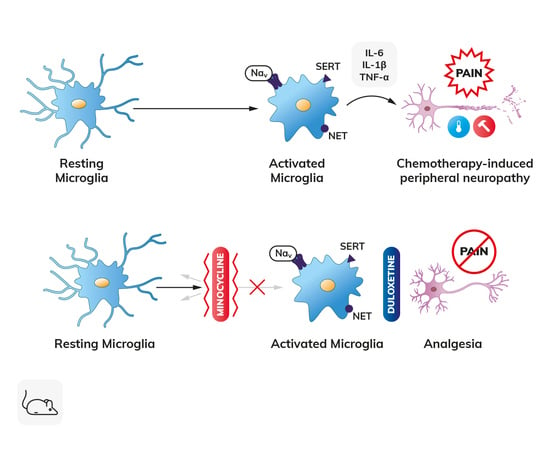
The Microglial Activation Inhibitor Minocycline, Used Alone and in Combination with Duloxetine, Attenuates Pain Caused by Oxaliplatin in Mice
Abstract
:1. Introduction
2. Results
2.1. Experimental Design
2.2. Effect of Test Drugs Used Alone in a Single- or Repeated-Dose Protocol on Tactile Allodynia in Oxaliplatin-Treated Mice (Von Frey Test)
2.2.1. Effect of Minocycline on Tactile Allodynia
2.2.2. Effect of Duloxetine on Tactile Allodynia
2.3. Effect of Test Drugs Used in Combination in a Single- or Repeated-Dose Protocol on Tactile Allodynia in Oxaliplatin-Treated Mice (Von Frey Test)
2.3.1. Effect of Minocycline Combined with Ambroxol on Tactile Allodynia
2.3.2. Effect of Minocycline Combined with Duloxetine on Tactile Allodynia
2.4. Duration of the Effect on Tactile Allodynia
2.5. Influence of Test Drugs Used Alone in a Single- or Repeated-Dose Protocols on Cold Hyperalgesia in Oxaliplatin-Treated Mice (Cold Plate Test)
2.5.1. Effect of Minocycline on Cold Hyperalgesia
2.5.2. Effect of Duloxetine on Cold Hyperalgesia
2.6. Influence of Test Drugs Used in Combination in a Single- or Repeated-Dose Protocol on Cold Hyperalgesia in Oxaliplatin-Treated Mice (Cold Plate Test)
2.6.1. Effect of Minocycline Combined with Ambroxol on Cold Hyperalgesia
2.6.2. Effect of Minocycline Combined with Duloxetine on Cold Hyperalgesia
2.7. Duration of the Effect on Cold Hyperalgesia
2.8. Influence on Motor Coordination-Rotarod Test
3. Discussion
4. Materials and Methods
4.1. Animals and Housing Conditions
4.2. Chemicals and Drug Administration Scheme
4.3. Behavioral Tests
4.3.1. Induction of CIPN
4.3.2. Assessment of Tactile Allodynia—Von Frey Test
4.3.3. Assessment of Cold Hyperalgesia—Cold Plate Test
4.3.4. Assessment of Motor Coordination—Rotarod Test
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cav | Voltage-gated calcium channel |
| CDT | Combination drug therapy |
| CIPN | Chemotherapy-induced peripheral neuropathy |
| Dulo | Duloxetine |
| Mino | Minocycline |
| Nav | Voltage-gated sodium channel |
| Oxa | Oxaliplatin |
| Veh | Vehicle |
References
- Flatters, S.J.L.; Dougherty, P.M.; Colvin, L.A. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): A narrative review. Br. J. Anaesth. 2017, 119, 737–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miltenburg, N.C.; Boogerd, W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat. Rev. 2014, 40, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Pachman, D.R.; Qin, R.; Seisler, D.; Smith, E.M.; Kaggal, S.; Novotny, P.; Ruddy, K.J.; Lafky, J.M.; Ta, L.E.; Beutler, A.S.; et al. Comparison of oxaliplatin and paclitaxel-induced neuropathy (Alliance A151505). Support. Care Cancer 2016, 24, 5059–5068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pachman, D.R.; Qin, R.; Seisler, D.K.; Smith, E.M.; Beutler, A.S.; Ta, L.E.; Lafky, J.M.; Wagner-Johnston, N.D.; Ruddy, K.J.; Dakhil, S.; et al. Clinical course of oxaliplatin-induced neuropathy: Results from the randomized phase III trial N08CB (Alliance). J. Clin. Oncol. 2015, 33, 3416–3422. [Google Scholar] [CrossRef] [Green Version]
- Staff, N.P.; Cavaletti, G.; Islam, B.; Lustberg, M.; Psimaras, D.; Tamburin, S. Platinum-induced peripheral neurotoxicity: From pathogenesis to treatment. J. Peripher. Nerv. Syst. 2019, 24, S26–S39. [Google Scholar] [CrossRef]
