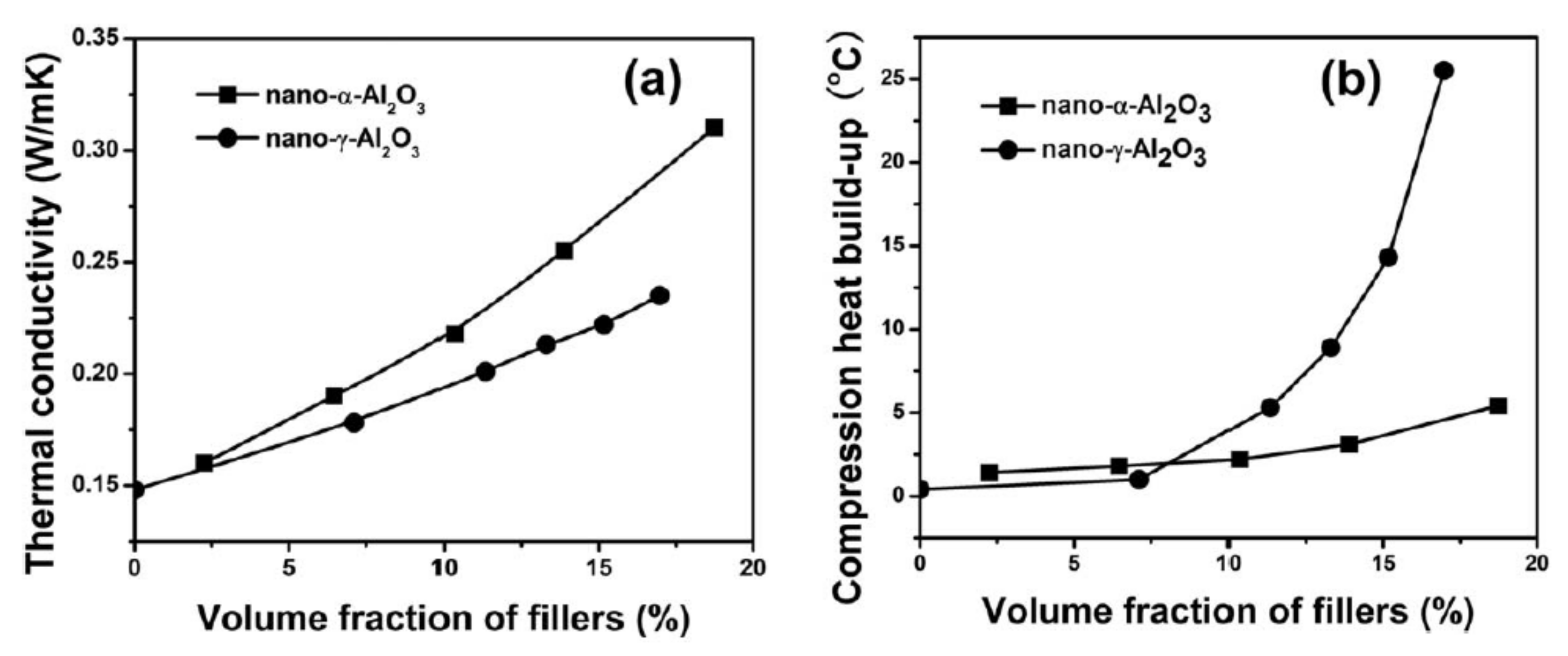
3.2.1. Alumina
3.2.2. Boron Nitride
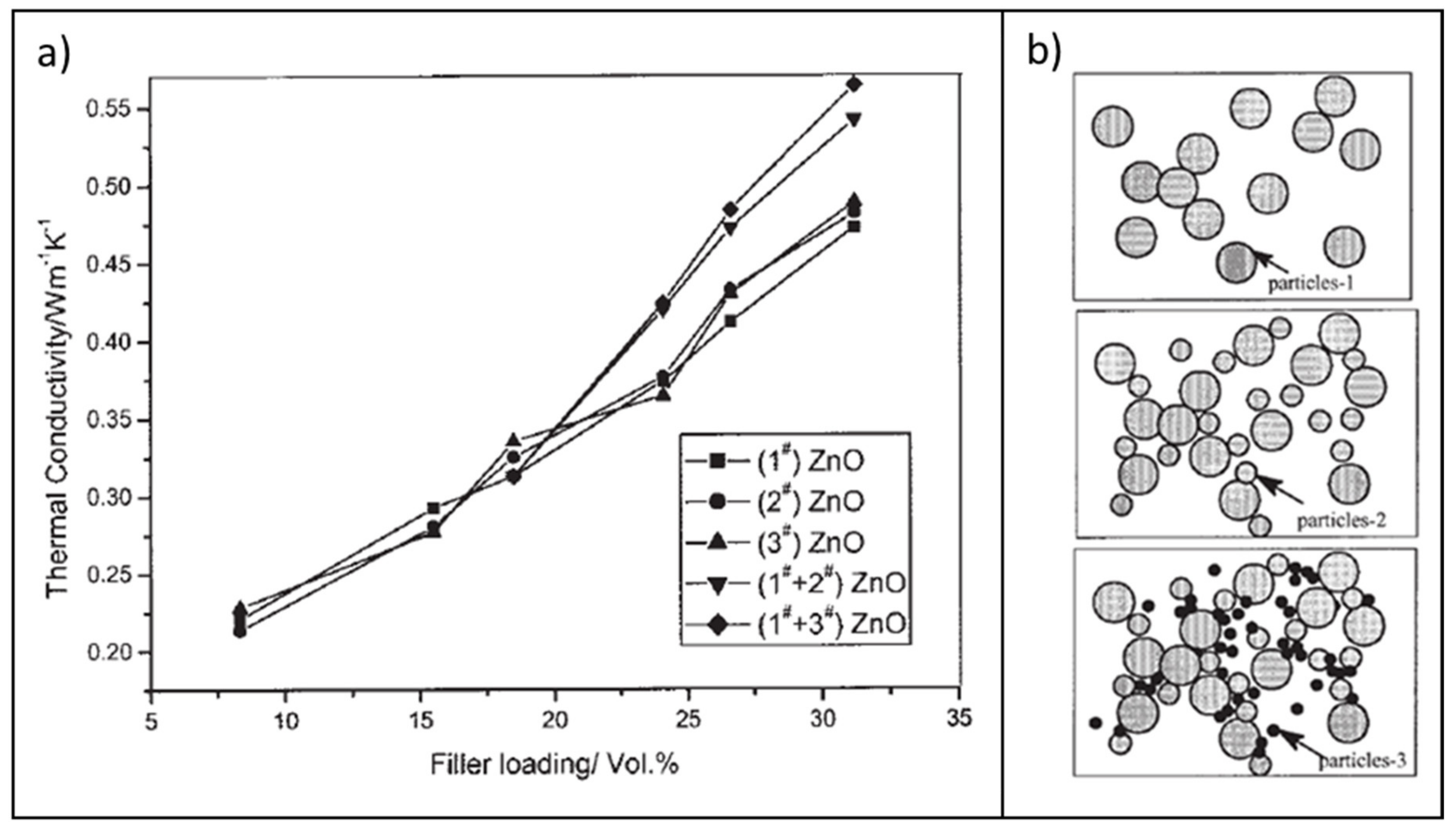
3.2.3. Zinc Oxide
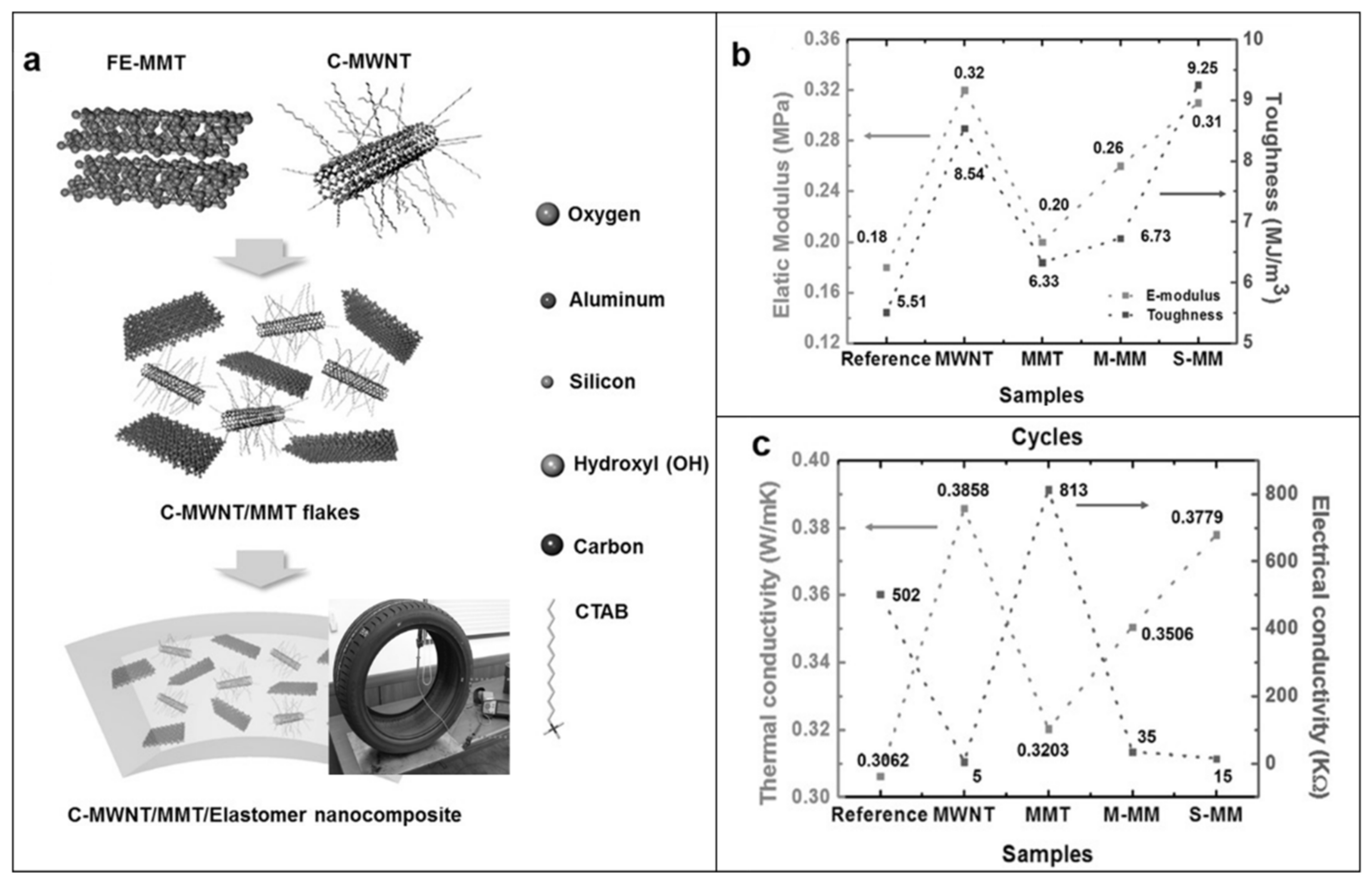
4. Hybrid Fillers: A New Frontier for the Thermal Management of Rubber Nanocomposites
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Idumah, C.I.; Hassan, A. Recently emerging trends in thermal conductivity of polymer nanocomposites. Rev. Chem. Eng. 2016, 32, 413–457. [Google Scholar] [CrossRef]
- Moore, A.L.; Shi, L. Emerging challenges and materials for thermal management of electronics. Mater. Today 2014, 17, 163–174. [Google Scholar] [CrossRef]
- Xu, Y.; Kraemer, D.; Song, B.; Jiang, Z.; Zhou, J.; Loomis, J.; Wang, J.; Li, M.; Ghasemi, H.; Huang, X.; et al. Nanostructured polymer films with metal-like thermal conductivity. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Dai, W.; Ma, T.; Yan, Q.; Gao, J.; Tan, X.; Lv, L.; Hou, H.; Wei, Q.; Yu, J.; Wu, J.; et al. Metal-Level Thermally Conductive yet Soft Graphene Thermal Interface Materials. ACS Nano 2019, 13, 11561–11571. [Google Scholar] [CrossRef]
- Suh, D.; Moon, C.M.; Kim, D.; Baik, S. Ultrahigh Thermal Conductivity of Interface Materials by Silver-Functionalized Carbon Nanotube Phonon Conduits. Adv. Mater. 2016, 28, 7220–7227. [Google Scholar] [CrossRef]
- Tong, X.C. Advanced Materials for Thermal Management of Electronic Packaging; Springer Series in Advanced Microelectronics; Springer New York: New York, NY, USA, 2011; Volume 30, ISBN 978-1-4419-7758-8. [Google Scholar]
- Li, N.; Ren, J.; Wang, L.; Zhang, G.; Hänggi, P.; Li, B. Colloquium: Phononics: Manipulating heat flow with electronic analogs and beyond. Rev. Mod. Phys. 2012, 84, 1045–1066. [Google Scholar] [CrossRef]
- Xu, X.; Chen, J.; Zhou, J.; Li, B. Thermal Conductivity of Polymers and Their Nanocomposites. Adv. Mater. 2018, 30, 1705544. [Google Scholar] [CrossRef]
- Yang, X.; Liang, C.; Ma, T.; Guo, Y.; Kong, J.; Gu, J.; Chen, M.; Zhu, J. A review on thermally conductive polymeric composites: Classification, measurement, model and equations, mechanism and fabrication methods. Adv. Compos. Hybrid. Mater. 2018, 1, 207–230. [Google Scholar] [CrossRef]
- Leung, S.N. Thermally conductive polymer composites and nanocomposites: Processing-structure-property relationships. Compos. Part B Eng. 2018, 150, 78–92. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Ngo, I.L.; Jeon, S.; Byon, C. Thermal conductivity of transparent and flexible polymers containing fillers: A literature review. Int. J. Heat Mass Transf. 2016, 98, 219–226. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, H.; Fu, Q. Recent progress on thermal conductive and electrical insulating polymer composites. Compos. Commun. 2018, 8, 74–82. [Google Scholar] [CrossRef]
- Guo, Y.; Ruan, K.; Shi, X.; Yang, X.; Gu, J. Factors affecting thermal conductivities of the polymers and polymer composites: A review. Compos. Sci. Technol. 2020, 193, 108134. [Google Scholar] [CrossRef]
- Ngo, I.L.; Byon, C. A generalized correlation for predicting the thermal conductivity of composites with heterogeneous nanofillers. Int. J. Heat Mass Transf. 2015, 90, 894–899. [Google Scholar] [CrossRef]
- Huang, C.; Qian, X.; Yang, R. Thermal conductivity of polymers and polymer nanocomposites. Mater. Sci. Eng. R Rep. 2018, 132, 1–22. [Google Scholar] [CrossRef]
- Hansson, J.; Nilsson, T.M.J.; Ye, L.; Liu, J. Novel nanostructured thermal interface materials: A review. Int. Mater. Rev. 2018, 63, 22–45. [Google Scholar] [CrossRef]
- He, X.; Wang, Y. Recent advances in the rational design of thermal conductive polymer composites. Ind. Eng. Chem. Res. 2021, 60, 1137–1154. [Google Scholar] [CrossRef]
- Cui, Y.; Li, M.; Hu, Y. Emerging interface materials for electronics thermal management: Experiments, modeling, and new opportunities. J. Mater. Chem. C 2020, 8, 10568–10586. [Google Scholar] [CrossRef]
- Burger, N.; Laachachi, A.; Ferriol, M.; Lutz, M.; Toniazzo, V.; Ruch, D. Review of thermal conductivity in composites: Mechanisms, parameters and theory. Prog. Polym. Sci. 2016, 61, 1–28. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Mingyuan, G.U.; Liu, X. Study of the thermal conduction mechanism of nano-SiC/DGEBA/EMI-2,4 composites. Polymer 2008, 49, 4666–4672. [Google Scholar] [CrossRef]
- Shen, D.; Zhan, Z.; Liu, Z.; Cao, Y.; Zhou, L.; Liu, Y.; Dai, W.; Nishimura, K.; Li, C.; Lin, C.T.; et al. Enhanced thermal conductivity of epoxy composites filled with silicon carbide nanowires. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Du, G.; Chen, N. A novel approach for Al2O3/epoxy composites with high strength and thermal conductivity. Compos. Sci. Technol. 2016, 124, 36–43. [Google Scholar] [CrossRef]
