The Botanical, Chemical and Ethnobotanical Diversity of Southern African Lamiaceae
Abstract
:1. Introduction
2. Botanical Diversity
- Subfamily 1. Prostantheroideae Luerssen. No southern African genera.
- Subfamily 2. Symphorematoideae Briquet. No southern African genera.
- Subfamily 3. Viticoideae Briquet. Vitex (ca. 250 spp., 10 in southern Africa).
- Subfamily 4. Nepetoideae (Dumortier) Luerssen. This is the largest subfamily and contains ca. 118 genera and ca, 3400 species [22]. Rosmarinic acid is a potential synapomorphy for the subfamily. The tribe Mentheae Dumort. includes the genera Killickia Bräuchler, Heubl and Doroszenko, Mentha L., Micromeria Benth., Salvia, while the tribe Ocimeae Dumort. has two subtribes: Ociminae J.A. Schmidt, with the genera Basilicum, Endostemon N.E. Br., Haumaniastrum, Hoslundia, Ocimum, Orthosiphon Benth., Platostoma and Syncolostemon; subtribe Plectranthinae Endl., with the genera Aeollanthus C.Mart. ex Spreng., Coleus, Equilabium Mwany., A.J. Paton and Culham, Plectranthus, Rabdosiella, Tetradenia Benth. and Thorncroftia N.E. Br.
- Subfamily 5. Premnoideae B. Li, R. G. Olmstead and P. D. Cantino. Premna (50–200 spp., only 1 in southern Africa).
- Subfamily 6. Ajugoideae Kosteletzky. Ajuga, Clerodendrum L., Kalaharia, Karomia, Rotheca Raf., Teucrium and Volkameria.
- Subfamily 7. Peronematoideae B. Li, R. G. Olmstead and P. D. Cantino. No southern African genera.
- Subfamily 8. Scutellarioideae (Dumortier) Caruel. Tinnea Kotschy ex Hook. f. (19 species, all endemic to Africa, 4 in southern Africa).
- Subfamily 9. Cymarioideae B. Li, R. G. Olmstead and P. D. Cantino. No southern African genera.
- Subfamily 10. Lamioideae Harley. This is the largest subfamily in the Old World and is second only to Nepetoideae in terms of the numbers of taxa, with more than 60 genera and ca. 1200 species. It has been suggested [19] that allenic components in the seed oil may be a synapomorphy for the subfamily. Of the 10 tribes recognized [19], only three are represented in southern Africa: Stachydeae with Stachys, Marrubieae with Pseudodictamnus Fabr. and Leucadeae with Acrotome Benth. ex Endl., Harmsiella Briq., Leonotis (Pers.) R.Br. and Leucas R.Br.
3. Chemical Compounds
3.1. Alkaloids
3.2. Coumarins
3.3. Phenolics
3.4. Pyrones
3.5. Terpenoids and Steroids
3.6. Volatile Oils
4. Traditional Uses
4.1. Medicinal Uses
4.2. Food Uses
4.2.1. Fruits and Vegetables
4.2.2. Beverages
4.3. Other Uses
5. Discussion
6. Materials and Methods
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prakash, O.; Chandra, M.; Pant, A.K.; Rawat, D.S. Mint (Mentha spicata L.) Oils; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780124166448. [Google Scholar]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World; CABI: Boston, MA, USA, 2017; ISBN 1786393255. [Google Scholar]
- Van Wyk, B.-E. Food Plants of the World: Identification, Culinary Uses and Nutritional Value; CABI: Boston, MA, USA, 2019; ISBN 1789241308. [Google Scholar]
- Van Wyk, B.-E. Culinary Herbs and Spices of the World; University of Chicago Press: Chicago, IL, USA, 2014; ISBN 022609183X. [Google Scholar]
- Van Wyk, B.-E. A family-level floristic inventory and analysis of medicinal plants used in Traditional African Medicine. J. Ethnopharmacol. 2020, 249, 112351. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, M. (Ed.) Lamiaceae Species: Biology, Ecology and Practical Uses; MDPI: Basel, Switzerland, 2020; ISBN 9783039284191. [Google Scholar]
- Tolley, K.A.; Da Silva, J.M.; Jansen van Vuuren, B. South African National Biodiversity Assessment 2018 Technical Report Volume 7: Genetic Diversity; SANBI: Pretoria, South Africa, 2019. [Google Scholar]
- Mamadalieva, N.Z.; Akramov, D.K.; Wessjohann, L.A.; Hussain, H.; Long, C.; Tojibaev, K.S.; Alshammari, E.; Ashour, M.L.; Wink, M. The Genus Lagochilus (Lamiaceae): A review of its diversity, ethnobotany, phytochemistry, and pharmacology. Plants 2021, 10, 132. [Google Scholar] [CrossRef]
