Ultraviolet Spectroscopic Detection of Nitrate and Nitrite in Seawater Simultaneously Based on Partial Least Squares
Abstract
:1. Introduction
2. Theory
2.1. PLS Regressions
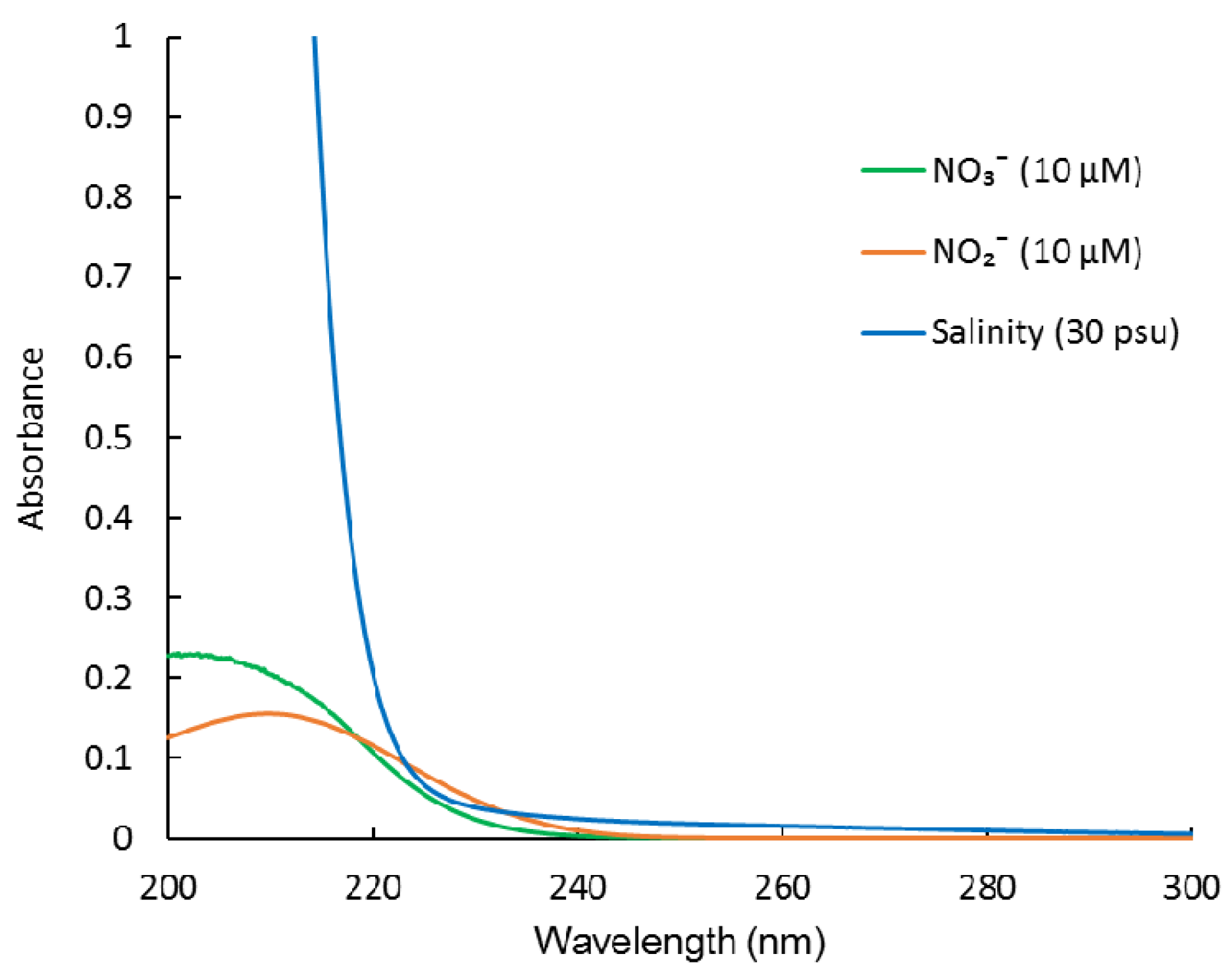
2.2. Interference from CDOM
2.3. CLS Regression
3. Experimental
3.1. Reagents
3.2. Apparatus and Software
3.3. Model Validation
3.4. The Calibration and Prediction Sample Sets
4. Results and Discussion
4.1. Selection of the Optimal Number of Factors
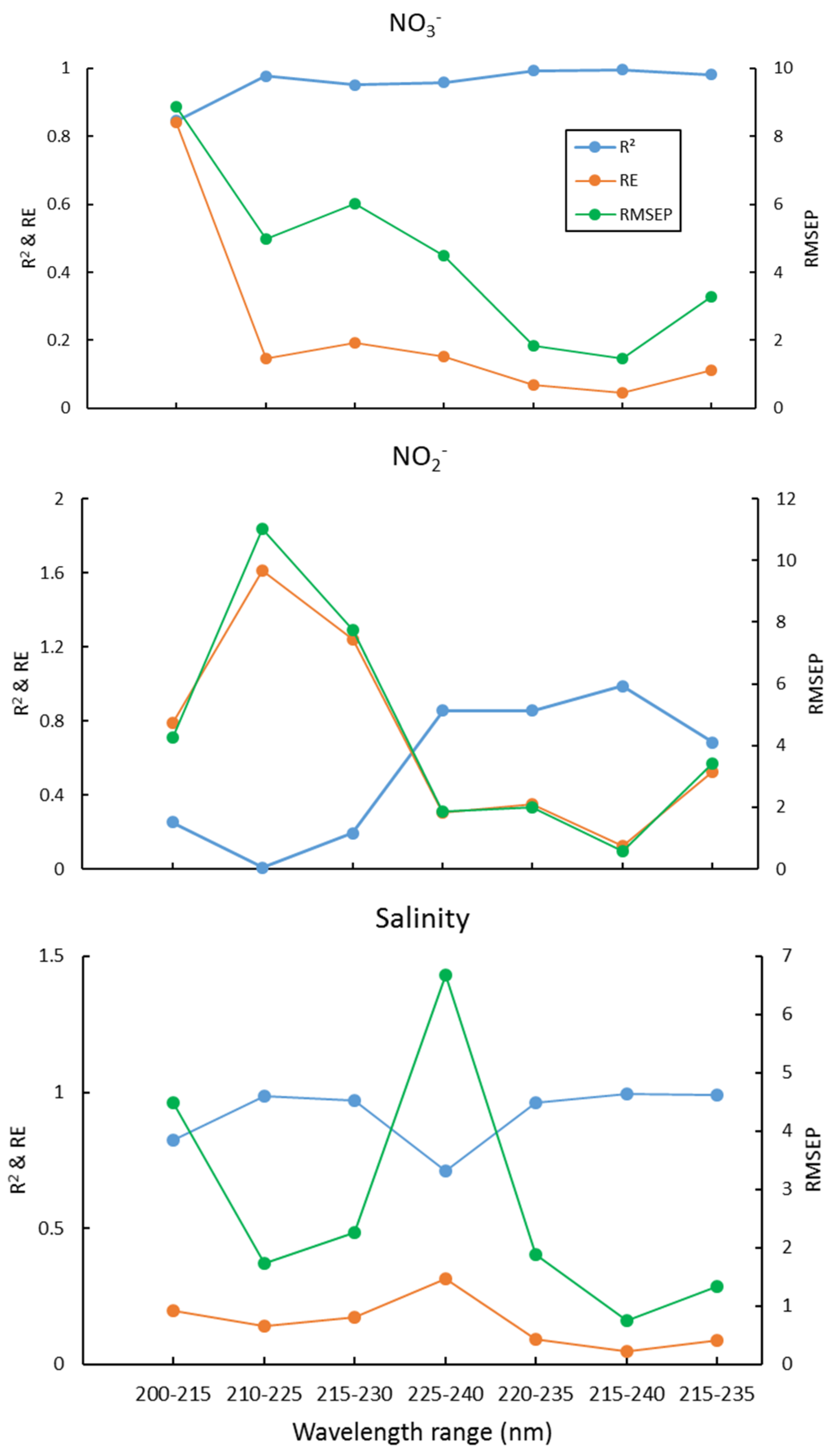
4.2. Wavelength Selection
4.3. Comparison of PLS2 and CLS Regressions
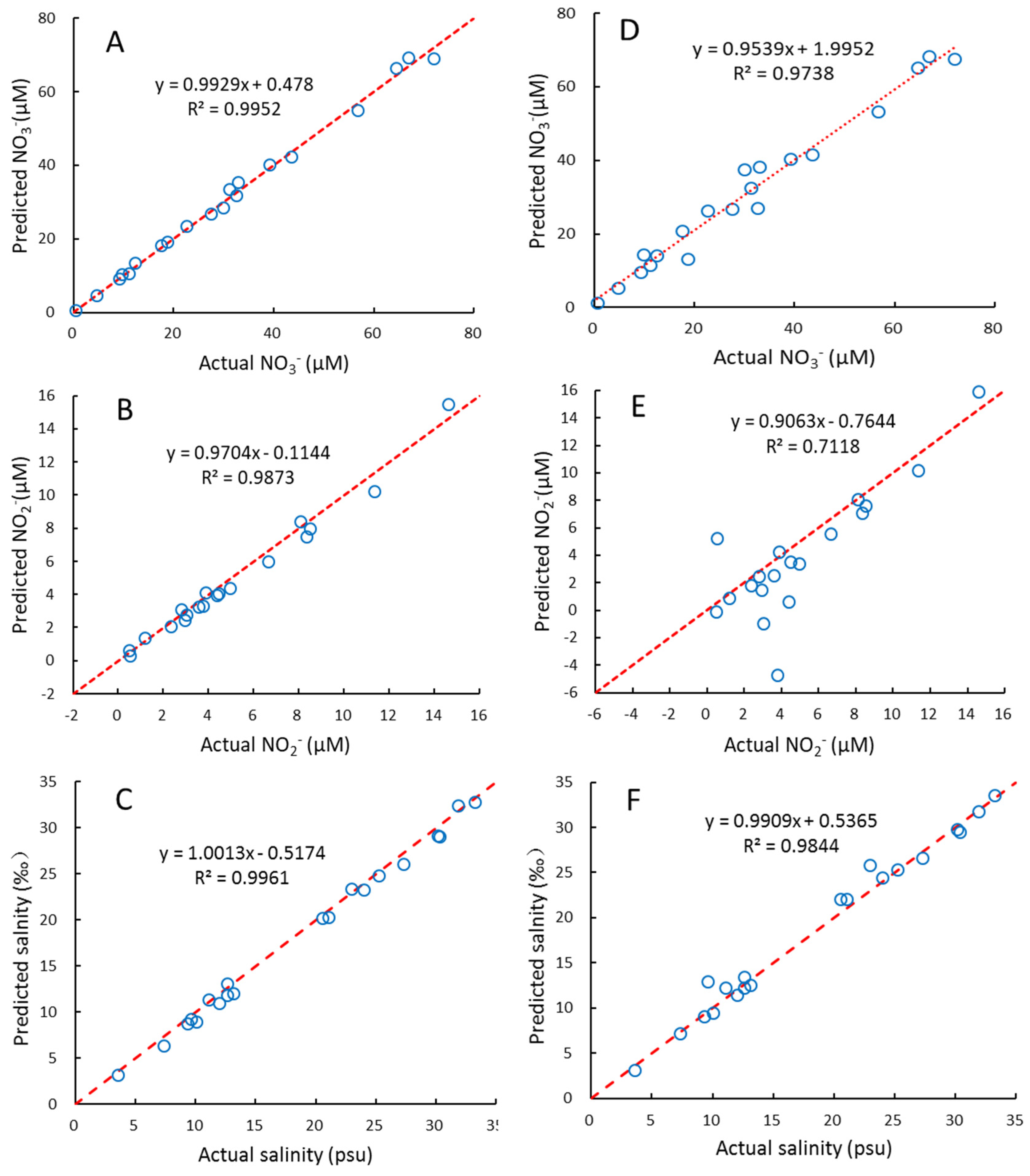
4.4. Evaluation of the PLS2 Model
4.5. Application and Comparison of the Predicted Results with Conventional Wet-Chemical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Arrigo, K.R. Marine microorganisms and global nutrient cycles. Nature 2005, 437, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.G.; Barber, R.T.; Smetacek, V.V. Biogeochemical Controls and Feedbacks on Ocean Primary Production. Science 1998, 281, 200–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollenweider, R.A.; Marchetti, R.; Viviani, R. The problems of the Emilia Romagna coastal waters: Facts and interpretations. Mar. Coast. Eutrophication 1992, 21–33. [Google Scholar] [CrossRef]
- Ma, J.; Adornato, L.; Byrne, R.H.; Yuan, D. Determination of nanomolar levels of nutrients in seawater. Trends Anal. Chem. 2014, 60, 1–15. [Google Scholar] [CrossRef]
- Singh, P.; Singh, M.K.; Beg, Y.R.; Nishad, G.R. A review on spectroscopic methods for determination of nitrite and nitrate in environmental samples. Talanta 2019, 191, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Yu, L.J.; Liu, Y.; Lin, L.; Lu, R.G.; Zhu, J.P.; He, L.; Lu, Z.L. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef]
- Armstrong, F.A.J. Determination of Nitrate in Water Ultraviolet Spectrophotometry. Anal. Chem. 1963, 35, 1292–1294. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1912. [Google Scholar]