- Yao, Y.; Zeng, X.; Guo, K.; Sun, R.; Xu, J. Bin The effect of interfacial state on the thermal conductivity of functionalized Al2O3 filled glass fibers reinforced polymer composites. Compos. Part. A Appl. Sci. Manuf. 2015, 69, 49–55. [Google Scholar] [CrossRef]
- Fang, H.; Bai, S.L.; Wong, C.P. “White graphene”—Hexagonal boron nitride based polymeric composites and their application in thermal management. Compos. Commun. 2016, 2, 19–24. [Google Scholar] [CrossRef]
- Mu, Q.; Feng, S.; Diao, G. Thermal conductivity of silicone rubber filled with ZnO. Polym. Compos. 2007, 28, 125–130. [Google Scholar] [CrossRef]
- Du, H.; Qi, Y.; Yu, W.; Yin, J.; Xie, H. T-shape ZnO whisker: A more effective thermal conductive filler than spherical particles for the thermal grease. Int. J. Heat Mass Transf. 2017, 112, 1052–1056. [Google Scholar] [CrossRef]
- Yorifuji, D.; Ando, S. Enhanced thermal conductivity over percolation threshold in polyimide blend films containing ZnO nano-pyramidal particles: Advantage of vertical double percolation structure. J. Mater. Chem. 2011, 21, 4402–4407. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, D.; Gan, X.; Xie, R.; Zhang, F.; Zhou, K. Preparation of high performance AlN/Hydantion composite by gelcasting and infiltration processes. Ceram. Int. 2014, 40, 2535–2538. [Google Scholar] [CrossRef]
- Ma, A.; Chen, W.; Hou, Y.; Zhang, G. The Preparation and Cure Kinetics Researches of Thermal Conductivity Epoxy/AlN Composites. Polym. Plast. Technol. Eng. 2010, 49, 354–358. [Google Scholar] [CrossRef]
- Nanda, J.; Maranville, C.; Bollin, S.C.; Sawall, D.; Ohtani, H.; Remillard, J.T.; Ginder, J.M. Thermal conductivity of single-wall carbon nanotube dispersions: Role of interfacial effects. J. Phys. Chem. C 2008, 112, 654–658. [Google Scholar] [CrossRef]
- Hauser, R.A.; King, J.A.; Pagel, R.M.; Keith, J.M. Effects of carbon fillers on the thermal conductivity of highly filled liquid-crystal polymer based resins. J. Appl. Polym. Sci. 2008, 109, 2145–2155. [Google Scholar] [CrossRef]
- Ji, T.; Feng, Y.; Qin, M.; Li, S.; Zhang, F.; Lv, F.; Feng, W. Thermal conductive and flexible silastic composite based on a hierarchical framework of aligned carbon fibers-carbon nanotubes. Carbon N. Y. 2018, 131, 149–159. [Google Scholar] [CrossRef]
- Afanasov, I.M.; Savchenko, D.V.; Ionov, S.G.; Rusakov, D.A.; Seleznev, A.N.; Avdeev, V.V. Thermal conductivity and mechanical properties of expanded graphite. Inorg. Mater. 2009, 45, 486–490. [Google Scholar] [CrossRef]
- Gu, J.; Yang, X.; Lv, Z.; Li, N.; Liang, C.; Zhang, Q. Functionalized graphite nanoplatelets/epoxy resin nanocomposites with high thermal conductivity. Int. J. Heat Mass Transf. 2016, 92, 15–22. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Song, S.H.; Park, K.H.; Kim, B.H.; Choi, Y.W.; Jun, G.H.; Lee, D.J.; Kong, B.-S.; Paik, K.-W.; Jeon, S. Enhanced Thermal Conductivity of Epoxy-Graphene Composites by Using Non-Oxidized Graphene Flakes with Non-Covalent Functionalization. Adv. Mater. 2013, 25, 732–737. [Google Scholar] [CrossRef]
- Liu, L.H.; Yan, M. Functionalization of pristine graphene with perfluorophenyl azides. J. Mater. Chem. 2011, 21, 3273–3276. [Google Scholar] [CrossRef]
- Hill, R.F.; Supancic, P.H. Thermal Conductivity of Platelet-Filled Polymer Composites. J. Am. Ceram. Soc. 2004, 85, 851–857. [Google Scholar] [CrossRef]
- Lee, G.W.; Park, M.; Kim, J.; Lee, J.I.; Yoon, H.G. Enhanced thermal conductivity of polymer composites filled with hybrid filler. Compos. Part A Appl. Sci. Manuf. 2006, 37, 727–734. [Google Scholar] [CrossRef]
- Teng, C.C.; Ma, C.C.M.; Chiou, K.C.; Lee, T.M. Synergetic effect of thermal conductive properties of epoxy composites containing functionalized multi-walled carbon nanotubes and aluminum nitride. Compos. Part B Eng. 2012, 43, 265–271. [Google Scholar] [CrossRef]
- Leung, S.N.; Khan, M.O.; Chan, E.; Naguib, H.E.; Dawson, F.; Adinkrah, V.; Lakatos-Hayward, L. Synergistic effects of hybrid fillers on the development of thermally conductive polyphenylene sulfide composites. J. Appl. Polym. Sci. 2013, 127, 3293–3301. [Google Scholar] [CrossRef]
- Zeng, J.; Fu, R.; Shen, Y.; He, H.; Song, X. High thermal conductive epoxy molding compound with thermal conductive pathway. J. Appl. Polym. Sci. 2009, 113, 2117–2125. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Y. Effect of an interface layer on thermal conductivity of polymer composites studied by the design of double-layered and triple-layered composites. Int. J. Heat Mass Transf. 2019, 141, 1049–1055. [Google Scholar] [CrossRef]
