Interaction of Natural Compounds in Licorice and Turmeric with HIV-NCp7 Zinc Finger Domain: Potential Relevance to the Mechanism of Antiviral Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Zinc Finger by CD Spectroscopy
2.2. Evaluation of the Binding between NCp7 ZF and Natural Compounds by Fluorescence Assay
2.3. Elucidation of the Binding Mode between NCp7 ZF and Natural Compounds by Mass Spectrometry
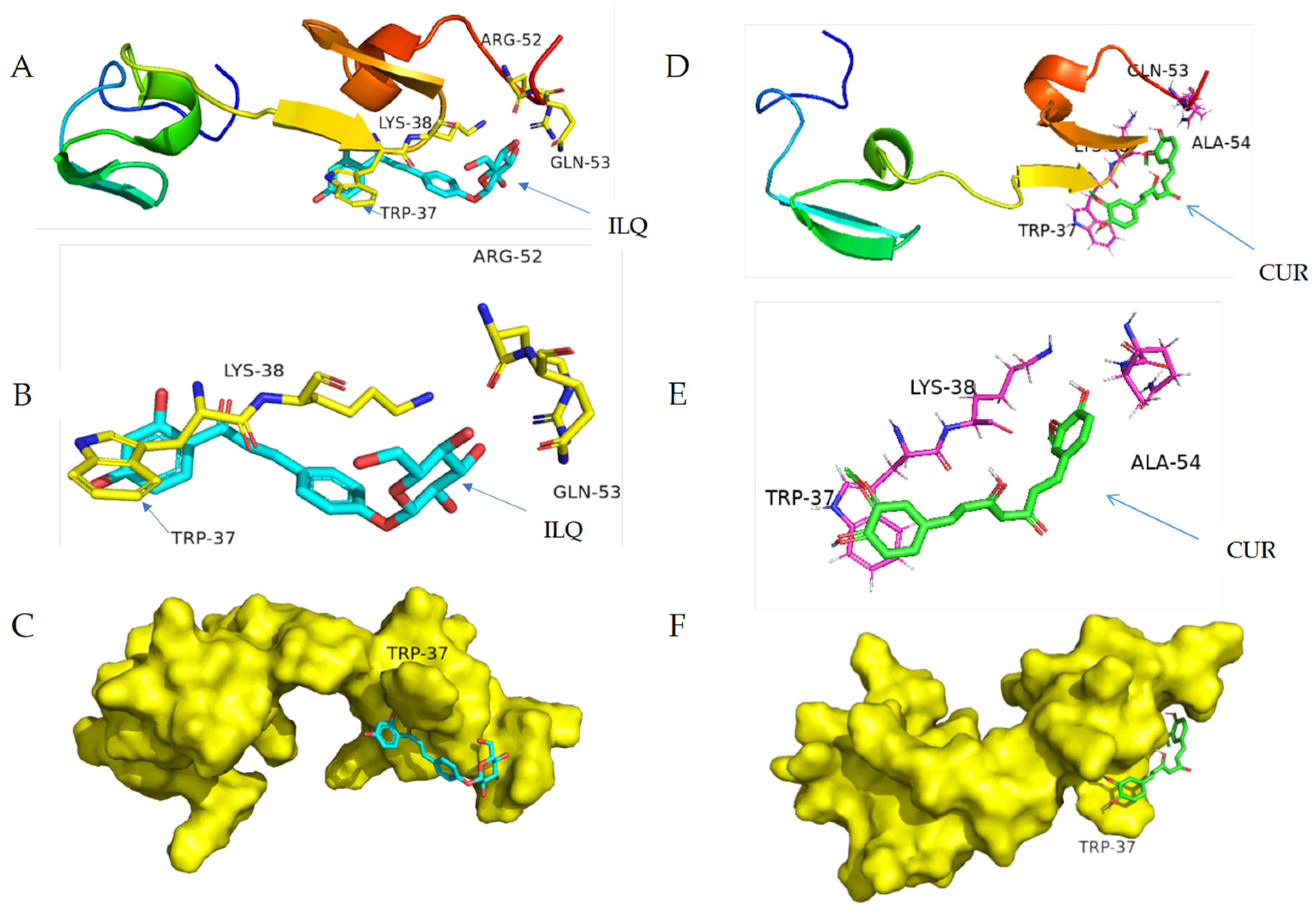
2.4. Structural Information of the Binding from CD Spectroscopy and Molecular Docking
3. Materials and Methods
3.1. Reagents and Equipment
3.2. Methods and Procedure
3.2.1. Preparation of Zinc Finger Protein
3.2.2. Fluorescence Assay
3.2.3. Mass Spectrometry
3.2.4. Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| ZF | zinc finger |
| LQ | liquiritin |
| ILQ | isoliquiritin |
| GL | glycyrrhizic acid |
| GA | glycyrrhetinic acid |
| CUR | curcumin |
| HIV-NCp7 | the human immunodeficiency virus nucleocapsid protein |
| NCIs | NC inhibitors |
| CD | circular dichroism |
| ESI-MS | electrospray ionization-mass spectrometer |
References
- Daniel, A.G.; Peterson, E.J.; Farrell, N.P. The bioinorganic chemistry of apoptosis: Potential inhibitory zinc binding sites in caspase-3. Angew. Chem. Int. Ed. 2014, 53, 4098–4101. [Google Scholar] [CrossRef]
- Anzellotti, A.I.; Liu, Q.; Bloemink, M.J.; Scarsdale, J.N.; Farrell, N. Targeting retroviral Zn finger-DNA interactions: A small-molecule approach using the electrophilic nature of trans-platinum-nucleobase compounds. Chem. Biol. 2006, 13, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Anzellotti, A.I.; Farrell, N.P. Zinc metalloproteins as medicinal targets. Chem. Soc. Rev. 2008, 37, 1629–1651. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, L.; Tugarinov, V.; Appella, D.H.; Clore, G.M. Targeting a dark excited state of HIV-1 nucleocapsid by antiretroviral thioesters revealed by NMR spectroscopy. Angew. Chem. Int. Ed. 2018, 57, 2687–2691. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Kovalenko, L.; Lyonnais, S.; Antaki, D.; Torbett, B.E.; Botta, M.; Mirambeau, G.; Mely, Y. Nucleocapsid protein: A desirable target for future therapies against HIV-1. Curr. Top. Microbiol. 2015, 389, 53–92. [Google Scholar]