- Johnson, K.S.; Coletti, L.J. In situ ultraviolet spectrophotometry for high resolution and long-term monitoring of nitrate, bromide and bisulfide in the ocean. Deep Sea Res. Part I 2002, 49, 1291–1305. [Google Scholar] [CrossRef]
- Thomas, O.; Gallot, S.; Mazas, N. Ultraviolet multiwavelength absorptiometry (UVMA) for the examination of natural waters and waste waters: Part II: Determination of nitrate. Fresenius J. Anal. Chem. 1990, 338, 238–240. [Google Scholar] [CrossRef]
- Huebsch, M.; Grimmeisen, F.; Zemann, M.; Fenton, O.; Richards, K.G.; Jordan, P.; Sawarieh, A.; Blum, P.; Goldscheider, N. Technical Note: Field experiences using UV/VIS sensors for high-resolution monitoring of nitrate in groundwater. Hydrol. Earth Syst. Sci. 2015, 19, 1589–1598. [Google Scholar] [CrossRef] [Green Version]
- Zielinski, O.; Fiedler, B.; Heuermann, R.; Kortzinger, A.; Munderloh, K. A new nitrate continuous observation sensor for autonomous sub-surface applications: Technical design and first results. In Proceedings of the Oceans 2007, Aberdeen, UK, 18–21 June 2007; pp. 1–4. [Google Scholar]
- Gruber, N. Chapter 1—The Marine Nitrogen Cycle: Overview and Challenges. In Nitrogen in the Marine Environment, 2nd ed.; Capone, D.G., Bronk, D.A., Mulholland, M.R., Carpenter, E.J., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 1–50. [Google Scholar]
- Casciotti, K.L.; Böhlke, J.K.; Mcilvin, M.R.; Mroczkowski, S.J.; Hannon, J.E. Oxygen Isotopes in Nitrite: Analysis, Calibration, and Equilibration. Anal. Chem. 2007, 79, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Casciotti, K.L.; Buchwald, C.; Mcilvin, M. Implications of nitrate and nitrite isotopic measurements for the mechanisms of nitrogen cycling in the Peru oxygen deficient zone. Deep Sea Res. Part I 2013, 80, 78–93. [Google Scholar] [CrossRef]