- Giri, A.; Hopkins, P.E. A Review of Experimental and Computational Advances in Thermal Boundary Conductance and Nanoscale Thermal Transport across Solid Interfaces. Adv. Funct. Mater. 2020, 30, 1903857. [Google Scholar] [CrossRef]
- Nan, C.W.; Birringer, R.; Clarke, D.R.; Gleiter, H. Effective thermal conductivity of particulate composites with interfacial thermal resistance. J. Appl. Phys. 1997, 81, 6692–6699. [Google Scholar] [CrossRef]
- Kotia, A.; Borkakoti, S.; Deval, P.; Ghosh, S.K. Review of interfacial layer’s effect on thermal conductivity in nanofluid. Heat Mass Transf. 2017, 53, 2199–2209. [Google Scholar] [CrossRef]
- Oluwalowo, A.; Nguyen, N.; Zhang, S.; Park, J.G.; Liang, R. Electrical and thermal conductivity improvement of carbon nanotube and silver composites. Carbon N. Y. 2019, 146, 224–231. [Google Scholar] [CrossRef]
- Aradhana, R.; Mohanty, S.; Nayak, S.K. Novel electrically conductive epoxy/reduced graphite oxide/silica hollow microspheres adhesives with enhanced lap shear strength and thermal conductivity. Compos. Sci. Technol. 2019, 169, 86–94. [Google Scholar] [CrossRef]
- Prasad, K.; Nikzad, M.; Doherty, C.M.; Sbarski, I. Predicting trends in structural and physical properties of a model polymer with embedded natural fibers: Viability of molecular dynamics studies for a bottom up design. J. Appl. Polym. Sci. 2019, 136, 48189. [Google Scholar] [CrossRef]
- Yu, L.; Yang, D.; Wei, Q.; Zhang, L. Constructing of strawberry-like core-shell structured Al2O3 nanoparticles for improving thermal conductivity of nitrile butadiene rubber composites. Compos. Sci. Technol. 2021, 209, 108786. [Google Scholar] [CrossRef]
- Shiva, M.; Lakhi, M. Studying the effects of silica/alumina and silica/boehmite binary filler on the mechanical properties and the non-isothermal curing time of carbon black filled tyre tread composite. Compos. Part B Eng. 2019, 175, 107124. [Google Scholar] [CrossRef]
- Suntako, R. Effect of synthesized ZnO nanoparticles on thermal conductivity and mechanical properties of natural rubber. In Proceedings of the 2nd International Conference on Innovative Engineering Materials (ICIEM 2017), Philadelphia, PA, USA, 21–23 October 2017; Volume 284. [Google Scholar]
- Danilova-Tret′yak, S.M. On Thermophysical Properties of Rubbers and Their Components. J. Eng. Phys. Thermophys. 2016, 89, 1388–1393. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; Ding, J.; Zhang, L.; Chan, T.W. Preparation of nano-reinforced thermal conductive natural rubber composites. Polym. Compos. 2016, 37, 771–781. [Google Scholar] [CrossRef]
- Leblanc, J.L. Rubber-filler interactions and rheological properties in filled compounds. Prog. Polym. Sci. 2002, 27, 627–687. [Google Scholar] [CrossRef]
- Uher, C. Thermal Conductivity of High. Tc Superconductors. J. Supercond. 1990, 3, 337–389. [Google Scholar] [CrossRef]
- Zhai, S.; Zhang, P.; Xian, Y.; Zeng, J.; Shi, B. Effective thermal conductivity of polymer composites: Theoretical models and simulation models. Int. J. Heat Mass Transf. 2018, 117, 358–374. [Google Scholar] [CrossRef]
- Keyes, R.W. High-temperature thermal conductivity of insulating crystals: Relationship to the melting point. Phys. Rev. 1959, 115, 564–567. [Google Scholar] [CrossRef]
- Wang, Z.L.; Mu, H.T.; Liang, J.G.; Tang, D.W. Thermal boundary resistance and temperature dependent phonon conduction in CNT array multilayer structure. Int. J. Therm. Sci. 2013, 74, 53–62. [Google Scholar] [CrossRef]
- Reddy, P.; Castelino, K.; Majumdar, A. Diffuse mismatch model of thermal boundary conductance using exact phonon dispersion. Appl. Phys. Lett. 2005, 87, 1–3. [Google Scholar] [CrossRef]
- Li, A.; Zhang, C.; Zhang, Y.F. Thermal Conductivity of Graphene-Polymer Composites: Mechanisms, Properties, and Applications. Polymers 2017, 9, 437. [Google Scholar] [CrossRef]
- Soga, K.; Saito, T.; Kawaguchi, T.; Satoh, I. Percolation effect on thermal conductivity of filler-dispersed polymer composites. J. Therm. Sci. Technol. 2017, 12, JTST0013. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, K.; Liu, Y.; Yu, B.; Zhang, Q.; Chen, F.; Fu, Q. Preparation of highly thermally conductive but electrically insulating composites by constructing a segregated double network in polymer composites. Compos. Sci. Technol. 2019, 175, 135–142. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Sun, B.; Pan, M.; Dai, G. Properties and Heat-Conduction Mechanism of Thermally Conductive Polymer Composites. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-HGSZ200910038.htm (accessed on 26 April 2021).
