Modelling and Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from Black Quinoa by Response Surface Methodology
Abstract
1. Introduction
2. Results
2.1. Optimization of Phenolic Compound Extraction by RSM
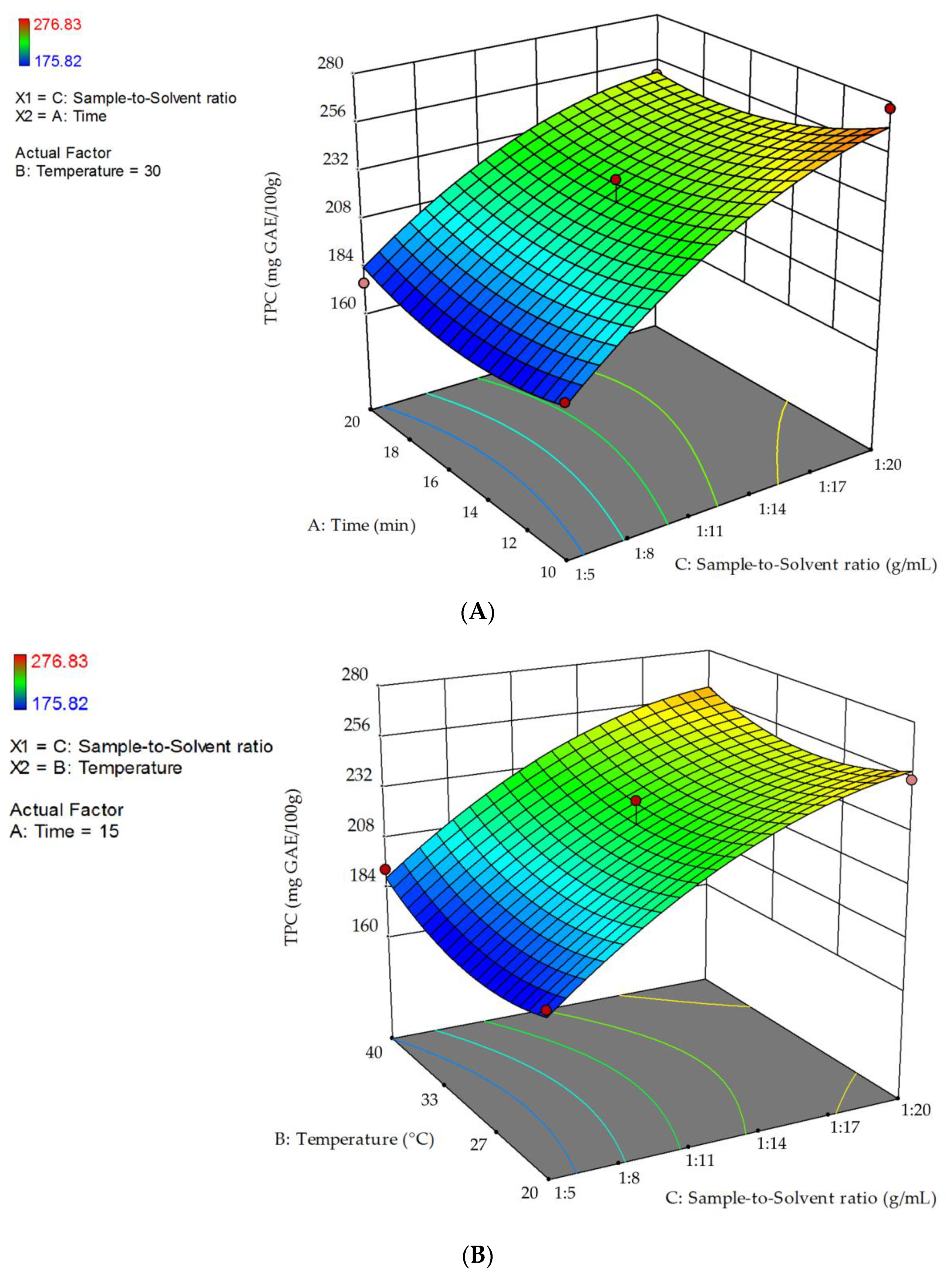
2.1.1. Optimization of Extraction with Methanol/Acetone Aqueous Solutions
2.1.2. Optimization of Extraction with Ethanol Aqueous Solutions
2.1.3. Determination and Experimental Validation of The Optimized Conditions
2.2. Phenolic Profile at Optimal Extraction Conditions
3. Discussion
3.1. Total Phenolic Content in Quinoa Seeds
3.2. Phenolic Profile
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Sample Preparation
4.2.2. Ultrasound-assisted Extraction of Free Phenolic Compounds
4.2.3. Determination of Total Phenolic Content
4.2.4. Experimental Design
4.2.5. Validation of the Model
4.2.6. HPLC Analysis of Quinoa Extracts at Optimal Extraction Conditions
4.2.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds: Health Benefits and Potential Applications; Woodhead Publishing Ltd.: Cambridge, UK, 2019; pp. 33–50. ISBN 9780128147757. [Google Scholar]
- Koch, W. Dietary Polyphenols-Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.; Martinoli, M.G. Considerations for the use of polyphenols as therapies in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 1883. [Google Scholar] [CrossRef] [PubMed]
- Melini, V.; Melini, F. Functional Components and Anti-Nutritional Factors in Gluten-Free Grains: A Focus on Quinoa Seeds. Foods 2021, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Melini, F.; Melini, V. Impact of Fermentation on Phenolic Compounds and Antioxidant Capacity of Quinoa. Fermentation 2021, 7, 20. [Google Scholar] [CrossRef]
- Wang, W.; Gao, Y.-T.; Wei, J.-W.; Chen, Y.-F.; Liu, Q.-L.; Liu, H.-M. Optimization of Ultrasonic Cellulase-Assisted Extraction and Antioxidant Activity of Natural Polyphenols from Passion Fruit. Molecules 2021, 26, 2494. [Google Scholar] [CrossRef] [PubMed]
- Rohilla, S.; Mahanta, C.L. Optimization of extraction conditions for ultrasound-assisted extraction of phenolic compounds from tamarillo fruit (Solanum betaceum) using response surface methodology. J. Food Meas. Charact. 2021, 15, 1763–1773. [Google Scholar] [CrossRef]
- Zhang, J.; Lee, T.G. Optimization of phenolics and flavonoids extraction from the fruit of Empetrum nigrum var. japonicum from Jeju Island in South Korea. J. Ind. Eng. Chem. 2021, 98, 350–357. [Google Scholar] [CrossRef]