- Hu, H.; Bourbonnais, A.; Larkum, J.; Bange, H.W.; Altabet, M.A. Nitrogen cycling in shallow low-oxygen coastal waters off Peru from nitrite and nitrate nitrogen and oxygen isotopes. Biogeosciences 2016, 13, 7257–7299. [Google Scholar] [CrossRef] [Green Version]
- Lam, P.; Lavik, G.; Jensen, M.M.; Vossenberg, J.V.D.; Schmid, M.; Woebken, D.; Gutiérrez, D.; Amann, R.; Jetten, M.S.M.; Kuypers, M.M.M. Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proc. Nat. Acad. Sci. USA 2009, 106, 4752–4757. [Google Scholar] [CrossRef] [Green Version]
- Morrison, J.M.; Codispoti, L.A.; Smith, S.L.; Wishner, K.; Flagg, C.; Gardner, W.D.; Gaurin, S.; Naqvi, S.W.A.; Manghnani, V.; Prosperie, L. The oxygen minimum zone in the Arabian Sea during 1995. Deep Sea Res. Part II 1999, 46, 1931. [Google Scholar] [CrossRef]
- Langergraber, G.; Fleischmann, N.; Hofstaedter, F. A multivariate calibration procedure for UV/VIS spectrometric quantification of organic matter and nitrate in wastewater. Water Sci. Technol. 2003, 47, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Rieger, L.; Langergraber, G.; Thomann, M.; Fleischmann, N.; Siegrist, H. Spectral in-situ analysis of NO2, NO3, COD, DOC and TSS in the effluent of a WWTP. Water Sci. Technol. 2004, 50, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Rieger, L.; Vanrolleghem, P.A.; Langergraber, G.; Kaelin, D.; Siegrist, H. Long-term evaluation of a spectral sensor for nitrite and nitrate. Water Sci. Technol. 2008, 57, 1563. [Google Scholar] [CrossRef]
- Haaland, D.M.; Thomas, E.V. Partial least-squares methods for spectral analyses. 1. Relation to other quantitative calibration methods and the extraction of qualitative information. Anal. Chem. 1988, 60, 1193–1202. [Google Scholar] [CrossRef]
- Otto, M. Chemometrics: Statistics and Computer Application in Analytical Chemistry; Wiley-VCH Verlag GmbH: Weinheim, Germany, 1999. [Google Scholar]
- Beebe, K.R.; Kowalski, B.R. An Introduction to Multivariate Calibration and Analysis. Anal. Chem. 1987, 59, 1007A–1017A. [Google Scholar] [CrossRef]