- Henry, A.; Chen, G. Anomalous heat conduction in polyethylene chains: Theory and molecular dynamics simulations. Phys. Rev. B Condens. Matter Mater. Phys. 2009, 79, 144305. [Google Scholar] [CrossRef]
- Yamamoto, O. Thermal Conductivity of Cross-linked Polymers. Polym. J. 1971, 2, 509–517. [Google Scholar] [CrossRef][Green Version]
- Araby, S.; Zhang, L.; Kuan, H.C.; Dai, J.B.; Majewski, P.; Ma, J. A novel approach to electrically and thermally conductive elastomers using graphene. Polymer 2013, 54, 3663–3670. [Google Scholar] [CrossRef]
- Ma, J.; Feng, Y.X.; Xu, J.; Xiong, M.L.; Zhu, Y.J.; Zhang, L.Q. Effects of compatibilizing agent and in situ fibril on the morphology, interface and mechanical properties of EPDM/nylon copolymer blends. Polymer 2001, 43, 937–945. [Google Scholar] [CrossRef]
- Fu, Y.X.; He, Z.X.; Mo, D.C.; Lu, S.S. Thermal conductivity enhancement with different fillers for epoxy resin adhesives. Appl. Therm. Eng. 2014, 66, 493–498. [Google Scholar] [CrossRef]
- Guiney, L.M.; Mansukhani, N.D.; Jakus, A.E.; Wallace, S.G.; Shah, R.N.; Hersam, M.C. Three-Dimensional Printing of Cytocompatible, Thermally Conductive Hexagonal Boron Nitride Nanocomposites. Nano Lett. 2018, 18, 3488–3493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.L.; Zha, J.W.; Li, W.K.; Li, C.Q.; Wang, S.J.; Wen, Y.; Dang, Z.M. Enhanced thermal conductivity and mechanical property through boron nitride hot string in polyvinylidene fluoride fibers by electrospinning. Compos. Sci. Technol. 2018, 156, 1–7. [Google Scholar] [CrossRef]
- Moradi, S.; Calventus, Y.; Román, F.; Hutchinson, J.M. Achieving High Thermal Conductivity in Epoxy Composites: Effect of Boron Nitride Particle Size and Matrix-Filler Interface. Polymers 2019, 11, 1156. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; France, D.M.; Routbort, J.L.; Choi, S.U.S. Review and comparison of nanofluid thermal conductivity and heat transfer enhancements. Heat Transf. Eng. 2008, 29, 432–460. [Google Scholar] [CrossRef]
- Zhou, W.; Zuo, J.; Ren, W. Thermal conductivity and dielectric properties of Al/PVDF composites. Compos. Part. A Appl. Sci. Manuf. 2012, 43, 658–664. [Google Scholar] [CrossRef]
- Zhu, B.L.; Ma, J.; Wu, J.; Yung, K.C.; Xie, C.S. Study on the properties of the epoxy-matrix composites filled with thermally conductive AlN and BN ceramic particles. J. Appl. Polym. Sci. 2010, 118, 2754–2764. [Google Scholar] [CrossRef]
- Ng, H.Y.; Lu, X.; Lau, S.K. Thermal conductivity of boron nitride-filled thermoplastics: Effect of filler characteristics and composite processing conditions. Polym. Compos. 2005, 26, 778–790. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, P.; Tanaka, T. A review of dielectric polymer composites with high thermal conductivity. IEEE Electr. Insul. Mag. 2011, 27, 8–16. [Google Scholar] [CrossRef]
- Gu, J.; Lv, Z.; Yang, X.; Wang, G.; Zhang, Q. Fabrication and properties of thermally conductive epoxy resin nanocomposites filled with fGNPs/PNBRs hybrid fillers. Sci. Adv. Mater. 2016, 8, 972–979. [Google Scholar] [CrossRef]
- Chen, S.; Feng, Y.; Qin, M.; Ji, T.; Feng, W. Improving thermal conductivity in the through-thickness direction of carbon fibre/SiC composites by growing vertically aligned carbon nanotubes. Carbon N. Y. 2017, 116, 84–93. [Google Scholar] [CrossRef]
- Lule, Z.; And, J.K. Thermally conductive and highly rigid polylactic acid (PLA) hybrid composite filled with surface treated alumina/nano-sized aluminum nitride. Compos. Part A Appl. Sci. 2019, 124, 105506. [Google Scholar] [CrossRef]
- Warzoha, R.J.; Fleischer, A.S. Heat flow at nanoparticle interfaces. Nano Energy 2014, 6, 137–158. [Google Scholar] [CrossRef]
- Wattanakul, K.; Manuspiya, H.; Yanumet, N. The adsorption of cationic surfactants on BN surface: Its effects on the thermal conductivity and mechanical properties of BN-epoxy composite. Colloids Surf. A Physicochem. Eng. Asp. 2010, 369, 203–210. [Google Scholar] [CrossRef]
- Huang, X.; Iizuka, T.; Jiang, P.; Ohki, Y.; Tanaka, T. Role of interface on the thermal conductivity of highly filled dielectric epoxy/AlN composites. J. Phys. Chem. C 2012, 116, 13629–13639. [Google Scholar] [CrossRef]
- Ganguli, S.; Roy, A.K.; Anderson, D.P. Improved thermal conductivity for chemically functionalized exfoliated graphite/epoxy composites. Carbon N. Y. 2008, 46, 806–817. [Google Scholar] [CrossRef]
- Hirano, H.; Kadota, J.; Yamashita, T.; Agari, Y. Treatment of Inorganic Filler Surface by Silane-Coupling Agent: Investigation of Treatment Condition and Analysis of Bonding State of Reacted Agent. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2012. [Google Scholar]
- Tan, F.; Qiao, X.; Chen, J.; Wang, H. Effects of coupling agents on the properties of epoxy-based electrically conductive adhesives. Int. J. Adhes. Adhes. 2006, 26, 406–413. [Google Scholar] [CrossRef]
- Xu, Y.; Chung, D.D.L. Increasing the thermal conductivity of boron nitride and aluminum nitride particle epoxy-matrix composites by particle surface treatments. Compos. Interfaces 2000, 7, 243–256. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, M.Q.; Zeng, H.M. Carbon-black-filled polyolefine as a positive temperature coefficient material: Effect of composition, processing, and filler treatment. J. Appl. Polym. Sci. 1998, 70, 559–566. [Google Scholar] [CrossRef]
- Yang, K.; Gu, M. Enhanced thermal conductivity of epoxy nanocomposites filled with hybrid filler system of triethylenetetramine-functionalized multi-walled carbon nanotube/silane-modified nano-sized silicon carbide. Compos. Part. A Appl. Sci. Manuf. 2010, 41, 215–221. [Google Scholar] [CrossRef]
- Yung, K.C.; Liem, H. Enhanced thermal conductivity of boron nitride epoxy-matrix composite through multi-modal particle size mixing. J. Appl. Polym. Sci. 2007, 106, 3587–3591. [Google Scholar] [CrossRef]
- Sato, K.; Ijuin, A.; Hotta, Y. Thermal conductivity enhancement of alumina/polyamide composites via interfacial modification. Ceram. Int. 2015, 41, 10314–10318. [Google Scholar] [CrossRef]
- Hong, J.-P.; Yoon, S.-W.; Hwang, T.-S.; Lee, Y.-K.; Won, S.-H.; Nam, J.-D. Interphase Control of Boron Nitride/Epoxy Composites for High Thermal Conductivity. Korea Aust. Rheol. J. 2010, 22, 259–264. [Google Scholar]
- Guo, J.; Saha, P.; Liang, J.; Saha, M.; Grady, B.P. Multi-walled carbon nanotubes coated by multi-layer silica for improving thermal conductivity of polymer composites. J. Therm. Anal. Calorim. 2013, 113, 467–474. [Google Scholar] [CrossRef]
- Lee, B.; Dai, G. Influence of interfacial modification on the thermal conductivity of polymer composites. J. Mater. Sci. 2009, 44, 4848–4855. [Google Scholar] [CrossRef]
- Zhou, W. Effect of coupling agents on the thermal conductivity of aluminum particle/epoxy resin composites. J. Mater. Sci. 2011, 46, 3883–3889. [Google Scholar] [CrossRef]
- Wang, Z.-H.H.; Lu, Y.-L.L.; Liu, J.; Dang, Z.-M.M.; Zhang, L.-Q.Q.; Wang, W. Preparation of nanoalumina/EPDM composites with good performance in thermal conductivity and mechanical properties. Polym. Adv. Technol. 2011, 22, 2302–2310. [Google Scholar] [CrossRef]
- Li, S.; Qi, S.; Liu, N.; Cao, P. Study on thermal conductive BN/novolac resin composites. Thermochim. Acta 2011, 523, 111–115. [Google Scholar] [CrossRef]