- Starobova, H.; Vetter, I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef]
- Colvin, L.A. Chemotherapy-induced peripheral neuropathy (CIPN): Where are we now? Pain 2019, 160 (Suppl 1), S1–S10. [Google Scholar] [CrossRef]
- Ibrahim, E.Y.; Ehrlich, B.E. Prevention of chemotherapy-induced peripheral neuropathy: A review of recent findings. Crit. Rev. Oncol. Hematol. 2020, 145, 102831. [Google Scholar] [CrossRef]
- Argyriou, A.A.; Bruna, J.; Park, S.B.; Cavaletti, G. Emerging pharmacological strategies for the management of chemotherapy-induced peripheral neurotoxicity (CIPN), based on novel CIPN mechanisms. Expert. Rev. Neurother. 2020, 20, 1005–1016. [Google Scholar] [CrossRef]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [Green Version]
- Trecarichi, A.; Flatters, S.J.L. Mitochondrial dysfunction in the pathogenesis of chemotherapy-induced peripheral neuropathy. Int. Rev. Neurobiol. 2019, 145, 83–126. [Google Scholar] [CrossRef]
- Caputi, F.F.; Di Cesare Mannelli, L.; Rullo, L.; Micheli, L.; Stamatakos, S.; Posa, L.; Ghelardini, C.; Romualdi, P.; Candeletti, S. The active second-generation proteasome inhibitor oprozomib reverts the oxaliplatin-induced neuropathy symptoms. Biochem. Pharmacol. 2020, 182, 114255. [Google Scholar] [CrossRef]
- Sisignano, M.; Baron, R.; Scholich, K.; Geisslinger, G. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat. Rev. Neurol. 2014, 10, 694–707. [Google Scholar] [CrossRef]
- Brewer, J.R.; Morrison, G.; Dolan, M.E.; Fleming, G.F. Chemotherapy-induced peripheral neuropathy: Current status and progress. Gynecol Oncol. 2016, 140, 176–183. [Google Scholar] [CrossRef] [Green Version]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef]
- Glimelius, B.; Manojlovic, N.; Pfeiffer, P.; Mosidze, B.; Kurteva, G.; Karlberg, M.; Mahalingam, D.; Buhl-Jensen, P.; Kowalski, J.; Bengtson, M.; et al. Persistent prevention of oxaliplatin-induced peripheral neuropathy using calmangafodipir (PledOx®): A placebo-controlled randomised phase II study (PLIANT). Acta. Oncol. 2018, 57, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Canta, A.; Chiorazzi, A.; Pozzi, E.; Fumagalli, G.; Monza, L.; Meregalli, C.; Carozzi, V.A.; Rodriguez-Menendez, V.; Oggioni, N.; Näsström, J.; et al. Calmangafodipir reduces sensory alterations and prevents intraepidermal nerve fibers loss in a mouse model of oxaliplatin induced peripheral neurotoxicity. Antioxidants 2020, 9, 594. [Google Scholar] [CrossRef]
- Ma, J.; Kavelaars, A.; Dougherty, P.M.; Heijnen, C.J. Beyond symptomatic relief for chemotherapy-induced peripheral neuropathy: Targeting the source. Cancer 2018, 124, 2289–2298. [Google Scholar] [CrossRef]
- Nishikawa, T.; Hasegawa, K.; Shintani, D.; Yano, Y.; Sato, S.; Yabuno, A.; Kurosaki, A.; Yoshida, H.; Fujiwara, K. Combination therapy of pregabalin with tramadol for treatment of peripheral neuropathy in patients with gynecological cancer receiving taxane containing chemotherapy. Gan Kagaku Ryoho 2017, 44, 227–231. [Google Scholar]
- Parvathy, S.S.; Masocha, W. Coadministration of indomethacin and minocycline attenuates established paclitaxel-induced neuropathic thermal hyperalgesia: Involvement of cannabinoid CB1 receptors. Sci. Rep. 2015, 5, 10541. [Google Scholar] [CrossRef] [Green Version]