- Mori, M.; Kovalenko, L.; Malancona, S.; Saladini, F.; De Forni, D.; Pires, M.; Humbert, N.; Real, E.; Botzanowski, T.; Cianferani, S.; et al. Structure-based identification of HIV-1 nucleocapsid protein inhibitors active against wild-type and drug-resistant HIV-1 strains. ACS Chem. Biol. 2018, 13, 253–266. [Google Scholar] [CrossRef]
- Tantillo, C.; Ding, J.; Jacobo-Molina, A.; Nanni, R.G.; Boyer, P.L.; Hughes, S.H.; Pauwels, R.; Andries, K.; Janssen, P.A.; Arnold, E. Locations of anti-aids drug binding sites and resistance mutations in the three-dimensional structure of HIV-1 reverse transcriptase: Implications for mechanisms of drug inhibition and resistance. J. Mol. Biol. 1994, 243, 369–387. [Google Scholar] [CrossRef]
- Remy, E.; de Rocquigny, H.; Petitjean, P.; Muriaux, D.; Theilleux, V.; Paoletti, J.; Roques, B.P. The annealing of tRNA3Lys to human immunodeficiency virus type 1 primer binding site is critically dependent on the ncp7 zinc fingers structure. J. Biol. Chem. 1998, 273, 4819–4822. [Google Scholar] [CrossRef]
- De Rocquigny, H.; Delaunay, T.; Petitjean, P.; Fournié-Zaluski, M.; Roques, B. La structure native de la ncp7 d’HIV-1 est nécessaire pour la reconnaissance spécifique de la séquence d’encapsidation psi de l’arn genomique: Etude par résonance plasmonique de surface. Regard. Biochim. 1998, 2, 44–50. [Google Scholar]
- Bailly, F.; Cotelle, P. Anti-HIV activities of natural antioxidant caffeic acid derivatives: Toward an antiviral supplementation diet. Curr. Med. Chem. 2005, 12, 1811–1818. [Google Scholar] [CrossRef]
- Singh, I.P.; Bodiwala, H.S. Recent advances in anti-HIV natural products. Nat. Prod. Rep. 2010, 27, 1781–1800. [Google Scholar] [CrossRef]