- Yu, J.H.; Duan, J.K.; Peng, W.Y.; Wang, L.C.; Peng, P.; Jiang, P.K. Influence of nano-ALN particles on thermal conductivity, thermal stability and cure behavior of cycloaliphatic epoxy/trimethacrylate system. Express Polym. Lett. 2011, 5, 132–141. [Google Scholar] [CrossRef]
- Hsieh, C.-Y.; Chung, S.-L. High thermal conductivity epoxy molding compound filled with a combustion synthesized AlN powder. J. Appl. Polym. Sci. 2006, 102, 4734–4740. [Google Scholar] [CrossRef]
- Peng, W.; Huang, X.; Yu, J.; Jiang, P.; Liu, W. Electrical and thermophysical properties of epoxy/aluminum nitride nanocomposites: Effects of nanoparticle surface modification. In Proceedings of the Composites Part A: Applied Science and Manufacturing; Elsevier: Amsterdam, The Netherlands, 2010; Volume 41, pp. 1201–1209. [Google Scholar]
- Wattanakul, K.; Manuspiya, H.; Yanumet, N. Effective surface treatments for enhancing the thermal conductivity of BN-filled epoxy composite. J. Appl. Polym. Sci. 2011, 119, 3234–3243. [Google Scholar] [CrossRef]
- Teng, C.C.; Ma, C.C.M.; Chiou, K.C.; Lee, T.M.; Shih, Y.F. Synergetic effect of hybrid boron nitride and multi-walled carbon nanotubes on the thermal conductivity of epoxy composites. Mater. Chem. Phys. 2011, 126, 722–728. [Google Scholar] [CrossRef]
- Zhang, M.; Gu, A.; Liang, G.; Yuan, L. Preparation of high thermal conductive aluminum nitride/cyanate ester nanocomposite using a new macromolecular coupling agent. Polym. Adv. Technol. 2012, 23, 1503–1510. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Wu, C.; Wu, X.; Wang, G.; Jiang, P. Interfacial modification of boron nitride nanoplatelets for epoxy composites with improved thermal properties. Polymer 2012, 53, 471–480. [Google Scholar] [CrossRef]
- Chen, C.; Tang, Y.; Ye, Y.S.; Xue, Z.; Xue, Y.; Xie, X.; Mai, Y.W. High-performance epoxy/silica coated silver nanowire composites as underfill material for electronic packaging. Compos. Sci. Technol. 2014, 105, 80–85. [Google Scholar] [CrossRef]
- Badi, N.; Mekala, R. Heat-Sink Solution Through Artificial Nanodielectrics for LED Lighting Application. In Proceedings of the COMSOL Conference, Boston, MA, USA, 3–5 October 2012; p. 56. [Google Scholar]
- Zheng, Y.; Wang, S. Synthesis of boron nitride coatings on quartz fibers: Thickness control and mechanism research. Appl. Surf. Sci. 2011, 257, 10752–10757. [Google Scholar] [CrossRef]
- Hwang, Y.; Kim, M.; Kim, J. Effect of Al2O3 coverage on SiC particles for electrically insulated polymer composites with high thermal conductivity. RSC Adv. 2014, 4, 17015–17021. [Google Scholar] [CrossRef]
- Im, H.; Kim, J. Effect of homogeneous Al(OH)3 covered MWCNT addition on the thermal conductivity of Al2O3/epoxy-terminated poly(dimethylsiloxane) composites. J. Mater. Sci. 2012, 47, 6025–6033. [Google Scholar] [CrossRef]
- Chiu, H.T.; Sukachonmakul, T.; Kuo, M.T.; Wang, Y.H.; Wattanakul, K. Surface modification of aluminum nitride by polysilazane and its polymer-derived amorphous silicon oxycarbide ceramic for the enhancement of thermal conductivity in silicone rubber composite. Appl. Surf. Sci. 2014, 292, 928–936. [Google Scholar] [CrossRef]
- Zhou, Y.; Bai, Y.; Yu, K.; Kang, Y.; Wang, H. Excellent thermal conductivity and dielectric properties of polyimide composites filled with silica coated self-passivated aluminum fibers and nanoparticles. Appl. Phys. Lett. 2013, 102, 252903. [Google Scholar] [CrossRef]
- Liang, Q.; Moon, K.S.; Zhang, Y.; Wong, C.P. Low stress and high thermal conductive underfill for Cu/low-k application. In Proceedings of the Electronic Components and Technology Conference, Lake Buena Vista, FL, USA, 27–30 May 2008; pp. 1958–1962. [Google Scholar]
- Choi, S.; Kim, K.; Nam, J.; Shim, S.E. Synthesis of silica-coated graphite by enolization of polyvinylpyrrolidone and its thermal and electrical conductivity in polymer composites. Carbon N. Y. 2013, 60, 254–265. [Google Scholar] [CrossRef]
- Zhao, J.; Du, F.; Cui, W.; Zhu, P.; Zhou, X.; Xie, X. Effect of silica coating thickness on the thermal conductivity of polyurethane/SiO2 coated multiwalled carbon nanotube composites. Compos. Part A Appl. Sci. Manuf. 2014, 58, 1–6. [Google Scholar] [CrossRef]
- Yu, S.; Park, B.I.; Park, C.; Hong, S.M.; Han, T.H.; Koo, C.M. RTA-treated carbon fiber/copper core/shell hybrid for thermally conductive composites. ACS Appl. Mater. Interfaces 2014, 6, 7498–7503. [Google Scholar] [CrossRef]
- Kim, S.W.; Choi, H.S.; Lee, K.S. Thermal conductivity of thermally conductive composites consisting of core-shell particles with nanostructured shell layers. Mater. Res. Bull. 2014, 60, 843–848. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, Y.; Sun, H.; Liu, S.; Chi, Z.; Chen, X.; Xu, J. Polyimide nanocomposites with boron nitride-coated multi-walled carbon nanotubes for enhanced thermal conductivity and electrical insulation. J. Mater. Chem. A 2014, 2, 20958–20965. [Google Scholar] [CrossRef]
- Gomathi, A.; Ramya Harika, M.; Rao, C.N.R. Urea route to coat inorganic nanowires, carbon fibers and nanotubes by boron nitride. Mater. Sci. Eng. A 2008, 476, 29–33. [Google Scholar] [CrossRef]
- Dong, J.; Sun, F.H.; Tang, H.; Hayashi, K.; Li, H.; Shang, P.P.; Miyazaki, Y.; Li, J.F. Reducing Lattice Thermal Conductivity of MnTe by Se Alloying toward High Thermoelectric Performance. ACS Appl. Mater. Interfaces 2019, 11, 28221–28227. [Google Scholar] [CrossRef]
- An, D.; Chen, S.; Lu, Z.; Li, R.; Chen, W.; Fan, W.; Wang, W.; Wu, Y. Low Thermal Conductivity and Optimized Thermoelectric Properties of p-Type Te-Sb2Se3: Synergistic Effect of Doping and Defect Engineering. ACS Appl. Mater. Interfaces 2019, 11, 27788–27797. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, K.; Yan, L.; Wei, C.; Zhang, Y.; Gong, C.; Guo, J.; Zhang, J.; Zhang, D.; Zhang, J. Efficient and scalable high-quality graphene nanodot fabrication through confined lattice plane electrochemical exfoliation. Chem. Commun. 2019, 55, 5805–5808. [Google Scholar] [CrossRef]
- Lo, S.-H.; He, J.; Biswas, K.; Kanatzidis, M.G.; Dravid, V.P. Phonon Scattering and Thermal Conductivity in p-Type Nanostructured PbTe-BaTe Bulk Thermoelectric Materials. Adv. Funct. Mater. 2012, 22, 5175–5184. [Google Scholar] [CrossRef]
- Watari, K.; Ishizaki, K.; Tsuchiya, F. Phonon scattering and thermal conduction mechanisms of sintered aluminium nitride ceramics. J. Mater. Sci. 1993, 28, 3709–3714. [Google Scholar] [CrossRef]
- Takahata, K.; Iguchi, Y.; Tanaka, D.; Itoh, T.; Terasaki, I. Low thermal conductivity of the layered oxide Another example of a phonon glass and an electron crystal. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 61, 12551–12555. [Google Scholar] [CrossRef]
- Hatui, G.; Bhattacharya, P.; Sahoo, S.; Dhibar, S.; Das, C.K. Combined effect of expanded graphite and multiwall carbon nanotubes on the thermo mechanical, morphological as well as electrical conductivity of in situ bulk polymerized polystyrene composites. Compos. Part A Appl. Sci. Manuf. 2014, 56, 181–191. [Google Scholar] [CrossRef]
- Gulotty, R.; Castellino, M.; Jagdale, P.; Tagliaferro, A.; Balandin, A.A. Effects of functionalization on thermal properties of single-wall and multi-wall carbon nanotube-polymer nanocomposites. ACS Nano 2013, 7, 5114–5121. [Google Scholar] [CrossRef]