- Martens, H.; Naes, T. Multivariate Calibration; Wiley & Sons: Chichester, UK, 1989. [Google Scholar]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Manne, R. Analysis of Two PLS Algorithms for Multivariate Calibration. Chemom. Intell. Lab. Syst. 1987, 2, 187–197. [Google Scholar] [CrossRef]
- Khajehsharifi, H.; Mousavi, M.F.; Ghasemi, J.; Shamsipur, M. Kinetic spectrophotometric method for simultaneous determination of selenium and tellurium using partial least squares calibration. Anal. Chim. Acta 2004, 512, 369–373. [Google Scholar] [CrossRef]
- Tewari, J.; Strong, R.; Boulas, P. At-line determination of pharmaceuticals small molecule’s blending end point using chemometric modeling combined with Fourier transform near infrared spectroscopy. Spectrochim. Acta Part A 2017, 173, 886–891. [Google Scholar] [CrossRef]
- Abdel-Rahman, E.M.; Mutanga, O.; Odindi, J.; Adam, E.; Odindo, A.; Ismail, R. Estimating Swiss chard foliar macro- and micronutrient concentrations under different irrigation water sources using ground-based hyperspectral data and four partial least squares (PLS)-based (PLS1, PLS2, SPLS1 and SPLS2) regression algorithms. Comput. Electron. Agric. 2017, 132, 21–33. [Google Scholar] [CrossRef]
- Andries, J.P.M.; Heyden, Y.V.; Buydens, L.M.C. Predictive-property-ranked variable reduction with final complexity adapted models in partial least squares modeling for multiple responses. Anal. Chem. 2013, 85, 5444–5453. [Google Scholar] [CrossRef]
- Thurman, E.M. Organic Geochemistry of Natural Waters; M.Nijhoff/Dr. W. Junk Publishers: Dordrecht, The Netherlands, 1985. [Google Scholar]
- Kirk, J.T. Light and Photosynthesis in Aquatic Ecosystems; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Hansell, D.A.; Carlson, C.A. Biogeochemistry of Marine Dissolved Organic Matter; Academic Press: London, UK, 2014. [Google Scholar]