- Chakraborty, S.; Uppaluri, R.; Das, C. Optimization of ultrasound-assisted extraction (UAE) process for the recovery of bioactive compounds from bitter gourd using response surface methodology (RSM). Food Bioprod. Process. 2020, 120, 114–122. [Google Scholar] [CrossRef]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chem. 2021, 334, 127521. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S.; Najari, Z. An integrated valorization of industrial waste of eggplant: Simultaneous recovery of pectin, phenolics and sequential production of pullulan. Waste Manag. 2019, 100, 101–111. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzalez, R.; Fernández-López, J.; Ricci, A.; Pérez-Álvarez, J.A.; Sterzo, C.L.; Viuda-Martos, M. Bioaccessibility of polyphenolic compounds of six quinoa seeds during in vitro gastrointestinal digestion. J. Funct. Foods 2017, 38, 77–88. [Google Scholar] [CrossRef]
- Melini, V.; Panfili, G.; Fratianni, A.; Acquistucci, R. Bioactive compounds in rice on Italian market: Pigmented varieties as a source of carotenoids, total phenolic compounds and anthocyanins, before and after cooking. Food Chem. 2019, 277, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Carciochi, R.A.; Galván-D’Alessandro, L.; Vandendriessche, P.; Chollet, S. Effect of Germination and Fermentation Process on the Antioxidant Compounds of Quinoa Seeds. Plant Foods Hum. Nutr. 2016, 71, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Carciochi, R.A.; Dimitrov, K.; Galván D’Alessandro, L. Effect of malting conditions on phenolic content, Maillard reaction products formation, and antioxidant activity of quinoa seeds. J. Food Sci. Technol. 2016, 53, 3978–3985. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A knowledge base for the recovery of natural phenols with different solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Muñiz-Márquez, D.B.; Aguilar-Zárate, P.; Ascacio-Valdés, J.A.; Cruz, K.; Reyes-Luna, C.; Rodríguez, R.; Aguilar, C.N. Extraction of Bioactive Phenolic Compounds by Alternative Technologies. In Ingredients Extraction by Physicochemical Methods in Food; Elsevier: San Diego, CA, USA, 2017; pp. 229–252. [Google Scholar]
- Stikić, R.I.; Milinčić, D.D.; Kostić, A.Ž.; Jovanović, Z.B.; Gašić, U.M.; Tešić, Ž.L.; Djordjević, N.Z.; Savić, S.K.; Czekus, B.G.; Pešić, M.B. Polyphenolic profiles, antioxidant, and in vitro anticancer activities of the seeds of Puno and Titicaca quinoa cultivars. Cereal Chem. 2020, 97, 626–633. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Galván D’Alessandro, L.; Manrique, G.D. Effect of roasting conditions on the antioxidant compounds of quinoa seeds. Int. J. Food Sci. Technol. 2016, 51, 1018–1025. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Zura, L.; Lutz, M.; Jagus, R.; Victoria Agüero, M.; Pastén, A.; Di Scala, K.; Uribe, E. Assessment of dietary fiber, isoflavones and phenolic compounds with antioxidant and antimicrobial properties of quinoa (Chenopodium quinoa Willd.). Chil. J. Agric. Anim. Sci. 2018, 34, 1–11. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J.; Peñas, E. Response surface optimisation of germination conditions to improve the accumulation of bioactive compounds and the antioxidant activity in quinoa. Int. J. Food Sci. Technol. 2018, 53, 516–524. [Google Scholar] [CrossRef]