- Pop, E.; Mann, D.; Wang, Q.; Goodson, K.; Dai, H. Thermal conductance of an individual single-wall carbon nanotube above room temperature. Nano Lett. 2006, 6, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Calizo, I.; Teweldebrhan, D.; Pokatilov, E.P.; Nika, D.L.; Balandin, A.A.; Bao, W.; Miao, F.; Lau, C.N. Extremely high thermal conductivity of graphene: Prospects for thermal management applications in nanoelectronic circuits. Appl. Phys. Lett. 2008, 92, 151911. [Google Scholar] [CrossRef]
- Donnet, J.-B. Carbon Black: Science and Technology; Routledge: London, UK, 2018; ISBN 1351462628. [Google Scholar]
- Harada, M.; Hamaura, N.; Ochi, M.; Agari, Y. Thermal conductivity of liquid crystalline epoxy/BN filler composites having ordered network structure. Compos. B 2013, 55, 306–313. [Google Scholar] [CrossRef]
- Ross, R.B. Metallic Materials Specification Handbook; Springer Science & Business Media: Berlin, Germany, 2013; ISBN 1461534828. [Google Scholar]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Danafar, F.; Kalantari, M. A Review of Natural Rubber Nanocomposites Based on Carbon Nanotubes. J. Rubber Res. 2018, 21, 293–310. [Google Scholar] [CrossRef]
- Ata, S.; Subramaniam, C.; Nishizawa, A.; Yamada, T.; Hata, K. Highly Thermally Conductive Yet Flexible Composite of Carbon Fiber, Carbon Nanotube, and Rubber Obtained by Decreasing the Thermal Resistivity at the Interface between Carbon Fiber and Carbon Nanotube . Adv. Eng. Mater. 2017, 19, 1600596. [Google Scholar] [CrossRef]
- Klonos, P.A.; Tegopoulos, S.N.; Koutsiara, C.S.; Kontou, E.; Pissis, P.; Kyritsis, A. Effects of CNTs on thermal transitions, thermal diffusivity and electrical conductivity in nanocomposites: Comparison between an amorphous and a semicrystalline polymer matrix. Soft Matter 2019, 15, 1813–1824. [Google Scholar] [CrossRef]
- Souier, T.; Stefancich, M.; Chiesa, M. Characterization of multi-walled carbon nanotube-polymer nanocomposites by scanning spreading resistance microscopy. Nanotechnology 2012, 23, 405704. [Google Scholar] [CrossRef]
- Ma, P.C.; Siddiqui, N.A.; Marom, G.; Kim, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part. A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Araby, S.; Meng, Q.; Zhang, L.; Zaman, I.; Majewski, P.; Ma, J. Elastomeric composites based on carbon nanomaterials. Nanotechnology 2015, 26, 112001. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Yin, B.; Wang, J.; Jia, H.; He, J.; Zhang, X.; Xu, Z. Enhanced mechanical properties and thermal conductivity of styrene-butadiene rubber reinforced with polyvinylpyrrolidone-modified graphene oxide. J. Mater. Sci. 2016, 5724–5737. [Google Scholar] [CrossRef]
- Yang, H.; Cai, F.; Luo, Y.; Ye, X.; Zhang, C.; Wu, S. The interphase and thermal conductivity of graphene oxide/butadiene-styrene-vinyl pyridine rubber composites: A combined molecular simulation and experimental study. Compos. Sci. Technol. 2020, 188, 107971. [Google Scholar] [CrossRef]
- Liang, W.; Ge, X.; Ge, J.; Li, T.; Zhao, T.; Chen, X.; Song, Y.; Cui, Y.; Khan, M.; Ji, J.; et al. Reduced Graphene Oxide Embedded with MQ Silicone Resin Nano-Aggregates for Silicone Rubber Composites with Enhanced Thermal Conductivity and Mechanical Performance. Polymers 2018, 10, 1254. [Google Scholar] [CrossRef]
- Zhong, B.; Luo, Y.; Chen, W.; Luo, Y.; Hu, D.; Dong, H.; Jia, Z.; Jia, D. Immobilization of rubber additive on graphene for high-performance rubber composites. J. Colloid Interface Sci. 2019, 550, 190–198. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, J.M.; Park, K.H.; Lee, D.J.; Kwon, O.S.; Kim, J.; Yoon, H.; Chen, X. High performance graphene embedded rubber composites. RSC Adv. 2015, 5, 81707–81712. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.T.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Mu, Q.; Feng, S. Thermal conductivity of graphite/silicone rubber prepared by solution intercalation. Thermochim. Acta 2007, 462, 70–75. [Google Scholar] [CrossRef]
- Song, J.; Ma, L.; He, Y.; Yan, H.; Wu, Z.; Li, W. Modified graphite filled natural rubber composites with good thermal conductivity. Chinese J. Chem. Eng. 2015, 23, 853–859. [Google Scholar] [CrossRef]
- Hill, R.F.; Supancic, P.H. Determination of the Thermal Resistance of the Polymer-Ceramic Interface of Alumina-Filled Polymer Composites. J. Am. Ceram. Soc. 2005, 87, 1831–1835. [Google Scholar] [CrossRef]
- Song, J.; Wu, L.; Zhang, Y. Thermal conductivity enhancement of alumina/silicone rubber composites through constructing a thermally conductive 3D framework. Polym. Bull. 2020, 77, 2139–2153. [Google Scholar] [CrossRef]
- Guo, B.; Tang, Z.; Zhang, L. Transport performance in novel elastomer nanocomposites: Mechanism, design and control. Prog. Polym. Sci. 2016, 61, 29–66. [Google Scholar] [CrossRef]
- Cheng, J.P.; Liu, T.; Zhang, J.; Wang, B.B.; Ying, J.; Liu, F.; Zhang, X.B. Influence of phase and morphology on thermal conductivity of alumina particle/silicone rubber composites. Appl. Phys. A Mater. Sci. Process. 2014, 117, 1985–1992. [Google Scholar] [CrossRef]
- Zhou, W.; Qi, S.; Tu, C.; Zhao, H.; Wang, C.; Kou, J. Effect of the particle size of Al2O3 on the properties of filled heat-conductive silicone rubber. J. Appl. Polym. Sci. 2007, 104, 1312–1318. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, X.; Ding, F.; Bai, L.; Yuan, F. Simultaneously enhance thermal conductive property and mechanical properties of silicon rubber composites by introducing ultrafine Al2O3 nanospheres prepared via thermal plasma. Compos. Sci. Technol. 2020, 190, 108019. [Google Scholar] [CrossRef]
- Hou, G.; Cheng, B.; Ding, F.; Yao, M.; Cao, Y.; Hu, P.; Ma, R.; Yuan, F. Well dispersed silicon nanospheres synthesized by RF thermal plasma treatment and their high thermal conductivity and dielectric constant in polymer nanocomposites. RSC Adv. 2015, 5, 9432–9440. [Google Scholar] [CrossRef]
- Wang, M.J. Effect of polymer-filler and filler-filler interactions on dynamic properties of filled vulcanizates. Rubber Chem. Technol. 1998, 71, 520–589. [Google Scholar] [CrossRef]
- Wang, M.J. The role of filler networking in dynamic properties of filled rubber. Rubber Chem. Technol. 1999, 72, 430–448. [Google Scholar] [CrossRef]
- Liu, X.; Han, Q.; Yang, D.; Yang, D.; Ni, Y.; Ni, Y.; Yu, L.; Yu, L.; Wei, Q.; Wei, Q.; et al. Thermally Conductive Elastomer Composites with Poly(catechol-polyamine)-Modified Boron Nitride. ACS Omega 2020, 5, 14006–14012. [Google Scholar] [CrossRef]
- Sarkarat, M.; Lanagan, M.; Ghosh, D.; Lottes, A.; Budd, K.; Rajagopalan, R. Improved thermal conductivity and AC dielectric breakdown strength of silicone rubber/BN composites. Compos. Part C Open Access 2020, 2, 100023. [Google Scholar] [CrossRef]
- Kemaloglu, S.; Ozkoc, G.; Aytac, A. Properties of thermally conductive micro and nano size boron nitride reinforced silicon rubber composites. Thermochim. Acta 2010, 499, 40–47. [Google Scholar] [CrossRef]
- Li, J.; Li, F.; Zhao, X.; Zhang, W.; Li, S.; Lu, Y.; Zhang, L. Jelly-Inspired Construction of the Three-Dimensional Interconnected BN Network for Lightweight, Thermally Conductive, and Electrically Insulating Rubber Composites. ACS Appl. Electron. Mater. 2020, 2, 1661–1669. [Google Scholar] [CrossRef]