- Nelson, N.B.; Siegel, D.A. The global distribution and dynamics of chromophoric dissolved organic matter. Annu. Rev. Mar. Sci. 2013, 5, 447–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twardowski, M.S.; Boss, E.; Sullivan, J.M.; Donaghay, P.L. Modeling the spectral shape of absorption by chromophoric dissolved organic matter. Mar. Chem. 2004, 89, 69–88. [Google Scholar] [CrossRef]
- Guenther, E.A.; Johnson, K.S.; Coale, K.H. Direct ultraviolet spectrophotometric determination of total sulfide and iodide in natural waters. Anal. Chem. 2001, 73, 3481–3487. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Hur, J. Utilization of UV-Vis spectroscopy and related data analyses for dissolved organic matter (DOM) studies: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 131–154. [Google Scholar] [CrossRef]
- Frank, C.; Meier, D.; Voß, D.; Zielinski, O. Computation of nitrate concentrations in coastal waters using an in situ ultraviolet spectrophotometer: Behavior of different computation methods in a case study a steep salinity gradient in the southern North Sea. Methods Oceanogr. 2014, 9, 34–43. [Google Scholar] [CrossRef]
- Sakamoto, C.M.; Johnson, K.S.; Coletti, L.J. Improved algorithm for the computation of nitrate concentrations in seawater using an in situ ultraviolet spectrophotometer. Limnol. Oceanogr. Methods 2009, 7, 32–143. [Google Scholar] [CrossRef]
- Zielinski, O.; Voß, D.; Saworski, B.; Fiedler, B.; Körtzinger, A. Computation of nitrate concentrations in turbid coastal waters using an in situ ultraviolet spectrophotometer. Sea Res. 2011, 65, 456–460. [Google Scholar] [CrossRef]
- Press, W.; Flannery, B.; Teukolsky, S.; Vetterling, W. Numerical Recipes: The Art of Scientific Computing; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Sen, A. Srivastava, M. Unequal Variances, Regression Analysis; Springer: Berlin, Germany, 1990; pp. 111–131. [Google Scholar]
- Stone, M.P. Cross-validatory choice and assessment of statistical predictions. Inst. Phys. Pub. 1974, 36, 111–133. [Google Scholar] [CrossRef]