- Wadia, R.J.; Stolar, M.; Grens, C.; Ehrlich, B.E.; Chao, H.H. The prevention of chemotherapy induced peripheral neuropathy by concurrent treatment with drugs used for bipolar disease: A retrospective chart analysis in human cancer patients. Oncotarget 2017, 9, 7322–7331. [Google Scholar] [CrossRef] [Green Version]
- Garrido-Mesa, N.; Zarzuelo, A.; Gálvez, J. Minocycline: Far beyond an antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Di Cesare Mannelli, L.; Pacini, A.; Micheli, L.; Femia, A.P.; Maresca, M.; Zanardelli, M.; Vannacci, A.; Gallo, E.; Bilia, A.R.; Caderni, G.; et al. Astragali radix: Could it be an adjuvant for oxaliplatin-induced neuropathy? Sci. Rep. 2017, 7, 42021. [Google Scholar] [CrossRef] [Green Version]
- Di Cesare Mannelli, L.; Pacini, A.; Micheli, L.; Tani, A.; Zanardelli, M.; Ghelardini, C. Glial role in oxaliplatin-induced neuropathic pain. Exp. Neurol. 2014, 261, 22–33. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, W. The role of satellite glial cells, astrocytes, and microglia in oxaliplatin-induced neuropathic pain. Biomedicines 2020, 8, 324. [Google Scholar] [CrossRef]
- Aromolaran, K.A.; Goldstein, P.A. Ion channels and neuronal hyperexcitability in chemotherapy-induced peripheral neuropathy. Mol. Pain 2017, 13, 174480691771469. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zhang, Y.-Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.-R. Microglia in pain: Detrimental and protective roles in pathogenesis and resolution of pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [Green Version]
- Deuis, J.R.; Zimmermann, K.; Romanovsky, A.A.; Possani, L.D.; Cabot, P.J.; Lewis, R.J.; Vetter, I. An animal model of oxaliplatin-induced cold allodynia reveals a crucial role for Nav1.6 in peripheral pain pathways. Pain 2013, 154, 1749–1757. [Google Scholar] [CrossRef] [Green Version]
- Di Cesare Mannelli, L.; Pacini, A.; Corti, F.; Boccella, S.; Luongo, L.; Esposito, E.; Cuzzocrea, S.; Maione, S.; Calignano, A.; Ghelardini, C. Antineuropathic profile of N-palmitoylethanolamine in a rat model of oxaliplatin-induced neurotoxicity. PLoS ONE 2015, 10, e0128080. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Chung, Y.; Choi, S.; Min, B.-I.; Kim, S. Duloxetine Protects against oxaliplatin-induced neuropathic pain and spinal neuron hyperexcitability in rodents. Int. J. Mol. Sci. 2017, 18, 2626. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Li, D.X.; Yoon, H.; Go, D.; Quan, F.S.; Min, B.-I.; Kim, S.K. Serotonergic mechanism of the relieving effect of bee venom acupuncture on oxaliplatin-induced neuropathic cold allodynia in rats. Bmc Complement. Altern. Med. 2014, 14, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Lee, J.; Choi, C.; Kim, J.; Kim, S.; Kim, W. The analgesic effect of venlafaxine and its mechanism on oxaliplatin-induced neuropathic pain in mice. Int. J. Mol. Sci. 2019, 20, 1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sittl, R.; Lampert, A.; Huth, T.; Schuy, E.T.; Link, A.S.; Fleckenstein, J.; Alzheimer, C.; Grafe, P.; Carr, R.W. Anticancer drug oxaliplatin induces acute cooling-aggravated neuropathy via sodium channel subtype NaV1.6-resurgent and persistent current. Proc. Natl. Acad. Sci. 2012, 109, 6704–6709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaida, W.; Klinder, K.; Arndt, K.; Weiser, T. Ambroxol, a Nav1.8-preferring Na+ channel blocker, effectively suppresses pain symptoms in animal models of chronic, neuropathic and inflammatory pain. Neuropharmacology 2005, 49, 1220–1227. [Google Scholar] [CrossRef]