- Li, Z.J.; Zhao, Y.R.; Lin, W.W.; Ye, M.; Ling, X.M. Rapid screening and identification of active ingredients in licorice extract interacting with v3 loop region of HIV-1 gp120 using ace and ce-ms. J. Pharm. Biomed. Anal. 2015, 111, 28–35. [Google Scholar] [CrossRef]
- Huang, M.L.; Cheng, Z.Z.; Wang, L.; Feng, Y.L.; Huang, J.G.; Du, Z.F.; Jiang, H.L. A targeted strategy to identify untargeted metabolites from in vitro to in vivo: Rapid and sensitive metabolites profiling of licorice in rats using ultra-high performance liquid chromatography coupled with triple quadrupole-linear ion trap mass spectrometry. J. Chromatogr. B 2018, 1092, 40–50. [Google Scholar]
- Watanbe, H.; Miyaji, C.; Makino, M.; Abo, T. Therapeutic effects of glycyrrhizin in mice infected with lp-bm5 murine retrovirus and mechanisms involved in the prevention of disease progression. Biotherapy 1996, 9, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Nakashima, H.; Baba, M.; Pauwels, R.; De Clercq, E.; Shigeta, S.; Yamamoto, N. Inhibitory effect of glycyrrhizin on the in vitro infectivity and cytopathic activity of the human immunodeficiency virus [HIV (HTLV-III/LAV)]. Antivir. Res. 1987, 3, 127–137. [Google Scholar] [CrossRef]
- Mori, K.; Sakai, H.; Suzuki, S.; Akutsu, Y.; Ishikawa, M.; Imaizumi, M.; Tada, K.; Aihara, M.; Sawada, Y.; Yokoyama, M. Effects of glycyrrhizin (SNMC: Stronger neo-minophagen c) in hemophilia patients with HIV-1 infection. Tohoku J. Exp. Med. 1990, 162, 183–193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abhijit, M.; Krishnamachari, R.; John, W.; Kurt, W.K.; Yves, P. Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem. Pharmacol. 1995, 49, 1165–1170. [Google Scholar]
- Jennings Morgan, R.; Parks Robin, J. Curcumin as an antiviral agent. Viruses 2020, 6, 3412–3419. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi Amit, K. Curcumin and its analogues: A potential natural compound against HIV infection and aids. Food Funct. 2015, 12, 1242. [Google Scholar] [CrossRef]