- Kaur, I.; Tanwar, B.; Reddy, M.; Chauhan, A. Vitamin C, total polyphenols and antioxidant activity in raw, domestically processed and industrially processed Indian Chenopodium quinoa seeds. J. Appl. Pharm. Sci. 2016, 6, 139–145. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, Y.J.; Kim, Y.H.; Yoon, K.S. Antioxidant and antimicrobial activities of Quinoa (Chenopodium quinoa Willd.) Seeds Cultivated in Korea. Prev. Nutr. Food Sci. 2017, 22, 195–202. [Google Scholar] [PubMed]
- Mhada, M.; Metougui, M.L.; El Hazzam, K.; El Kacimi, K.; Yasri, A. Variations of Saponins, Minerals and Total Phenolic Compounds Due to Processing and Cooking of Quinoa (Chenopodium quinoa Willd.) Seeds. Foods 2020, 9, 660. [Google Scholar] [CrossRef] [PubMed]
- Navarro del Hierro, J.; Herrera, T.; García-Risco, M.R.; Fornari, T.; Reglero, G.; Martin, D. Ultrasound-assisted extraction and bioaccessibility of saponins from edible seeds: Quinoa, lentil, fenugreek, soybean and lupin. Food Res. Int. 2018, 109, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, X.; Zhang, B.; Chen, P.X.; Liu, R.; Tsao, R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar] [CrossRef]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Greiner, R.; Orlien, V.; Lebovka, N.I. Negative pressure cavitation extraction: A novel method for extraction of food bioactive compounds from plant materials. Trends Food Sci. Technol. 2016, 52, 98–108. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F.; Luziatelli, F.; Ruzzi, M. Functional ingredients from agri-food waste: Effect of inclusion thereof on phenolic compound content and bioaccessibility in bakery products. Antioxidants 2020, 9, 1216. [Google Scholar] [CrossRef]
- Diaz-Valencia, Y.K.; Alca, J.J.; Calori-Domingues, M.A.; Zanabria-Galvez, S.J.; Da Cruz, S.H. Nutritional composition, total phenolic compounds and antioxidant activity of quinoa (Chenopodium quinoa Willd.) of different colours. Nov. Biotechnol. Chim. 2018, 17, 74–85. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, K.; Yao, Y.; Chen, Y.; Guo, H.; Ren, G.; Yang, X.; Li, J. Antioxidant, anti-inflammatory, and antitumor activities of phenolic compounds from white, red, and black Chenopodium quinoa seed. Cereal Chem. 2020, 97, 703–713. [Google Scholar] [CrossRef]
- Škrovánková, S.; Válková, D.; Mlček, J. Polyphenols and antioxidant activity in pseudocereals and their products. Potravin. Slovak, J. Food Sci. 2020, 14, 365–370. [Google Scholar] [CrossRef]