- Leffler, A.; Reckzeh, J.; Nau, C. Block of sensory neuronal Na+ channels by the secreolytic ambroxol is associated with an interaction with local anesthetic binding sites. Eur. J. Pharmacol. 2010, 630, 19–28. [Google Scholar] [CrossRef]
- Weiser, T. Comparison of the effects of four Na+ channel analgesics on TTX-resistant Na+ currents in rat sensory neurons and recombinant Nav1.2 channels. Neurosci. Lett. 2006, 395, 179–184. [Google Scholar] [CrossRef]
- Horishita, T.; Yanagihara, N.; Ueno, S.; Okura, D.; Horishita, R.; Minami, T.; Ogata, Y.; Sudo, Y.; Uezono, Y.; Sata, T.; et al. Antidepressants inhibit Nav1.3, Nav1.7, and Nav1.8 neuronal voltage-gated sodium channels more potently than Nav1.2 and Nav1.6 channels expressed in Xenopus oocytes. Naunyn. Schmiedebergs Arch. Pharmacol. 2017, 390, 1255–1270. [Google Scholar] [CrossRef]
- Stoetzer, C.; Papenberg, B.; Doll, T.; Völker, M.; Heineke, J.; Stoetzer, M.; Wegner, F.; Leffler, A. Differential inhibition of cardiac and neuronal Na+ channels by the selective serotonin-norepinephrine reuptake inhibitors duloxetine and venlafaxine. Eur. J. Pharmacol. 2016, 783, 1–10. [Google Scholar] [CrossRef]
- Pappalardo, L.W.; Black, J.A.; Waxman, S.G. Sodium channels in astroglia and microglia. Glia 2016, 64, 1628–1645. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.A.; Yamauchi, Y.; Horikawa, H.; Monji, A.; Mizoguchi, Y.; Seki, Y.; Hayakawa, K.; Utsumi, H.; Kanba, S. Neurotransmitters, psychotropic drugs and microglia: Clinical implications for psychiatry. Curr. Med. Chem. 2013, 20, 331–344. [Google Scholar] [CrossRef]
- Furgała-Wojas, A.; Kowalska, M.; Nowaczyk, A.; Fijałkowski, Ł.; Sałat, K. Comparison of bromhexine and its active metabolite-ambroxol as potential analgesics reducing oxaliplatin-induced neuropathic pain-pharmacodynamic and molecular docking studies. Curr. Drug Metab. 2020, 21, 548–561. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef] [Green Version]
- Sałat, K.; Podkowa, A.; Kowalczyk, P.; Kulig, K.; Dziubina, A.; Filipek, B.; Librowski, T. Anticonvulsant active inhibitor of GABA transporter subtype 1, tiagabine, with activity in mouse models of anxiety, pain and depression. Pharmacol. Rep. 2015, 67, 465–472. [Google Scholar] [CrossRef]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [Green Version]
- Möller, T.; Bard, F.; Bhattacharya, A.; Biber, K.; Campbell, B.; Dale, E.; Eder, C.; Gan, L.; Garden, G.A.; Hughes, Z.A.; et al. Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor. Glia 2016, 64, 1788–1794. [Google Scholar] [CrossRef]
- Hershman, D.L.; Lacchetti, C.; Dworkin, R.H.; Lavoie Smith, E.M.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Gavin, P.; Lavino, A.; Lustberg, M.B.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2014, 32, 1941–1967. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Liang, S.; Fang, L.; Wu, M.; Cheng, H.; Mi, X.; Ding, Y. Low-dose minocycline mediated neuroprotection on retinal ischemia-reperfusion injury of mice. Mol. Vis. 2018, 24, 367–378. [Google Scholar]