- Tenenhaus, M. La Régression PLS: Théorie et Pratique; Editions Technip: Paris, France, 1998. [Google Scholar]
- Abdi, H. Partial least squares regression and projection on latent structure regression (PLS-regression). Comput. Stat. 2010, 2, 97–106. [Google Scholar] [CrossRef]
- Chang, H.; Zhu, L.; Lou, X.; Meng, X.; Guo, Y.; Wang, Z. Local Strategy Combined with a Wavelength Selection Method for Multivariate Calibration. Sensors 2016, 16, 827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamouei, M.; Budidha, K.; Baishya, N.; Qassem, M.; Kyriacou, P. Comparison of wavelength selection methods for in-vitro estimation of lactate: A new unconstrained, genetic algorithm-based wavelength selection. Sci. Rep. 2020, 10, 16905. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.P.; Munck, L.; Engelsen, S.B.; Norgaard, L.; Saudland, A.; Wagner, J. Interval Partial Least-Squares Regression (iPLS): A Comparative Chemometric Study with an Example from Near-Infrared Spectroscopy. Appl. Spectrosc. 2000, 54, 413–419. [Google Scholar]
- Sakamoto, C.M.; Johnson, K.S.; Coletti, L.J.; Jannasch, H.W. Pressure correction for the computation of nitrate concentrations in seawater using an in situ ultraviolet spectrophotometer. Limnol. Ocanogr. Methods 2017, 15, 897–902. [Google Scholar] [CrossRef] [Green Version]
| Calibration Set Samples | |||||||
|---|---|---|---|---|---|---|---|
| No. | NO3− (μM) | NO2− (μM) | Salinity (psu) | No. | NO3− (μM) | NO2− (μM) | Salinity (psu) |
| 1 | 0.00 | 0.00 | 0.00 | 18 | 0.97 | 0.00 | 33.24 |
| 2 | 0.50 | 0.00 | 0.00 | 19 | 0.00 | 9.15 | 12.33 |
| 3 | 1.98 | 0.00 | 0.00 | 20 | 0.00 | 1.93 | 26.08 |
| 4 | 9.92 | 0.00 | 0.00 | 21 | 0.00 | 0.50 | 33.56 |
| 5 | 49.61 | 0.00 | 0.00 | 22 | 4.75 | 0.96 | 32.44 |
| 6 | 0.00 | 0.50 | 0.00 | 23 | 20.89 | 5.30 | 26.76 |
| 7 | 0.00 | 2.01 | 0.00 | 24 | 0.95 | 0.97 | 26.08 |
| 8 | 0.00 | 10.06 | 0.00 | 25 | 5.25 | 1.06 | 17.94 |
| 9 | 0.00 | 20.12 | 0.00 | 26 | 11.67 | 2.96 | 19.94 |
| 10 | 0.00 | 0.00 | 6.78 | 27 | 8.27 | 1.05 | 14.13 |