- Noubigh, A.; Aydi, A.; Abderrabba, M. Experimental measurement and correlation of solubility data and thermodynamic properties of protocatechuic acid in four organic solvents. J. Chem. Eng. Data 2015, 60, 514–518. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, G.; Zhang, W.; Goltsev, V.; Sun, S.; Wang, J.; Li, P.; Ma, F. Anthocyanin concentration depends on the counterbalance between its synthesis and degradation in plum fruit at high temperature. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Yao, G.-L.; Ma, X.-H.; Cao, X.-Y.; Chen, J. Effects of Power Ultrasound on Stability of Cyanidin-3-glucoside Obtained from Blueberry. Molecules 2016, 21, 1564. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chi, J.; Zhang, M.; Zhang, R.; Fan, S.; Dong, L.; Huang, F.; Liu, L. Changes in saponins, phenolics and antioxidant activity of quinoa (Chenopodium quinoa willd) during milling process. LWT 2019, 114, 108381. [Google Scholar] [CrossRef]
- Vilas-Boas, S.M.; Vieira, V.; Brandão, P.; Alves, R.S.; Coutinho, J.A.P.; Pinho, S.P.; Ferreira, O. Solvent and temperature effects on the solubility of syringic, vanillic or veratric acids: Experimental, modeling and solid phase studies. J. Mol. Liq. 2019, 289, 111089. [Google Scholar] [CrossRef]
- Tabart, J.; Kevers, C.; Sipel, A.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Optimisation of extraction of phenolics and antioxidants from black currant leaves and buds and of stability during storage. Food Chem. 2007, 105, 1268–1275. [Google Scholar] [CrossRef]
- Zi, J.; Peng, B.; Yan, W. Solubilities of rutin in eight solvents at T = 283.15, 298.15, 313.15, 323.15, and 333.15 K. Fluid Phase Equilib. 2007, 261, 111–114. [Google Scholar] [CrossRef]
- Sompong, R.; Siebenhandl-Ehn, S.; Linsberger-Martin, G.; Berghofer, E. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chem. 2011, 124, 132–140. [Google Scholar] [CrossRef]



| Run Order | Independent Variables | Response for Extraction with Methanol/Acetone Aqueous Solutions | ||
|---|---|---|---|---|
| Extraction Time (min), X1 | Extraction Temperature (°C), X2 | Sample-to-Solvent Ratio (g mL−1), X3 | TPC (mg GAE 100 g−1 dm) | |
| 1 | 10 | 30 | 1:20 | 276.83 ± 6.81 |
| 2 | 15 | 40 | 1:5 | 193.01 ± 5.36 |
| 3 | 10 | 20 | 1:12.5 | 246.03 ± 5.23 |
| 4 | 20 | 40 | 1:12.5 | 253.23 ± 5.95 |
| 5 | 15 | 30 | 1:12.5 | 216.57 ± 7.84 |
| 6 | 20 | 30 | 1:5 | 175.82 ± 5.31 |
| 7 | 20 | 20 | 1:12.5 | 241.93 ± 6.25 |
| 8 | 15 | 20 | 1:20 | 254.93 ± 5.98 |
| 9 | 15 | 30 | 1:12.5 | 223.24 ± 7.01 |
| 10 | 15 | 40 | 1:20 | 258.80 ± 6.26 |
| 11 | 10 | 40 | 1:12.5 | 245.23 ± 6.89 |
| 12 | 20 | 30 | 1:20 | 248.54 ± 5.45 |