- Meuwissen, N.; Van Zundert, J.; Boer, W.; Heylen, R.; Vissers, K.; Vanelderen, P. Minocycline dose-dependently reduces neuropathic pain behavior in a rat chonic constriction injury model. Eur. J. Anaesthesiol. 2013, 30, 213. [Google Scholar] [CrossRef]
- Banno, T.; Omura, T.; Masaki, N.; Arima, H.; Xu, D.; Okamoto, A.; Costigan, M.; Latremoliere, A.; Matsuyama, Y.; Setou, M. Arachidonic acid containing phosphatidylcholine increases due to microglial activation in ipsilateral spinal dorsal horn following spared sciatic nerve injury. PLoS ONE 2017, 12, e0177595. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Tsuda, M. Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 2018, 19, 138–152. [Google Scholar] [CrossRef]
- Kosaka, Y.; Yafuso, T.; Shimizu-Okabe, C.; Kim, J.; Kobayashi, S.; Okura, N.; Ando, H.; Okabe, A.; Takayama, C. Development and persistence of neuropathic pain through microglial activation and KCC2 decreasing after mouse tibial nerve injury. Brain Res. 2020, 1733, 146718. [Google Scholar] [CrossRef]
- Lei, Y.; Sun, Y.; Lu, C.; Ma, Z.; Gu, X. Activated glia increased the level of proinflammatory cytokines in a resiniferatoxin-induced neuropathic pain rat model. Reg. Anesth. Pain Med. 2016, 41, 744–749. [Google Scholar] [CrossRef]
- Salat, K.; Gryzlo, B.; Kulig, K. Experimental drugs for neuropathic pain. Curr. Neuropharmacol. 2018, 16, 1193–1209. [Google Scholar] [CrossRef]
- Tsuda, M. Microglia in the CNS and neuropathic pain. Adv. Exp. Med. Biol. 2018, 1099, 77–91. [Google Scholar] [CrossRef]
- Miyamoto, K.; Kume, K.; Ohsawa, M. Role of microglia in mechanical allodynia in the anterior cingulate cortex. J. Pharmacol. Sci. 2017, 134, 158–165. [Google Scholar] [CrossRef]
- Yasui, M.; Yoshimura, T.; Takeuchi, S.; Tokizane, K.; Tsuda, M.; Inoue, K.; Kiyama, H. A chronic fatigue syndrome model demonstrates mechanical allodynia and muscular hyperalgesia via spinal microglial activation. Glia 2014, 62, 1407–1417. [Google Scholar] [CrossRef]
- Wang, D.; Couture, R.; Hong, Y. Activated microglia in the spinal cord underlies diabetic neuropathic pain. Eur. J. Pharmacol. 2014, 728, 59–66. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Xue, R.; Fan, S.-Y.; Fan, Q.-Y.; An, L.; Li, J.; Zhu, L.; Ran, Y.-H.; Zhang, L.-M.; Zhong, B.-H.; et al. Ammoxetine attenuates diabetic neuropathic pain through inhibiting microglial activation and neuroinflammation in the spinal cord. J. Neuroinflammation 2018, 15, 176. [Google Scholar] [CrossRef] [Green Version]
- Qin, B.; Luo, N.; Li, Y.; Gong, D.; Zheng, J.; Tan, X.; Zheng, W. Protective effect of gastrodin on peripheral neuropathy induced by anti-tumor treatment with vincristine in rat models. Drug Chem. Toxicol. 2018, 44, 84–91. [Google Scholar] [CrossRef]
- Hu, L.-Y.; Zhou, Y.; Cui, W.-Q.; Hu, X.-M.; Du, L.-X.; Mi, W.-L.; Chu, Y.-X.; Wu, G.-C.; Wang, Y.-Q.; Mao-Ying, Q.-L. Triggering receptor expressed on myeloid cells 2 (TREM2) dependent microglial activation promotes cisplatin-induced peripheral neuropathy in mice. Brain Behav. Immun. 2018, 68, 132–145. [Google Scholar] [CrossRef]