- Gu, J.; Guo, Y.; Yang, X.; Liang, C.; Geng, W.; Tang, L.; Li, N.; Zhang, Q. Synergistic improvement of thermal conductivities of polyphenylene sulfide composites filled with boron nitride hybrid fillers. Compos. Part A Appl. Sci. Manuf. 2017, 95, 267–273. [Google Scholar] [CrossRef]
- Li, L.H.; Chen, Y. Atomically Thin Boron Nitride: Unique Properties and Applications. Adv. Funct. Mater. 2016, 26, 2594–2608. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Huang, Y.; Terao, T.; Mitome, M.; Tang, C.; Zhi, C. Boron nitride nanotubes and nanosheets. ACS Nano 2010, 4, 2979–2993. [Google Scholar] [CrossRef]
- Cai, Q.; Scullion, D.; Gan, W.; Falin, A.; Zhang, S.; Watanabe, K.; Taniguchi, T.; Chen, Y.; Santos, E.J.G.; Li, L.H. High thermal conductivity of high-quality monolayer boron nitride and its thermal expansion. Sci. Adv. 2019, 5, eaav0129. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, K.; Liu, Z.; Wang, B.; Yan, C.; Wang, Z.; Lin, C.T.; Jiang, N.; Yu, J. Cotton Candy-Templated Fabrication of Three-Dimensional Ceramic Pathway within Polymer Composite for Enhanced Thermal Conductivity. ACS Appl. Mater. Interfaces 2019, 11, 44700–44707. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Luo, X.; Zheng, N.; Ma, T.; Tan, J.; Li, C.; Zhang, Q.; Gu, J. Self-healing, recoverable epoxy elastomers and their composites with desirable thermal conductivities by incorporating BN fillers via in-situ polymerization. Compos. Sci. Technol. 2018, 164, 59–64. [Google Scholar] [CrossRef]
- Wu, K.; Wang, J.; Liu, D.; Lei, C.; Liu, D.; Lei, W.; Fu, Q. Highly Thermoconductive, Thermostable, and Super-Flexible Film by Engineering 1D Rigid Rod-Like Aramid Nanofiber/2D Boron Nitride Nanosheets. Adv. Mater. 2020, 32, 1906939. [Google Scholar] [CrossRef]
- Han, J.; Du, G.; Gao, W.; Bai, H. An Anisotropically High Thermal Conductive Boron Nitride/Epoxy Composite Based on Nacre-Mimetic 3D Network. Adv. Funct. Mater. 2019, 29, 1900412. [Google Scholar] [CrossRef]
- Davis, R.F. III-V Nitrides for Electronic and Optoelectronic Applications. Proc. IEEE 1991, 79, 702–712. [Google Scholar] [CrossRef]
- Kuang, Z.; Chen, Y.; Lu, Y.; Liu, L.; Hu, S.; Wen, S.; Mao, Y.; Zhang, L. Fabrication of Highly Oriented Hexagonal Boron Nitride Nanosheet/Elastomer Nanocomposites with High Thermal Conductivity. Small 2015, 11, 1655–1659. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.J.; Wang, W.Y.; Lin, T.; Chen, X.J.; Zhang, Y.T.; Yang, J.H.; Wang, Y.; Zhou, Z.W. Largely Enhanced Thermal Conductivity and High Dielectric Constant of Poly(vinylidene fluoride)/Boron Nitride Composites Achieved by Adding a Few Carbon Nanotubes. J. Phys. Chem. C 2016, 120, 6344–6355. [Google Scholar] [CrossRef]
- Pan, C.; Kou, K.; Jia, Q.; Zhang, Y.; Wu, G.; Ji, T. Improved thermal conductivity and dielectric properties of hBN/PTFE composites via surface treatment by silane coupling agent. Compos. Part. B Eng. 2017, 111, 83–90. [Google Scholar] [CrossRef]
- Cheng, H.; Zhao, K.; Gong, Y.; Wang, X.; Wang, R.; Wang, F.; Hu, R.; Wang, F.; Zhang, X.; He, J.; et al. Covalent coupling regulated thermal conductivity of poly(vinyl alcohol)/boron nitride composite film based on silane molecular structure. Compos. Part. A Appl. Sci. Manuf. 2020, 137, 106026. [Google Scholar] [CrossRef]
- Chen, T.; Liu, B. Improvement of thermal conductivities for silicone nanocomposite via incorporating poly(Γ-methacryloxypropyltrimethoxy silane) grafted graphene fillers. Chem. Phys. Lett. 2018, 693, 121–126. [Google Scholar] [CrossRef]
- Zhang, Y.; Choi, J.R.; Park, S.J. Enhancing the heat and load transfer efficiency by optimizing the interface of hexagonal boron nitride/elastomer nanocomposites for thermal management applications. Polymer 2018, 143, 1–9. [Google Scholar] [CrossRef]
- Quiles-Díaz, S.; Martínez-Rubí, Y.; Guan, J.; Kim, K.S.; Couillard, M.; Salavagione, H.J.; Gómez-Fatou, M.A.; Simard, B. Enhanced Thermal Conductivity in Polymer Nanocomposites via Covalent Functionalization of Boron Nitride Nanotubes with Short Polyethylene Chains for Heat-Transfer Applications. ACS Appl. Nano Mater. 2019, 2, 440–451. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Z.; Wu, Y.; Liu, X.; He, Y.B.; Kim, J.K. Multilayer Graphene Enables Higher Efficiency in Improving Thermal Conductivities of Graphene/Epoxy Composites. Nano Lett. 2016, 16, 3585–3593. [Google Scholar] [CrossRef]
- Yang, D.; Ni, Y.; Kong, X.; Gao, D.; Wang, Y.; Hu, T.; Zhang, L. Mussel-inspired modification of boron nitride for natural rubber composites with high thermal conductivity and low dielectric constant. Compos. Sci. Technol. 2019, 177, 18–25. [Google Scholar] [CrossRef]
- Yang, D.; Kong, X.; Ni, Y.; Gao, D.; Yang, B.; Zhu, Y.; Zhang, L. Novel nitrile-butadiene rubber composites with enhanced thermal conductivity and high dielectric constant. Compos. Part. A Appl. Sci. Manuf. 2019, 124, 105447. [Google Scholar] [CrossRef]
- Wu, X.; Liu, H.; Tang, Z.; Guo, B. Scalable fabrication of thermally conductive elastomer/boron nitride nanosheets composites by slurry compounding. Compos. Sci. Technol. 2016, 123, 179–186. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, L.; Dai, Q.; Cui, L.; Liu, S.; Qi, Y.; Dong, W.; He, J.; Bai, C. Fabrication of b-cyclodextrin-crosslinked epoxy polybutadiene/hydroxylated boron nitride nanocomposites with improved mechanical and thermal-conducting properties. J. Mater. Res. Technol. 2019, 8, 5853–5861. [Google Scholar] [CrossRef]
- Park, S.W.; Joo, O.S.; Jung, K.D.; Kim, H.; Han, S.H. Development of ZnO/Al2O3 catalyst for reverse-water-gas-shift reaction of CAMERE (carbon dioxide hydrogenation to form methanol via a reverse-water-gas-shift reaction) process. Appl. Catal. A Gen. 2001, 211, 81–90. [Google Scholar] [CrossRef]
- Crapanzano, R.; Villa, I.; Mostoni, S.; D’Arienzo, M.; Di Credico, B.; Fasoli, M.; Scotti, R.; Vedda, A. Morphology Related Defectiveness in ZnO Luminescence: From Bulk to Nano-Size. Nanomaterials 2020, 10, 1983. [Google Scholar] [CrossRef] [PubMed]
- D’Arienzo, M.; Armelao, L.; Cacciamani, A.; Mari, C.M.; Polizzi, S.; Ruffo, R.; Scotti, R.; Testino, A.; Wahba, L.; Morazzoni, F. One-step preparation of SnO2 and Pt-doped SnO2 as inverse opal thin films for gas sensing. Chem. Mater. 2010, 22, 4083–4089. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Cristofori, D.; Scotti, R.; Morazzoni, F. New insights into the SnO2 sensing mechanism based on the properties of shape controlled tin oxide nanoparticles. Chem. Mater. 2013, 25, 3675–3686. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef]
- Lewicka, Z.A.; Benedetto, A.F.; Benoit, D.N.; Yu, W.W.; Fortner, J.D.; Colvin, V.L. The structure, composition, and dimensions of TiO2 and ZnO nanomaterials in commercial sunscreens. J. Nanoparticle Res. 2011, 13, 3607–3617. [Google Scholar] [CrossRef]
- Ponnamma, D.; Cabibihan, J.J.; Rajan, M.; Pethaiah, S.S.; Deshmukh, K.; Gogoi, J.P.; Pasha, S.K.K.; Ahamed, M.B.; Krishnegowda, J.; Chandrashekar, B.N.; et al. Synthesis, optimization and applications of ZnO/polymer nanocomposites. Mater. Sci. Eng. C 2019, 98, 1210–1240. [Google Scholar] [CrossRef]
- International Rubber Study Group—Reports. Available online: http://www.rubberstudy.org/reports (accessed on 30 April 2021).