| 13 | 15 | 30 | 1:12.5 | 236.03 ± 8.18 |
| 14 | 15 | 20 | 1:5 | 182.32 ± 6.47 |
| 15 | 10 | 30 | 1:5 | 183.25 ± 5.76 |
| Term | Regression Coefficients | Standard Error | T-Value | p-Value | |
|---|---|---|---|---|---|
| TPC model— methanol/acetone aqueous extraction | |||||
| β0 | 225.28 | 5.72 | 39.37 | <0.0001 | |
| β1 | −3.98 | 3.50 | −1.14 | 0.3078 | |
| β2 | 3.13 | 3.50 | 0.89 | 0.4123 | |
| β3 | 38.09 | 3.50 | 10.87 | 0.0001 | |
| β12 | 10.08 | 5.16 | 1.96 | 0.1079 | |
| β22 | 11.24 | 5.16 | 2.18 | 0.0812 | |
| β32 | −14.26 | 5.16 | −2.76 | 0.0397 | |
| β12 | 3.03 | 4.96 | 0.61 | 0.5683 | |
| β13 | −5.21 | 4.96 | −1.05 | 0.3408 | |
| β23 | −1.71 | 4.96 | −0.34 | 0.7478 | |
| TPC model— ethanol aqueous extraction | |||||
| β0 | 160.82 | 2.39 | 67.25 | <0.0001 | |
| β1 | −3.21 | 1.46 | −2.19 | 0.0799 | |
| β2 | 17.11 | 1.46 | 11.69 | 0.0001 | |
| β3 | 26.00 | 1.46 | 17.75 | <0.0001 | |
| β12 | 3.48 | 2.16 | 1.62 | 0.1670 | |
| β22 | 19.78 | 2.16 | 9.18 | 0.0003 | |
| β32 | −8.50 | 2.16 | −3.94 | 0.0109 | |
| β12 | −5.25 | 2.07 | −2.53 | 0.0523 | |
| β13 | −0.99 | 2.07 | −0.48 | 0.6520 | |
| β23 | 3.25 | 2.07 | 1.57 | 0.1771 |
| Source of Variation | DF | Adj SS | Adj MS | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| TPC model—methanol/acetone aqueous extraction | ||||||
| Regression | 9 | 13679.0 | 1519.9 | 15.47 | 0.0038 | |
| Residuals | 5 | 4912 | 98.2 | |||
| Lack-of-Fit | 3 | 295.6 | 98.5 | 1.01 | 0.5332 | |
| Pure Error | 2 | 195.6 | 97.8 | |||
| Total | 14 | 14170.2 | ||||
| R2 = 0.9653 | ||||||
| adj R2 = 0.9029 | ||||||
| TPC model—ethanol aqueous extraction | ||||||
| Regression | 9 | 9832.36 | 1092.48 | 63.69 | <0.0001 | |
| Residuals | 5 | 85.77 | 17.15 | |||
| Lack-of-Fit | 3 | 80.76 | 26.92 | 10.75 | 0.0863 | |
| Pure Error | 2 | 5.01 | 2.50 | |||
| Total | 14 | 9918.12 | ||||
| R2 = 0.9914 | ||||||
| adj R2 = 0.9758 | ||||||
| Run Order | Independent Variables | Response for Extraction with Ethanol Aqueous Solutions | ||
|---|---|---|---|---|
| Extraction Time (min), X1 | Extraction Temperature (°C), X2 | Sample-to-Solvent Ratio (g mL−1), X3 | TPC (mg GAE 100 g−1 dm) | |
| 1 | 10 | 30 | 1:20 | 183.69 ± 2.64 |
| 2 | 15 | 30 | 1:12.5 | 160.45 ± 3.18 |
| 3 | 20 | 40 | 1:12.5 | 193.80 ± 3.10 |
| 4 | 15 | 20 | 1:20 | 181.11 ± 2.88 |
| 5 | 20 | 20 | 1:12.5 | 169.45 ± 2.69 |
| 6 | 20 | 30 | 1:20 | 173.77 ± 2.83 |
| 7 | 15 | 30 | 1:12.5 | 162.55 ± 3.22 |
| 8 | 15 | 40 | 1:5 | 156.58 ± 2.91 |
| 9 | 20 | 30 | 1:5 | 129.90 ± 2.66 |
| 10 | 15 | 30 | 1:12.5 | 159.45 ± 2.58 |
| 11 | 15 | 40 | 1:20 | 221.22 ± 2.73 |
| 12 | 10 | 40 | 1:12.5 | 209.20 ± 2.62 |
| 13 | 10 | 20 | 1:12.5 | 163.86 ± 3.06 |
| 14 | 15 | 20 | 1:5 | 129.48 ± 2.89 |
| 15 | 10 | 30 | 1:5 | 135.85 ± 2.69 |