- Wen, Y.-R.; Tan, P.-H.; Cheng, J.-K.; Liu, Y.-C.; Ji, R.-R. Microglia: A promising target for treating neuropathic and postoperative pain, and morphine tolerance. J. Med. Assoc. 2011, 110, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Sung, C.-S.; Cherng, C.-H.; Wen, Z.-H.; Chang, W.-K.; Huang, S.-Y.; Lin, S.-L.; Chan, K.-H.; Wong, C.-S. Minocycline and fluorocitrate suppress spinal nociceptive signaling in intrathecal IL-1β-induced thermal hyperalgesic rats. Glia 2012, 60, 2004–2017. [Google Scholar] [CrossRef] [PubMed]
- Zama, M.; Fujita, S.; Nakaya, Y.; Tonogi, M.; Kobayashi, M. Preceding administration of minocycline suppresses plastic changes in cortical excitatory propagation in the model rat with partial infraorbital nerve ligation. Front. Neurol. 2019, 10, 1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastos, L.F.S.; Merlo, L.A.; Rocha, L.T.S.; Coelho, M.M. Characterization of the antinociceptive and anti-inflammatory activities of doxycycline and minocycline in different experimental models. Eur. J. Pharmacol. 2007, 576, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ismail, C.A.N.; Suppian, R.; Aziz, C.B.A.; Long, I. Minocycline attenuates the development of diabetic neuropathy by modulating DREAM and BDNF protein expression in rat spinal cord. J. Diabetes Metab. Disord. 2019, 18, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wei, H.; Piirainen, S.; Chen, Z.; Kalso, E.; Pertovaara, A.; Tian, L. Spinal versus brain microglial and macrophage activation traits determine the differential neuroinflammatory responses and analgesic effect of minocycline in chronic neuropathic pain. Brain Behav. Immun. 2016, 58, 107–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starobova, H.; Mueller, A.; Allavena, R.; Lohman, R.J.; Sweet, M.J.; Vetter, I. Minocycline prevents the development of mechanical allodynia in mouse models of vincristine-induced peripheral neuropathy. Front. Neurosci. 2019, 13, 653. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.-Q.; Liu, D.-Q.; Chen, S.-P.; Sun, J.; Wang, X.-M.; Tian, Y.-K.; Wu, W.; Ye, D.-W. Minocycline as a promising therapeutic strategy for chronic pain. Pharmacol. Res. 2018, 134, 305–310. [Google Scholar] [CrossRef]
- Starobova, H.; Mueller, A.; Deuis, J.R.; Carter, D.A.; Vetter, I. Inflammatory and Neuropathic Gene Expression Signatures of Chemotherapy-Induced Neuropathy Induced by Vincristine, Cisplatin, and Oxaliplatin in C57BL/6J Mice. J. Pain 2020, 21, 182–194. [Google Scholar] [CrossRef]
- Pachman, D.R.; Dockter, T.; Zekan, P.J.; Fruth, B.; Ruddy, K.J.; Ta, L.E.; Lafky, J.M.; Dentchev, T.; Le-Lindqwister, N.A.; Sikov, W.M.; et al. A pilot study of minocycline for the prevention of paclitaxel-associated neuropathy: ACCRU study RU221408I. Suppor. Care Cancer 2017, 25, 3407–3416. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Pacini, A.; Bonaccini, L.; Zanardelli, M.; Mello, T.; Ghelardini, C. Morphologic features and glial activation in rat oxaliplatin-dependent neuropathic pain. J. Pain 2013, 14, 1585–1600. [Google Scholar] [CrossRef]
- Boyette-Davis, J.; Dougherty, P.M. Protection against oxaliplatin-induced mechanical hyperalgesia and intraepidermal nerve fiber loss by minocycline. Exp. Neurol. 2011, 229, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Padi, S.S.V.; Kulkarni, S.K. Minocycline prevents the development of neuropathic pain, but not acute pain: Possible anti-inflammatory and antioxidant mechanisms. Eur. J. Pharmacol. 2008, 601, 79–87. [Google Scholar] [CrossRef]