- Sergei, N.; Charlee, M.; Evaristus, N.; Xionghao, L.; MariaMananita, H.; Amol, K.; Andrey, I.; Namita, K.; Tatiana, A. Inhibition of HIV-1 by curcumin a, a novel curcumin analog. Drug Des. Dev. Ther. 2015, 9, 5051–5060. [Google Scholar]
- Sui, Z.; Salto, R.; Li, J.; Craik, C.; Ortiz de Montellano Paul, R. Inhibition of the HIV-1 and HIV-2 proteases by curcumin and curcumin boron complexes. Bioorg. Med. Chem. 1993, 1, 415–422. [Google Scholar] [CrossRef]
- James, J.S. Curcumin: Clinical trial finds no antiviral effect. AIDS treatment news. 1996, 2, 1–2. [Google Scholar]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.J.; Feng, M.Y.; Liu, H.; Ni, W.; Rauwolf, T.; Porco, J.A.; Yan, H.; He, L.; Liu, H.Y. Eucalyptusdimers a-c, dimeric phloroglucinol phellandrene meroterpenoids from eucalyptus robusta. Org. Lett. 2018, 20, 5066–5070. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, G.; Chen, X.; Chen, S.-X.; Gan, L.-S.; Yuan, T. Colocynthenins a–d, ring-a seco-cucurbitane triterpenoids from the fruits of citrullus colocynthis. J. Nat. Prod. 2018, 81, 2115–2119. [Google Scholar] [CrossRef]
- Hofstadler, S.A.; Sannes-Lowery, K.A. Applications of ESI-MS in drug discovery: Interrogation of noncovalent complexes. Nat. Rev. Drug Discov. 2006, 5, 585–595. [Google Scholar] [CrossRef]
- Haris, P.I. Can infrared spectroscopy provide information on protein-protein interactions? Biochem. Soc. Trans. 2010, 38, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Karthick, T.; Balachandran, V.; Perumal, S. Spectroscopic investigations, molecular interactions, and molecular docking studies on the potential inhibitor “thiophene-2-carboxylicacid”. Spectrochim. Acta A 2015, 141, 104–112. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Ali, S.; Badshah, A. Drug-DNA interactions and their study by uv-visible, fluorescence spectroscopies and cyclic voltametry. J. Photochem. Photobiol. B 2013, 124, 1–19. [Google Scholar] [CrossRef]
