Cytoprotective Activity of p-Terphenyl Polyketides and Flavuside B from Marine-Derived Fungi against Oxidative Stress in Neuro-2a Cells
Abstract
:1. Introduction
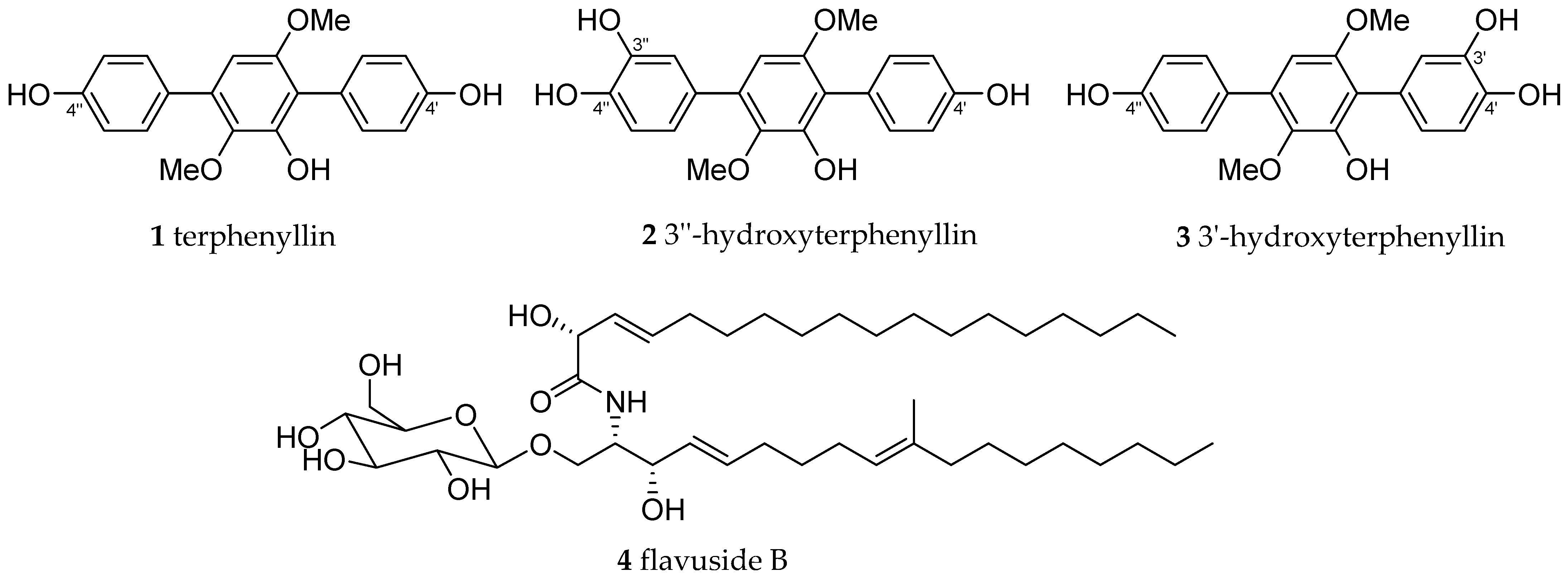
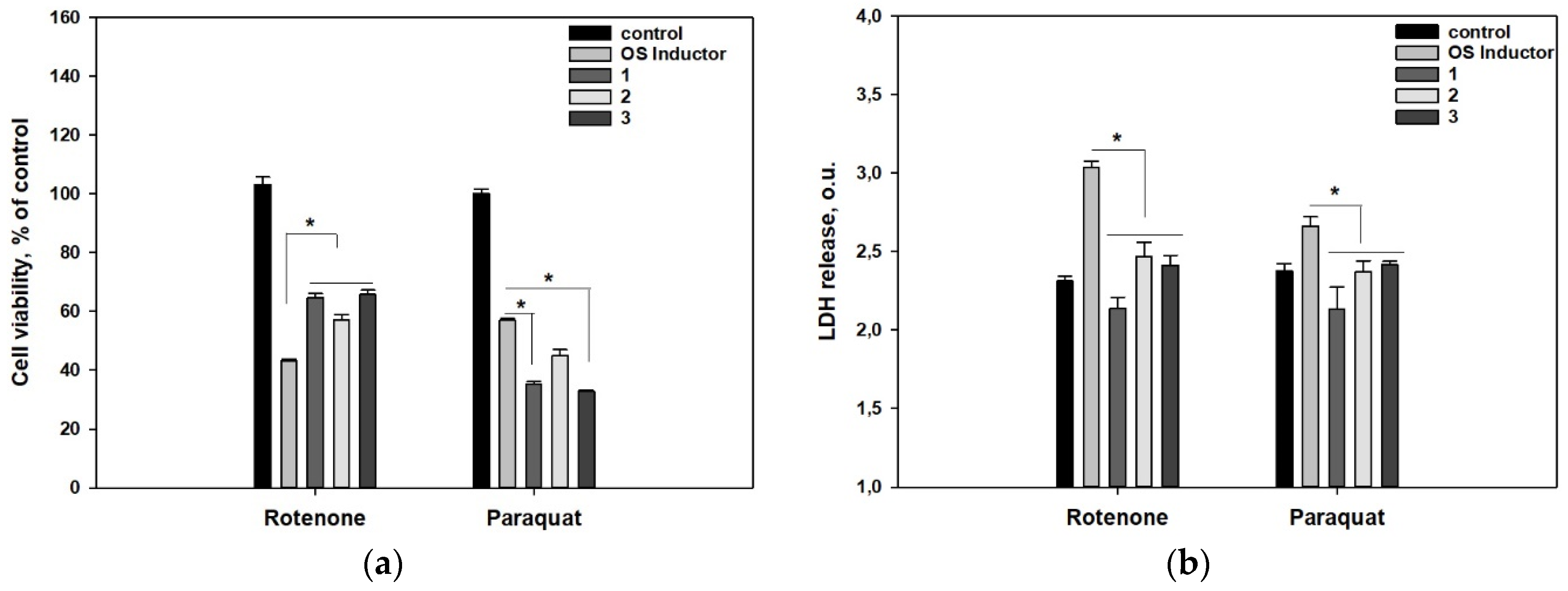
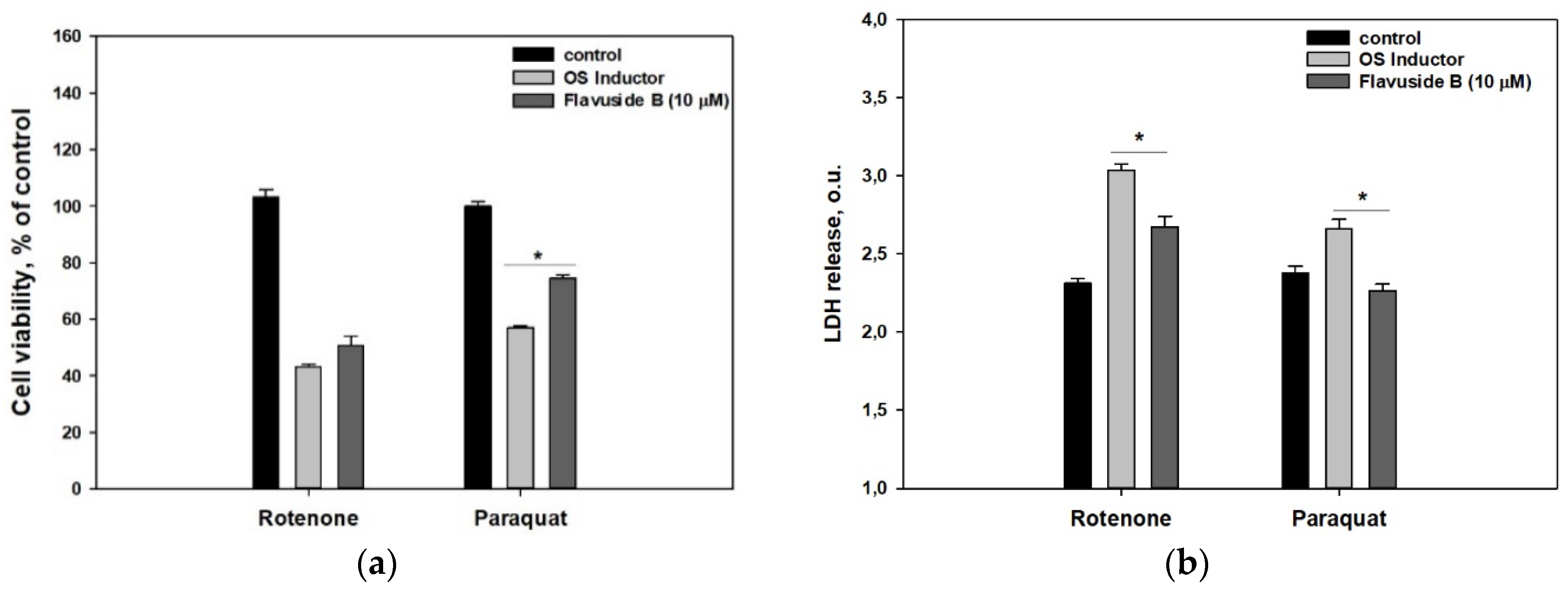
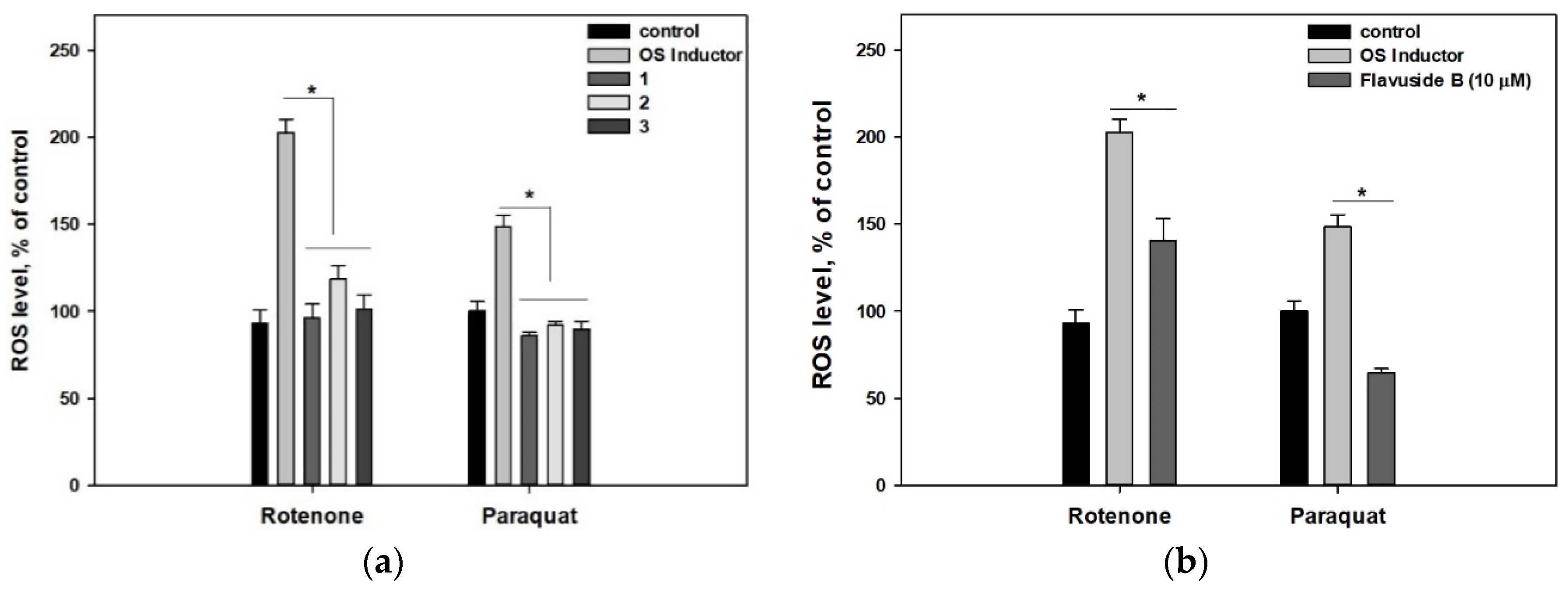
2. Results
3. Discussion
4. Materials and Methods
4.1. Compounds
4.2. DPPH Radical Scavenging Assay
4.3. Neuro-2a Cell Culture
4.4. MTT Cell Viability Assay
4.5. Paraquat- and Rotenone-Induced Neurotoxicity
4.6. Lactate Dehydrogenase (LDH) Release Detection
4.7. Reactive Oxygen Species (ROS) Level Analysis
4.8. Data Evaluation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Vitale, G.A.; Coppola, D.; Esposito, F.P.; Buonocore, C.; Ausuri, J.; Tortorella, E.; de Pascale, D. Antioxidant molecules from marine fungi: Methodologies and perspectives. Antioxidants 2020, 9, 1183. [Google Scholar] [CrossRef]
- Leutou, A.S.; Yun, K.; Son, B.W. Induced production of 6,9-dibromoflavasperone, a new radical scavenging naphthopyranone in the marine-mudflat-derived fungus Aspergillus niger. Arch. Pharm. Res. 2016, 39, 806–810. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Liu, D.; Proksch, P.; Yu, S.; Lin, W. Antioxidative phenolic compounds from a marine-derived fungus Aspergillus versicolor. Tetrahedron 2016, 72, 50–57. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, H.J.; Sohn, J.H.; Yim, J.H.; Kim, Y.C.; Oh, H. Anti-neuroinflammatory effect of 6,8,1’-tri-O-methylaverantin, a metabolite from a marine-derived fungal strain Aspergillus sp., via upregulation of heme oxygenase-1 in lipopolysaccharide-activated microglia. Neurochem. Int. 2017, 113, 8–22. [Google Scholar] [CrossRef]
- Shadnia, S.; Ebadollahi-Natanzi, A.; Ahmadzadeh, S.; Karami-Mohajeri, S.; Pourshojaei, Y.; Rahimi, H.R. Delayed death following paraquat poisoning: Three case reports and a literature review. Toxicol. Res. 2018, 7, 745–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segura-Aguilar, J. Neurotoxins as Preclinical Models for Parkinson’s Disease. Neurotoxicity Res. 2018, 34, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Liu, G.; Li, J.; Li, G.; Li, T.; Zhao, H.; Li, L.; Yang, W.; Bai, N.; Zheng, M.; et al. Rotenone and 3-bromopyruvate toxicity impacts electrical and structural cardiac remodeling in rats. Toxicol. Lett. 2020, 318, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Satpute, R.M.; Pawar, P.P.; Puttewar, S.; Sawale, S.D.; Ambhore, P.D. Effect of resveratrol and tetracycline on the subacute paraquat toxicity in mice. Hum. Exp. Toxicol. 2017, 36, 1303–1314. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Antonov, A.S.; Trang, V.T.D.; Pivkin, M.V.; Khudyakova, Y.V.; Denisenko, V.A.; Popov, R.S.; Kim, N.Y.; Yurchenko, E.A.; Gerasimenko, A.V.; et al. New Deoxyisoaustamide Derivatives from the Coral-Derived Fungus Penicillium dimorphosporum KMM 4689. Mar. Drugs 2021, 19, 32. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Smetanina, O.F.; Ivanets, E.V.; Phan, T.T.H.; Ngo, N.T.D.; Zhuravleva, O.I.; Rasin, A.B.; Dyshlovoy, S.A.; Menchinskaya, E.S.; Pislyagin, E.A.; et al. Auroglaucin-related neuroprotective compounds from Vietnamese marine sediment-derived fungus Aspergillus niveoglaucus. Nat. Prod. Res. 2020, 34, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Smetanina, O.F.; Yurchenko, A.N.; Girich (Ivanets), E.V.; Trinh, P.T.H.; Antonov, A.S.; Dyshlovoy, S.A.; von Amsberg, G.; Kim, N.Y.; Chingizova, E.A.; Pislyagin, E.A.; et al. Biologically Active Echinulin-Related Indolediketopiperazines from the Marine Sediment-Derived Fungus Aspergillus niveoglaucus. Molecules 2020, 25, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girich, E.V.; Yurchenko, A.N.; Smetanina, O.F.; Trinh, P.T.H.; Ngoc, N.T.D.; Pivkin, M.V.; Popov, R.S.; Pislyagin, E.A.; Menchinskaya, E.S.; Chingizova, E.A.; et al. Neuroprotective Metabolites from Vietnamese Marine Derived Fungi of Aspergillus and Penicillium Genera. Mar. Drugs 2020, 18, 608. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, E.; Menchinskaya, E.; Pislyagin, E.; Trinh, P.; Ivanets, E.; Smetanina, O.; Yurchenko, A. Neuroprotective Activity of Some Marine Fungal Metabolites in the 6-Hydroxydopamin- and Paraquat-Induced Parkinson’s Disease Models. Mar. Drugs 2018, 16, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yurchenko, A.N.; Ivanets, E.V.; Smetanina, O.F.; Pivkin, M.V.; Dyshlovoi, S.A.; von Amsberg, G.; Afiyatullov, S.S. Metabolites of the Marine Fungus Aspergillus candidus KMM 4676 Associated with a Kuril Colonial Ascidian. Chem. Nat. Compd. 2017, 53, 747–749. [Google Scholar] [CrossRef]