- Hama, A.T.; Plum, A.W.; Sagen, J. Antinociceptive effect of ambroxol in rats with neuropathic spinal cord injury pain. Pharmacol. Biochem. Behav. 2010, 97, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Kern, K.; Weiser, T. Topical ambroxol for the treatment of neuropathic pain. Der. Schmerz 2015, 29, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Kern, K.; Weiser, T. Topical ambroxol for the treatment of neuropathic pain: A first clinical observation. German version. Der. Schmerz 2015, 29, 632–640. [Google Scholar] [CrossRef] [Green Version]
- Kern, K.; Schwickert-Nieswandt, M.; Maihöfner, C.; Gaul, C. Topical ambroxol 20% for the treatment of classical trigeminal neuralgia-a new option? initial clinical case observations. Headache 2019, 59, 418–429. [Google Scholar] [CrossRef]
- Amin, B.; Hajhashemi, V.; Hosseinzadeh, H. Minocycline potentiates the anti-hyperalgesic effect of ceftriaxone in CCI-induced neuropathic pain in rats. Res. Pharmacol. Sci. 2015, 10, 34–42. [Google Scholar]
- Finsterer, J.; Habitzl, W. Incapacitating generalised myalgias and muscle stiffness under duloxetine and aripiprazole. Int. J. Clin. Pract. 2020, 74, e13487. [Google Scholar] [CrossRef]
- Masocha, W.; Thomas, A. Indomethacin plus minocycline coadministration relieves chemotherapy and antiretroviral drug-induced neuropathic pain in a cannabinoid receptors-dependent manner. J. Pharmacol. Sci. 2019, 139, 325–332. [Google Scholar] [CrossRef]
- Sałat, K.; Furgała, A.; Malikowska-Racia, N. Searching for analgesic drug candidates alleviating oxaliplatin-induced cold hypersensitivity in mice. Chem. Biol. Drug Des. 2019, 93, 1061–1072. [Google Scholar] [CrossRef]
- Jiang, B.; Chen, J.-L.; Lou, H.-G.; Yu, L.-Y.; Shen, H.-H.; Ruan, Z.R. Pharmacokinetic and bioequivalence study of three oral formulations of Ambroxol 30 mg: A randomized, three-period crossover comparison in healthy volunteers. Int. J. Clin. Pharmacol. 2014, 52, 920–926. [Google Scholar] [CrossRef]
- Scientific discussion. Available online: https://www.ema.europa.eu/en/documents/scientific-discussion/cymbalta-epar-scientific-discussion_en.pdf (accessed on 25 May 2021).
- Drugbank. Available online: https://go.drugbank.com/drugs/DB01017 (accessed on 25 May 2021).
- Sałat, K.; Furgała, A.; Sałat, R. Interventional and preventive effects of aripiprazole and ceftriaxone used alone or in combination on oxaliplatin-induced tactile and cold allodynia in mice. Biomed. Pharmacol. 2019, 111, 882–890. [Google Scholar] [CrossRef]
- Kremer, M.; Yalcin, I.; Goumon, Y.; Wurtz, X.; Nexon, L.; Daniel, D.; Megat, S.; Ceredig, R.A.; Ernst, C.; Turecki, G.; et al. A Dual Noradrenergic Mechanism for the Relief of Neuropathic Allodynia by the Antidepressant Drugs Duloxetine and Amitriptyline. J. Neurosci. 2018, 38, 9934–9954. [Google Scholar] [CrossRef] [Green Version]
- Sałat, K.; Gawlik, K.; Witalis, J.; Pawlica-Gosiewska, D.; Filipek, B.; Solnica, B.; Więckowski, K.; Malawska, B. Evaluation of antinociceptive and antioxidant properties of 3-[4-(3-trifluoromethyl-phenyl)-piperazin-1-yl]-dihydrofuran-2-one in mice. Naunyn. Schmiedebergs Arch. Pharmacol. 2013, 386, 493–505. [Google Scholar] [CrossRef] [Green Version]