- Stephen, A.G.; Worthy, K.M.; Towler, E.; Mikovits, J.A.; Sei, S.; Roberts, P.; Yang, Q.E.; Akee, R.K.; Klausmeyer, P.; McCloud, T.G.; et al. Identification of HIV-1 nucleocapsid protein: Nucleic acid antagonists with cellular anti-HIV activity. Biochem. Biophys. Res. Commun. 2002, 296, 1228–1237. [Google Scholar] [CrossRef]
- De Paula, Q.A.; Mangrum, J.B.; Farrell, N.P. Zinc finger proteins as templates for metal ion exchange: Substitution effects on the c-finger of HIV nucleocapsid ncp7 using m(chelate) species (m = pt, pd, au). J. Inorg. Biochem. 2009, 103, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Tsotsoros, S.D.; Lutz, P.B.; Daniel, A.G.; Peterson, E.J.; de Paiva, R.E.F.; Rivera, E.; Qu, Y.; Bayse, C.A.; Farrell, N.P. Enhancement of the physicochemical properties of [pt(dien)(nucleobase)](2+) for HIV-NCp7 targeting. Chem. Sci. 2017, 8, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Tarui, M.; Doi, M.; Ishida, T. Enhancement of aromatic amino acid-nucleic acid base stacking interaction by metal coordination to base: Fluorescence study on a tryptophan-pt (ii)-guanine ternary complex. FEBS Lett. 1995, 370, 193–196. [Google Scholar] [CrossRef]
- Jinxia, D.; James, A.K.; Joseph, J.B.; Tino, S.; Raveendra, D.; Yves, P.; Nouri, N. Mining the NCI antiviral compounds for HIV-1 integrase inhibitors. Bioorg. Med. Chem. 2006, 14, 3785–3792. [Google Scholar]
- Sheng, Y.P.; Cao, K.M.; Li, J.; Hou, Z.H.; Yuan, S.M.; Huang, G.M.; Liu, H.K.; Liu, Y.Z. Selective targeting of the zinc finger domain of HIV nucleocapsid protein ncp7 with ruthenium complexes. Chem. Eur. J. 2018, 24, 19146–19151. [Google Scholar] [CrossRef]
- Demicheli, C.; Frezard, F.; Mangrum, J.B.; Farrell, N.P. Interaction of trivalent antimony with a cchc zinc finger domain: Potential relevance to the mechanism of action of antimonial drugs. Chem. Commun. 2008, 39, 4828–4830. [Google Scholar] [CrossRef]