- Ivanets, E.V.; Yurchenko, A.N.; Smetanina, O.F.; Rasin, A.B.; Zhuravleva, O.I.; Pivkin, M.V.; Popov, R.S.; von Amsberg, G.; Afiyatullov, S.S.; Dyshlovoy, S.A. Asperindoles A–D and a p-terphenyl derivative from the ascidian-derived fungus Aspergillus sp. KMM 4676. Mar. Drugs 2018, 16, 232. [Google Scholar] [CrossRef] [Green Version]
- Yurchenko, A.N.; Smetanina, O.F.; Ivanets, E.V.; Kirichuk, N.N.; Khudyakova, Y.V.; Yurchenko, E.A.; Afiyatullov, S.S. Metabolites from the Facultative Marine Fungus Penicillium islandicum. Chem. Nat. Compd. 2016, 52, 365–367. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Dmitrenko, V.V. Pitfalls of the MTT assay: Direct and off-target effects of inhibitors can result in over/underestimation of cell viability. Gene 2015, 574, 193–203. [Google Scholar] [CrossRef]
- Kepp, O.; Galluzzi, L.; Lipinski, M.; Yuan, J.; Kroemer, G. Cell death assays for drug discovery. Nat. Rev. Drug Discov. 2011, 10, 221–237. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Mou, X.F.; Mao, N.; Hao, J.J.; Liu, M.; Zheng, J.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. Design, semisynthesis, alpha-glucosidase inhibitory, cytotoxic, and antibacterial activities of p-terphenyl derivatives. Eur. J. Med. Chem. 2018, 146, 232–244. [Google Scholar] [CrossRef]