- Sałat, K. Chemotherapy-induced peripheral neuropathy: Part 1-current state of knowledge and perspectives for pharmacotherapy. Pharmacol. Rep. 2020, 72, 486–507. [Google Scholar] [CrossRef]
- Sałat, K. Chemotherapy-induced peripheral neuropathy-part 2: Focus on the prevention of oxaliplatin-induced neurotoxicity. Pharmacol. Rep. 2020, 72, 508–527. [Google Scholar] [CrossRef]
- Mogil, J.S.; Bailey, A.L. Sex and gender differences in pain and analgesia. Prog. Brain Res. 2010, 186, 141–157. [Google Scholar] [CrossRef]
- Sorge, R.E.; Totsch, S.K. Sex Differences in Pain. J. Neurosci. Res. 2017, 95, 1271–1281. [Google Scholar] [CrossRef]
- Guindon, J.; Blanton, H.; Brauman, S.; Donckels, K.; Narasimhan, M.; Benamar, K. Sex Differences in a rodent model of HIV-1-associated neuropathic pain. Int. J. Mol. Sci. 2019, 20, 1196. [Google Scholar] [CrossRef] [Green Version]







| Treatment (Drug, Dose, Protocol) | Mean Time [s] (±SEM) on the Rotarod Revolving at: | ||
|---|---|---|---|
| 6 rpm | 18 rpm | 24 rpm | |
| Veh | 60.0 ± 0.0 | 60.0 ± 0.0 | 47.0 ± 8.5 |
| Veh + oxa | 60.0 ± 0.0 | 60.0 ± 0.0 | 60.0 ± 0.0 |
| Oxa + Mino 50 single day 1 | 54.8 ± 5.3 | 60.0 ± 0.0 | 53.0 ± 7.0 |
| Oxa + Mino 50 single day 7 | 60.0 ± 0.0 | 60.0 ± 0.0 | 60.0 ± 0.0 |
| Oxa + Mino 50 repeated day 7 | 60.0 ± 0.0 | 60.0 ± 0.0 | 60.0 ± 0.0 |
| Oxa + Mino 100 single day 1 | 46.8 ± 8.7 | 48.3 ± 7.7 | 36.5 ± 9.1 |
| Oxa + Mino 100 single day 7 | 46.0 ± 5.6 | 43.8 ± 10.2 | 42.7 ± 9.6 |
| Oxa + Mino 100 repeated day 7 | 51.6 ± 5.5 | 60.0 ± 0.0 | 55.8 ± 2.8 |
| Oxa + Mino 50 + Am 90 single day 1 | 57.5 ± 2.5 | 60.0 ± 0.0 | 58.3 ± 1.8 |
| Oxa + Mino 50 + Am 90 single day 7 | 60.0 ± 0.0 | 60.0 ± 0.0 | 60.0 ± 0.0 |
| Oxa + Mino 50 + Am 90 repeated day 7 | 60.0 ± 0.0 | 60.0 ± 0.0 | 60.0 ± 0.0 |
| Oxa + Dulo 10 single day 1 | 60.0 ± 0.0 | 49.6 ± 5.2 | 54.3 ± 3.8 |
| Oxa + Dulo 10 single day 7 | 52.9 ± 4.7 | 16.8 ± 3.5 **** | 9.6 ± 1.7 **** |
| Oxa + Dulo 10 repeated day 7 | 60.0 ± 0.0 | 56.5 ± 2.3 | 56.0 ± 2.6 |
| Oxa + Dulo 30 single day 1 | 49.3 ± 7.0 | 36.0 ± 6.8 ** | 23.5 ± 8.3 *** |
| Oxa + Dulo 30 single day 7 | 44.7 2 6.0 | 49.5 ± 5.6 | 16.0 ± 2.8 **** |
| Oxa + Dulo 30 repeated day 7 | 54.8 ± 3.4 | 60.0 ± 0.0 | 28.8 ± 8.2 ** |
| Oxa + Mino 50 + Dulo 10 single day 1 | 60.0 ± 0.0 | 49.8 ± 6.7 | 57.4 ± 1.7 |
| Oxa + Mino 50 + Dulo 10 single day 7 | 60.0 ± 0.0 | 60.0 ± 0.0 | 48.9 ± 7.3 |
| Oxa + Mino 50 + Dulo 10 repeated day 7 | 60.0 ± 0.0 | 49.4 ± 7.0 | 47.8 ± 7.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sałat, K.; Furgała-Wojas, A.; Sałat, R. The Microglial Activation Inhibitor Minocycline, Used Alone and in Combination with Duloxetine, Attenuates Pain Caused by Oxaliplatin in Mice. Molecules 2021, 26, 3577. https://doi.org/10.3390/molecules26123577
Sałat K, Furgała-Wojas A, Sałat R. The Microglial Activation Inhibitor Minocycline, Used Alone and in Combination with Duloxetine, Attenuates Pain Caused by Oxaliplatin in Mice. Molecules. 2021; 26(12):3577. https://doi.org/10.3390/molecules26123577
Chicago/Turabian StyleSałat, Kinga, Anna Furgała-Wojas, and Robert Sałat. 2021. "The Microglial Activation Inhibitor Minocycline, Used Alone and in Combination with Duloxetine, Attenuates Pain Caused by Oxaliplatin in Mice" Molecules 26, no. 12: 3577. https://doi.org/10.3390/molecules26123577