| Free Phenolic Compound | λmax | Rt | Concentration (Methanol/Acetone Aqueous Extract) | Concentration (Ethanol Aqueous Extract) |
|---|---|---|---|---|
| nm | min | mg 100 g−1 dm | mg 100 g−1 dm | |
| Gallic acid | 270, 228 | 5.50 | 0.49 ± 0.01 a | 0.52 ± 0.02 a |
| Protocatechuic acid | 291, 257, 228 | 10.73 | 3.23 ± 0.05 a | 2.52 ± 0.05 b |
| (+)-Catechin | 276, 233 | 15.21 | 2.26 ± 0.03 a | 1.95 ± 0.03 b |
| 4-Hydroxybenzoic acid | 252 | 15.88 | 0.65 ± 0.01 a | 0.69 ± 0.02 a |
| Vanillic acid | 289, 258 | 20.12 | 1.17 ± 0.03 a | 1.12 ± 0.02 a |
| t-Ferulic acid | 321, 241 | 34.12 | 4.98 ± 0.08 a | 4.11 ± 0.05 b |
| Rutin | 352, 254 | 34.45 | 14.19 ± 0.41 a | 15.50 ± 0.34 a |
| o-Coumaric acid | 322, 274 | 38.00 | 0.24 ± 0.01 a | 0.39 ± 0.01 b |
| 3,4-Dimethoxycinnamic acid | 318, 245 | 40.29 | 0.29 ± 0.01 a | 0.34 ± 0.01 b |
| Factors | Symbols | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Extraction time (min) | X1 | 10 | 15 | 20 |
| Extraction temperature (°C) | X2 | 20 | 30 | 40 |
| Sample-to-solvent ratio (g mL−1) | X3 | 1:5 | 1:12.5 | 1:20 |
| Phenolic Compounds | Regression Equation | R2 | LOD (µg mL−1) | LOQ (µg mL−1) |
|---|---|---|---|---|
| Gallic acid | Y = 0.7961 X + 0.2967 | 0.992 | 0.35 | 1.07 |
| Protocatechuic acid | Y = 1.1062 X + 0.3549 | 0.991 | 1.76 | 5.32 |
| (+)-Catechin | Y = 0.1621 X − 0.0133 | 0.994 | 0.99 | 2.99 |
| 4-Hydroxybenzoic acid | Y = 1.4774 X + 0.1550 | 0.995 | 0.49 | 1.48 |
| Vanillic acid | Y = 1.1100 X + 0.2324 | 0.998 | 0.38 | 1.15 |
| t-Ferulic acid | Y = 1.6521 X + 0.4750 | 0.997 | 1.58 | 4.79 |
| Rutin | Y = 0.7759 X − 5.3949 | 0.997 | 5.21 | 15.77 |
| o-Coumaric acid | Y = 1.3527 X + 0.2021 | 0.999 | 0.16 | 0.49 |
| 3,4-Dimethoxycinnamic acid | Y = 1.4616 X + 0.1248 | 0.996 | 0.24 | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melini, V.; Melini, F. Modelling and Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from Black Quinoa by Response Surface Methodology. Molecules 2021, 26, 3616. https://doi.org/10.3390/molecules26123616
Melini V, Melini F. Modelling and Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from Black Quinoa by Response Surface Methodology. Molecules. 2021; 26(12):3616. https://doi.org/10.3390/molecules26123616
Chicago/Turabian StyleMelini, Valentina, and Francesca Melini. 2021. "Modelling and Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from Black Quinoa by Response Surface Methodology" Molecules 26, no. 12: 3616. https://doi.org/10.3390/molecules26123616
APA StyleMelini, V., & Melini, F. (2021). Modelling and Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from Black Quinoa by Response Surface Methodology. Molecules, 26(12), 3616. https://doi.org/10.3390/molecules26123616