- Chia, S.J.; Tan, E.-K.; Chao, Y.-X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef] [Green Version]
- Bastias-Candia, S.; Zolezzi, J.M.; Inestrosa, N.C. Revisiting the Paraquat-Induced Sporadic Parkinson’s Disease-Like Model. Mol. Neurobiol. 2019, 56, 1044–1055. [Google Scholar] [CrossRef]
- Yen, G.C.; Chiang, H.C.; Wu, C.H.; Yeh, C.T. The protective effects of Aspergillus candidus metabolites against hydrogen peroxide-induced oxidative damage to Int 407 cells. Food Chem. Toxicol. 2003, 41, 1561–1567. [Google Scholar] [CrossRef]
- Yang, G.; Sandjo, L.; Yun, K.; Leutou, A.S.; Kim, G.D.; Choi, H.D.; Kang, J.S.; Hong, J.; Son, B.W. Flavusides A and B, antibacterial cerebrosides from the marine-derived fungus Aspergillus flavus. Chem. Pharm. Bull. 2011, 59, 1174–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Fan, J.T.; Gu, X.L.; Zhang, L.Y.; Han, J.; Du, S.H.; Zhang, A.X. Neuroprotective Activity of Cerebrosides from Typhonium giganteum by Regulating Caspase-3 and Bax/Bcl-2 Signaling Pathways in PC12 Cells. J. Nat. Prod. 2017, 80, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.P.; Liu, S.C.; Tang, C.H.; Chan, Y.; El-Shazly, M.; Lee, C.L.; Du, Y.C.; Wu, T.Y.; Chang, F.R.; Wu, Y.C. Anti-inflammatory Cerebrosides from Cultivated Cordyceps militaris. J. Agric. Food Chem. 2016, 64, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, E.A.; Kolesnikova, S.A.; Lyakhova, E.G.; Menchinskaya, E.S.; Pislyagin, E.A.; Chingizova, E.A.; Aminin, D.L. Lanostane Triterpenoid Metabolites from a Penares sp. Marine Sponge Protect Neuro-2a Cells against Paraquat Neurotoxicity. Molecules 2020, 25, 5397. [Google Scholar] [CrossRef] [PubMed]