- Mostoni; Milana; Credico; D’Arienzo; Scotti Zinc-Based Curing Activators: New Trends for Reducing Zinc Content in Rubber Vulcanization Process. Catalysts 2019, 9, 664. [CrossRef]
- Sim, L.C.; Ramanan, S.R.; Ismail, H.; Seetharamu, K.N.; Goh, T.J. Thermal characterization of Al2O3 and ZnO reinforced silicone rubber as thermal pads for heat dissipation purposes. Thermochim. Acta 2005, 430, 155–165. [Google Scholar] [CrossRef]
- Li, Z.; Wu, W.; Chen, H.; Zhu, Z.; Wang, Y.; Zhang, Y. Thermal conductivity of micro/nano filler filled polymeric composites. RSC Adv. 2013, 3, 6417–6428. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; Liu, J.; Dang, Z.; Zhang, L.; Wang, W. Preparation of nano-zinc oxide/EPDM composites with both good thermal conductivity and mechanical properties. J. Appl. Polym. Sci. 2011, 119, 1144–1155. [Google Scholar] [CrossRef]
- Susanna, A.; D’Arienzo, M.; Di Credico, B.; Giannini, L.; Hanel, T.; Grandori, R.; Morazzoni, F.; Mostoni, S.; Santambrogio, C.; Scotti, R. Catalytic effect of ZnO anchored silica nanoparticles on rubber vulcanization and cross-link formation. Eur. Polym. J. 2017, 93, 63–74. [Google Scholar] [CrossRef]
- Arani, A.A.A.; Pourmoghadam, F. Experimental investigation of thermal conductivity behavior of MWCNTS-Al2O3/ethylene glycol hybrid Nanofluid: Providing new thermal conductivity correlation. Heat Mass Transf. und Stoffuebertragung 2019, 55, 2329–2339. [Google Scholar] [CrossRef]
- Huang, J.; Yang, W.; Zhu, J.; Fu, L.; Li, D.; Zhou, L. Silver nanoparticles decorated 3D reduced graphene oxides as hybrid filler for enhancing thermal conductivity of polystyrene composites. Compos. Part. A Appl. Sci. Manuf. 2019, 123, 79–85. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, S.; Qin, Y.; Chen, H.; Zhao, W.; Sun, F.; Zhu, X. Thermally conductive cyanate ester nanocomposites filled with graphene nanosheets and multiwalled carbon nanotubes. Polym. Adv. Technol. 2014, 25, 1546–1551. [Google Scholar] [CrossRef]
- Lv, F.; Qin, M.; Zhang, F.; Yu, H.; Gao, L.; Lv, P.; Wei, W.; Feng, Y.; Feng, W. High cross-plane thermally conductive hierarchical composite using graphene-coated vertically aligned carbon nanotubes/graphite. Carbon N. Y. 2019, 149, 281–289. [Google Scholar] [CrossRef]
- Li, Z.; Ju, D.; Han, L.; Dong, L. Formation of more efficient thermally conductive pathways due to the synergistic effect of boron nitride and alumina in poly(3-hydroxylbutyrate). Thermochim. Acta 2017, 652, 9–16. [Google Scholar] [CrossRef]
- Zheng, X.; Kim, S.; Park, C.W. Enhancement of thermal conductivity of carbon fiber-reinforced polymer composite with copper and boron nitride particles. Compos. Part. A Appl. Sci. Manuf. 2019, 121, 449–456. [Google Scholar] [CrossRef]
- Li, C.; Liu, B.; Gao, Z.; Wang, H.; Liu, M.; Wang, S.; Xiong, C. Electrically insulating ZnOs/ZnOw/silicone rubber nanocomposites with enhanced thermal conductivity and mechanical properties. J. Appl. Polym. Sci. 2018, 135, 46454. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Zhang, Z.; Xian, Y.; Lin, Y.; Ji, X.; Lu, Y.; Zhang, L. Construction of interconnected Al2O3 doped rGO network in natural rubber nanocomposites to achieve significant thermal conductivity and mechanical strength enhancement. Compos. Sci. Technol. 2020, 186, 107930. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, D.; Yu, L.; Ni, Y.; Zhang, L. Fabrication of carboxyl nitrile butadiene rubber composites with high dielectric constant and thermal conductivity using Al2O3@PCPA@GO hybrids. Compos. Sci. Technol. 2020, 199, 108344. [Google Scholar] [CrossRef]
- Yang, D.; Ni, Y.; Liang, Y.; Li, B.; Ma, H.; Zhang, L. Improved thermal conductivity and electromechanical properties of natural rubber by constructing Al2O3-PDA-Ag hybrid nanoparticles. Compos. Sci. Technol. 2019, 180, 86–93. [Google Scholar] [CrossRef]
- Krainoi, A.; Kummerlöwe, C.; Vennemann, N.; Nakaramontri, Y.; Pichaiyut, S.; Nakason, C. Effect of carbon nanotubes decorated with silver nanoparticles as hybrid filler on properties of natural rubber nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47281. [Google Scholar] [CrossRef]
- Scotti, R.; Conzatti, L.; D’Arienzo, M.; Di Credico, B.; Giannini, L.; Hanel, T.; Stagnaro, P.; Susanna, A.; Tadiello, L.; Morazzoni, F. Shape controlled spherical (0D) and rod-like (1D) silica nanoparticles in silica/styrene butadiene rubber nanocomposites: Role of the particle morphology on the filler reinforcing effect. Polymer 2014, 55, 1497–1506. [Google Scholar] [CrossRef]
- Galimberti, M.; Agnelli, S.; Cipolletti, V. 11—Hybrid filler systems in rubber nanocomposites. In Woodhead Publishing Series in Composites Science and Engineering; Thomas, S., Maria, H.J., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 349–414. ISBN 978-0-08-100409-8. [Google Scholar]
- Di Credico, B.; Cobani, E.; Callone, E.; Conzatti, L.; Cristofori, D.; D’Arienzo, M.; Dirè, S.; Giannini, L.; Hanel, T.; Scotti, R.; et al. Size-controlled self-assembly of anisotropic sepiolite fibers in rubber nanocomposites. Appl. Clay Sci. 2018, 152, 51–64. [Google Scholar] [CrossRef]
- Shiva, M.; Akhtari, S.S.; Shayesteh, M. Effect of mineral fillers on physico-mechanical properties and heat conductivity of carbon black-filled SBR/butadiene rubber composite. Iran. Polym. J. 2020, 29, 957–974. [Google Scholar] [CrossRef]
- Song, S.H. Synergistic Effect of Clay Platelets and Carbon Nanotubes in Styrene-Butadiene Rubber Nanocomposites. Macromol. Chem. Phys. 2016, 217, 2617–2625. [Google Scholar] [CrossRef]
- Seo, J.G.; Lee, C.K.; Lee, D.; Song, S.H. High-performance tires based on graphene coated with Zn-free coupling agents. J. Ind. Eng. Chem. 2018, 66, 78–85. [Google Scholar] [CrossRef]
- Song, S.H. Graphene-Silica Hybrids Fillers for Multifunctional Solution Styrene Butadiene Rubber. J. Polym. Res. 2020, 27, 155. [Google Scholar] [CrossRef]



















| Material | λ W m−1 K−1) | Type | Reference |
|---|---|---|---|
| Single-wall Carbon Nanotubes | 3500 (single tube) | Carbon-Based | [128] |
| Graphene | 3080–5150 (in plane) | Carbon-Based | [129] |
| Graphite | 500–1000 | Carbon-Based | [130] |
| Alumina | 38–42 | Ceramic | [78] |
| Hexagonal Boron Nitride | in-plane: 400; smakalout of plane: 2 | Ceramic | [131] |
| Zinc Oxide | 50 | Ceramic | [1] |
| Silver | 417–427 | Metal | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirizzi, L.; Carnevale, M.; D’Arienzo, M.; Milanese, C.; Di Credico, B.; Mostoni, S.; Scotti, R. Tailoring the Thermal Conductivity of Rubber Nanocomposites by Inorganic Systems: Opportunities and Challenges for Their Application in Tires Formulation. Molecules 2021, 26, 3555. https://doi.org/10.3390/molecules26123555
Mirizzi L, Carnevale M, D’Arienzo M, Milanese C, Di Credico B, Mostoni S, Scotti R. Tailoring the Thermal Conductivity of Rubber Nanocomposites by Inorganic Systems: Opportunities and Challenges for Their Application in Tires Formulation. Molecules. 2021; 26(12):3555. https://doi.org/10.3390/molecules26123555
Chicago/Turabian StyleMirizzi, Lorenzo, Mattia Carnevale, Massimiliano D’Arienzo, Chiara Milanese, Barbara Di Credico, Silvia Mostoni, and Roberto Scotti. 2021. "Tailoring the Thermal Conductivity of Rubber Nanocomposites by Inorganic Systems: Opportunities and Challenges for Their Application in Tires Formulation" Molecules 26, no. 12: 3555. https://doi.org/10.3390/molecules26123555
APA StyleMirizzi, L., Carnevale, M., D’Arienzo, M., Milanese, C., Di Credico, B., Mostoni, S., & Scotti, R. (2021). Tailoring the Thermal Conductivity of Rubber Nanocomposites by Inorganic Systems: Opportunities and Challenges for Their Application in Tires Formulation. Molecules, 26(12), 3555. https://doi.org/10.3390/molecules26123555