| 11 | 0.00 | 0.00 | 16.95 | 28 | 25.44 | 10.32 | 13.91 |
| 12 | 0.00 | 0.00 | 27.12 | 29 | 4.75 | 0.96 | 8.11 |
| 13 | 0.00 | 0.00 | 33.90 | 30 | 11.81 | 2.40 | 10.09 |
| 14 | 4.75 | 0.96 | 0.00 | 31 | 20.89 | 5.30 | 8.92 |
| 15 | 20.89 | 5.30 | 0.00 | 32 | 52.22 | 10.59 | 8.92 |
| 16 | 55.12 | 0.00 | 15.07 | 33 | 85.62 | 2.22 | 1.51 |
| 17 | 9.45 | 0.00 | 25.83 | 34 | 26.85 | 6.69 | 8.86 |
| Prediction Set Samples | |||||||
| 1 | 9.23 | 1.17 | 12.61 | 11 | 67.18 | 11.04 | 7.04 |
| 2 | 0.49 | 0.49 | 33.24 | 12 | 71.87 | 4.86 | 20.69 |
| 3 | 4.61 | 2.34 | 25.23 | 13 | 66.87 | 5.07 | 20.54 |
| 4 | 11.02 | 2.79 | 30.13 | 14 | 18.66 | 0.52 | 9.58 |
| 5 | 27.54 | 7.95 | 9.32 | 15 | 32.56 | 15.97 | 3.56 |
| 6 | 34.20 | 6.65 | 12.59 | 16 | 12.35 | 8.68 | 23.98 |
| 7 | 43.52 | 8.50 | 10.06 | 17 | 29.95 | 3.76 | 32 |
| 8 | 39.12 | 14.6 | 11.25 | 18 | 56.71 | 3.56 | 12.54 |
| 9 | 23.24 | 4.26 | 29.32 | 19 | 18.11 | 2.83 | 23.65 |
| 10 | 9.68 | 4.05 | 11.23 | 20 | 35.9 | 2.91 | 26.57 |
| Factors | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| PLS1 | |||||||
| PRESS-NO3− | 1962 | 1391 | 327.6 | 2.39 | 2.51 | 2.53 | 3.96 |
| Qh2-NO3− | - | 0.17 | 0.77 | 0.99 | −0.36 | −0.75 | −2.13 |
| Cumulative Contribution Rate -NO3− (%) | 91.34 | 99.50 | 99.95 | 99.99 | |||
| PRESS-NO2− | 614.3 | 504.6 | 191.7 | 7.24 | 8.11 | 9.13 | 5.81 |
| Qh2-NO2− | - | 0.02 | 0.48 | 0.95 | −1.30 | −2.59 | −2.35 |
| Cumulative Contribution Rate -NO2− (%) | 90.52 | 99.60 | 99.95 | 99.99 | |||
| PRESS-Salinity | 3552 | 951.9 | 61.34 | 9.64 | 11.67 | 12.01 | 11.67 |
| Qh2-Salinity | - | 0.72 | 0.92 | 0.77 | −0.53 | −0.78 | −1.00 |
| Cumulative Contribution Rate -Salinity (%) | 76.89 | 99.62 | 99.96 | 99.99 | |||
| PLS2 | |||||||
| PRESS | 7432 | 3651 | 543.4 | 19.27 | 21.89 | 23.17 | 18.79 |
| Qh2 | - | 0.47 | 0.80 | 0.95 | −0.68 | −0.95 | −0.91 |
| Cumulative Contribution Rate (%) | 91.30 | 99.62 | 99.95 | 99.99 | |||
| Spiked (μM) | Found (μM) | Recovery (%) | |||