| Compound | % of Radical Scavenging at 100 µM | Compound | % of Radical Scavenging at 100 µM |
|---|---|---|---|
| 1 | 33.1 ± 0.9 | 3 | 41.3 ± 2.1 |
| 2 | 64.6 ± 1.1 | 4 | 10.3 ± 5.9 |
| Ascorbic acid | 67.3 ± 3.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurchenko, E.A.; Menchinskaya, E.S.; Pislyagin, E.A.; Chingizova, E.A.; Girich, E.V.; Yurchenko, A.N.; Aminin, D.L.; Mikhailov, V.V. Cytoprotective Activity of p-Terphenyl Polyketides and Flavuside B from Marine-Derived Fungi against Oxidative Stress in Neuro-2a Cells. Molecules 2021, 26, 3618. https://doi.org/10.3390/molecules26123618
Yurchenko EA, Menchinskaya ES, Pislyagin EA, Chingizova EA, Girich EV, Yurchenko AN, Aminin DL, Mikhailov VV. Cytoprotective Activity of p-Terphenyl Polyketides and Flavuside B from Marine-Derived Fungi against Oxidative Stress in Neuro-2a Cells. Molecules. 2021; 26(12):3618. https://doi.org/10.3390/molecules26123618
Chicago/Turabian StyleYurchenko, Ekaterina A., Ekaterina S. Menchinskaya, Evgeny A. Pislyagin, Ekaterina A. Chingizova, Elena V. Girich, Anton N. Yurchenko, Dmitry L. Aminin, and Valery V. Mikhailov. 2021. "Cytoprotective Activity of p-Terphenyl Polyketides and Flavuside B from Marine-Derived Fungi against Oxidative Stress in Neuro-2a Cells" Molecules 26, no. 12: 3618. https://doi.org/10.3390/molecules26123618