- Abbehausen, C.; Peterson, E.J.; de Paiva, R.E.F.; Corbi, P.P.; Formiga, A.L.B.; Qu, Y.; Farrell, N.P. Gold(i)-phosphine-n-heterocycles: Biological activity and specific (ligand) interactions on the C-terminal HIV-NCp7 zinc finger. Inorg. Chem. 2013, 52, 11280–11287. [Google Scholar] [CrossRef] [PubMed]
- Quintal, S.M.; de Paula, Q.A.; Farrell, N.P. Zinc finger proteins as templates for metal ion exchange and ligand reactivity. Chemical and biological consequences. Metallomics 2011, 3, 121–139. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
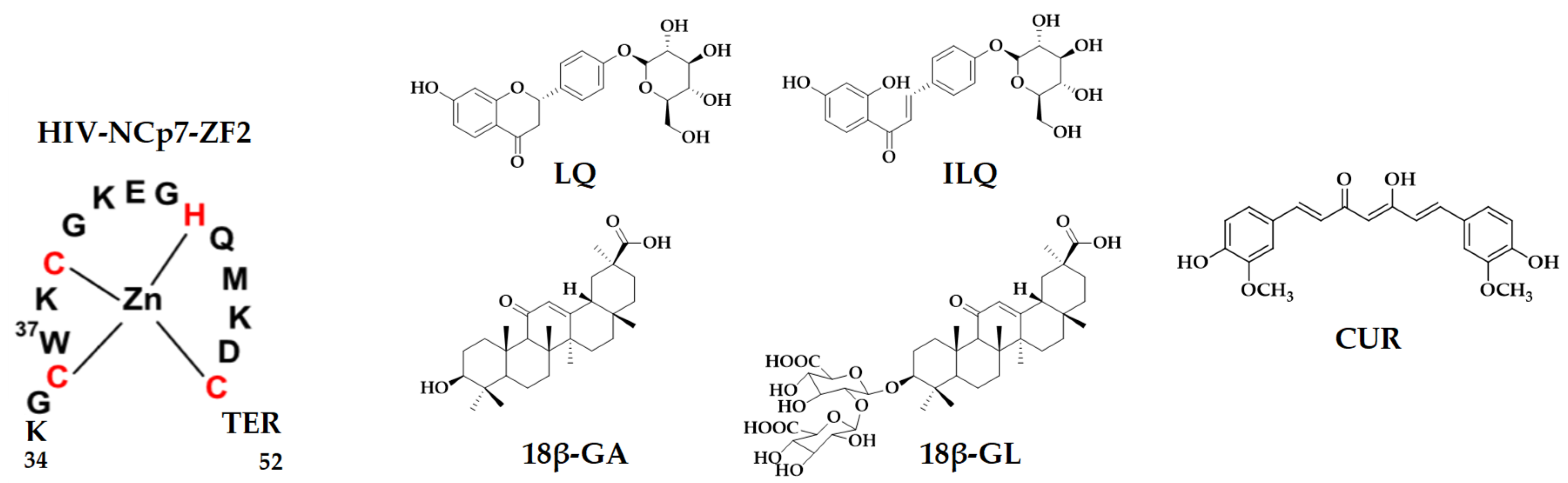


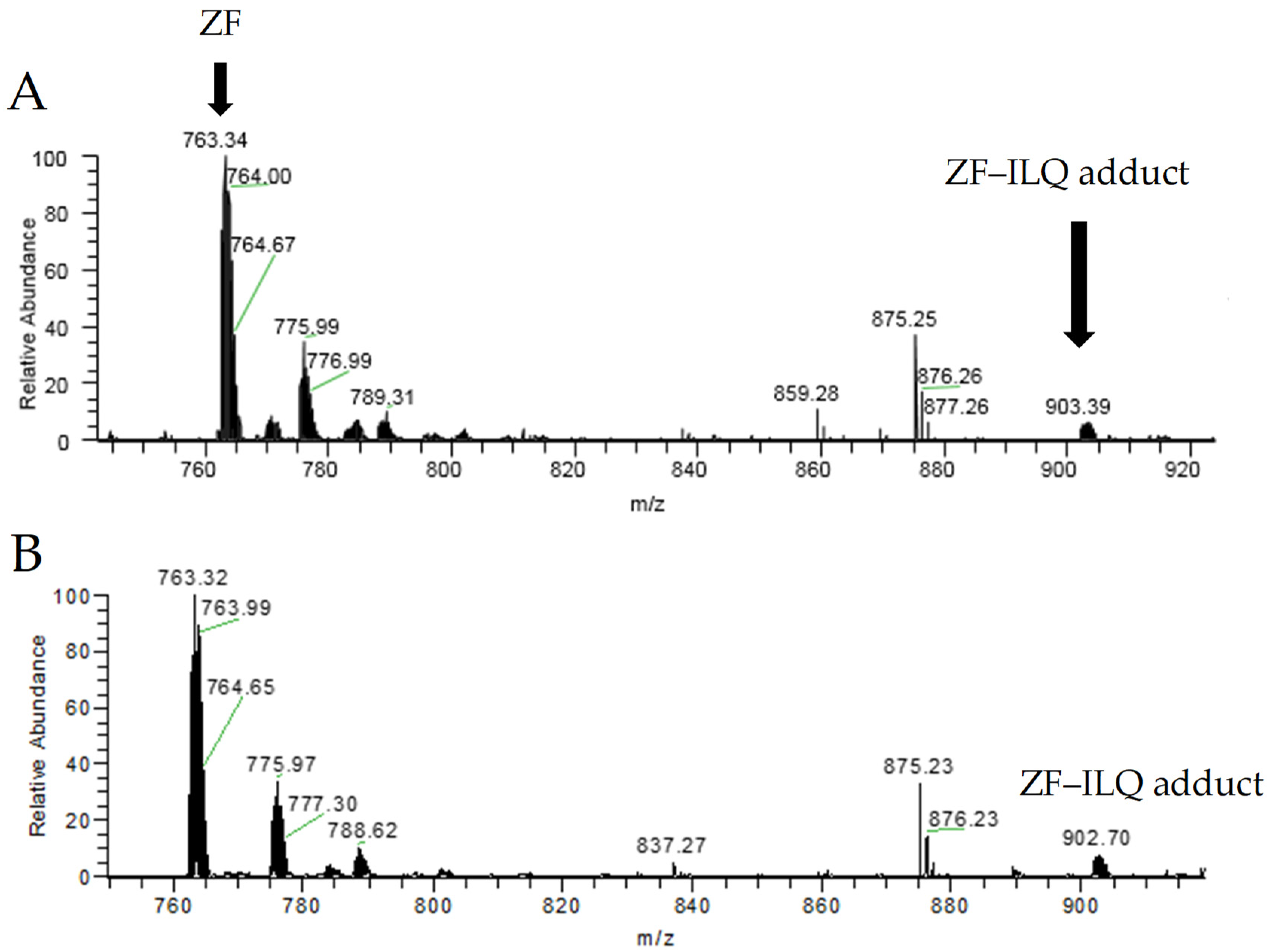

| Species | Formula | Mono Observed m/z |
|---|---|---|
| ZF | C90H144N30O28S4Zn | 762.62 |
| ZF–ILQ adduct | C111H166N30O37S4Zn | 902.02 |
| Oxipeptide–ILQ | C111H166N30O37S4 | 880.71 |
| ZF–CUR adduct | C111H164N30O37S4Zn | 885.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Wei, Y.; Wang, M.; Yan, P.; Jiang, H.; Du, Z. Interaction of Natural Compounds in Licorice and Turmeric with HIV-NCp7 Zinc Finger Domain: Potential Relevance to the Mechanism of Antiviral Activity. Molecules 2021, 26, 3563. https://doi.org/10.3390/molecules26123563
Wang R, Wei Y, Wang M, Yan P, Jiang H, Du Z. Interaction of Natural Compounds in Licorice and Turmeric with HIV-NCp7 Zinc Finger Domain: Potential Relevance to the Mechanism of Antiviral Activity. Molecules. 2021; 26(12):3563. https://doi.org/10.3390/molecules26123563
Chicago/Turabian StyleWang, Runjing, Yinyu Wei, Meiqin Wang, Pan Yan, Hongliang Jiang, and Zhifeng Du. 2021. "Interaction of Natural Compounds in Licorice and Turmeric with HIV-NCp7 Zinc Finger Domain: Potential Relevance to the Mechanism of Antiviral Activity" Molecules 26, no. 12: 3563. https://doi.org/10.3390/molecules26123563
APA StyleWang, R., Wei, Y., Wang, M., Yan, P., Jiang, H., & Du, Z. (2021). Interaction of Natural Compounds in Licorice and Turmeric with HIV-NCp7 Zinc Finger Domain: Potential Relevance to the Mechanism of Antiviral Activity. Molecules, 26(12), 3563. https://doi.org/10.3390/molecules26123563