|---|---|---|---|---|---|
| NO3− | NO2− | NO3− | NO2− | NO3− | NO2− |
| 3.53 | 2.10 | 3.22 | 1.92 | 109.74 | 109.36 |
| 10.95 | 14.20 | 11.23 | 13.83 | 97.50 | 102.66 |
| 19.46 | 1.94 | 18.83 | 2.43 | 103.36 | 79.97 |
| 28.15 | 6.28 | 29.26 | 6.35 | 96.22 | 98.85 |
| 28.78 | 3.83 | 28.18 | 4.27 | 102.11 | 89.75 |
| 37.82 | 5.66 | 37.17 | 5.98 | 101.76 | 94.69 |
| 46.62 | 7.44 | 45.90 | 7.45 | 101.57 | 99.93 |
| 55.17 | 9.18 | 54.54 | 9.26 | 101.16 | 99.13 |
| Sample | NO3− (μM) | NO2− (μM) | Salinity (psu) |
|---|---|---|---|
| 1 | 11.96 ± 0.13 (10.55 ± 0.54) | 4.63 ± 0.13 (3.59 ± 0.12) | 23.53 ± 0.04 |
| 2 | 75.73 ± 0.32 (74.68 ± 1.26) | 3.73 ± 0.17 (3.14 ± 0.09) | 17.07 ± 0.37 |
| 3 | 51.60 ± 0.26 (52.19 ± 1.77) | 2.47 ± 0.19 (1.57 ± 0.21) | 21.30 ± 0.43 |
| 4 | 33.36 ± 0.08 (31.85 ± 0.89) | 7.41 ± 0.08 (6.53 ± 1.12) | 23.83 ± 0.01 |
| 5 | 19.36 ± 0.12 (20.73 ± 0.63) | 1.27 ± 0.11 (0.67 ± 0.09) | 35.29 ± 0.21 |
| 6 | 21.27 ± 0.11 (20.06 ± 0.42) | 0.11 ± 0.16 (0.26 ± 0.04) | 29.13 ± 0.21 |
| 7 | 73.67 ± 0.09 (72.12 ± 1.02) | 0.47 ± 0.13 (1.03 ± 0.13) | 3.66 ± 0.04 |
| 8 | 14.18 ± 0.14 (12.53 ± 0.56) | −0.35 ± 0.11 (0.49 ± 0.08) | 31.99 ± 0.21 |
| 9 | 68.88 ± 0.28 (67.18 ± 1.55) | 5.92 ± 0.22 (4.25 ± 0.06) | 20.56 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ju, A.; Wang, L. Ultraviolet Spectroscopic Detection of Nitrate and Nitrite in Seawater Simultaneously Based on Partial Least Squares. Molecules 2021, 26, 3685. https://doi.org/10.3390/molecules26123685
Wang H, Ju A, Wang L. Ultraviolet Spectroscopic Detection of Nitrate and Nitrite in Seawater Simultaneously Based on Partial Least Squares. Molecules. 2021; 26(12):3685. https://doi.org/10.3390/molecules26123685
Chicago/Turabian StyleWang, Hu, Aobo Ju, and Lequan Wang. 2021. "Ultraviolet Spectroscopic Detection of Nitrate and Nitrite in Seawater Simultaneously Based on Partial Least Squares" Molecules 26, no. 12: 3685. https://doi.org/10.3390/molecules26123685