- Arnold, T.H.; Prentice, C.A.; Hawker, L.C.; Snyman, E.E.; Tomalin, M.; Crouch, N.R.; Pottas-Bircher, C. Medicinal Magical Plants of Southern AFRICA: An Annotated Checklist; National Botanical Institute: Pretoria, South Africa, 2002; ISBN 9780874216561. [Google Scholar]
- Nsuala, B.N.; Enslin, G.; Viljoen, A. “Wild cannabis”: A review of the traditional use and phytochemistry of Leonotis leonurus. J. Ethnopharmacol. 2015, 174, 520–539. [Google Scholar] [CrossRef] [PubMed]
- Aston Philander, L. An ethnobotany of Western Cape Rasta bush medicine. J. Ethnopharmacol. 2011, 138, 578–594. [Google Scholar] [CrossRef]
- Van Wyk, B.-E. The potential of South African plants in the development of new food and beverage products. S. Afr. J. Bot. 2011, 77, 857–868. [Google Scholar] [CrossRef] [Green Version]
- Asowata-Ayodele, A.M.; Afolayan, A.J.; Otunola, G.A. Ethnobotanical survey of culinary herbs and spices used in the traditional medicinal system of Nkonkobe Municipality, Eastern Cape, South Africa. S. Afr. J. Bot. 2016, 104, 69–75. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Gorelik, B. The history and ethnobotany of Cape herbal teas. S. Afr. J. Bot. 2017, 110, 18–38. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Van Oudtshoorn, B.; Gericke, N. Medicinal Plants of South Africa, 2nd ed.; Smit, J., Ed.; Briza Publications: Pretoria, South Africa, 2009; ISBN 978-1-8-75093-37-3. [Google Scholar]
- Harley, R.M.; Atkins, S.; Budantsev, A.L.; Cantino, P.D.; Conn, B.J.; Grayer, R.; Harley, M.M.; de Kok, R.; Krestovskaja, T.; Morales, R.; et al. Labiatae BT—Flowering Plants Dicotyledons: Lamiales (except Acanthaceae including Avicenniaceae); Kadereit, J.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 167–275. ISBN 978-3-642-18617-2. [Google Scholar]
- Codd, L.E. Flora of Southern Africa: Part 4 Lamiaceae; Leistner, O.A., Ed.; Botanical Research Institute, Departmet of Agriculture and Water Supply, Republic of South Africa: Pretoria, South Africa, 1985; ISBN 9780621082685. [Google Scholar]
- Paton, A.J.; Mwanyambo, M.; Govaerts, R.H.A.; Smitha, K.; Suddee, S.; Phillipson, P.B.; Wilson, T.C.; Forster, P.I.; Culham, A. Nomenclatural changes in Coleus and Plectranthus (Lamiaceae): A tale of more than two genera. PhytoKeys 2019, 129, 1–158. [Google Scholar] [CrossRef]
- Hiltunen, R.; Holm, Y. Basil: The Genus Ocimum; Harwood Academic Publishers: Amsterdam, The Netherlands, 1999; ISBN 9057024322. [Google Scholar]
- Otieno, D.F.; Balkwill, K.; Paton, A.J.; Savolainen, V. A reassessment of Hemizygia and Syncolostemon (Ocimeae—Lamiaceae). Taxon 2006, 55, 941–958. [Google Scholar] [CrossRef]
- Marx, H.E.; O’Leary, N.; Yuan, Y.W.; Lu-Irving, P.; Tank, D.C.; Múlgura, M.E.; Olmstead, R.G. A molecular phylogeny and classification of Verbenaceae. Am. J. Bot. 2010, 97, 1647–1663. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Olmstead, R.G.; Zahra, N.; Shinwari, Z.; Kubitzki, K.; Huelsenbeck, J.P.; Ronquist, F.; Boachon, B.; Buell, C.R.; Crisovan, E.; et al. A large-scale chloroplast phylogeny of the Lamiaceae sheds new light on its subfamilial classification. Mol. Phylogenet. Evol. 2016, 6, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frezza, C.; Venditti, A.; Serafini, M.; Bianco, A. Phytochemistry, Chemotaxonomy, Ethnopharmacology, and Nutraceutics of Lamiaceae, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 62, ISBN 9780444641854. [Google Scholar]
- Vladimir-Knezevic, S.; Blazekovic, B.; Kindl, M.; Vladic, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Štefan, M.B.; Vuković Rodríguez, J.; Blažeković, B.; Kindl, M.; Vladimir-Knežević, S. Total Hydroxycinnamic Acids Assay: Prevalidation and Application on Lamiaceae Species. Food Anal. Methods 2014, 7, 326–336. [Google Scholar] [CrossRef]
- Dhifi, W.; Litaiem, M.; Jelali, N.; Hamdi, N.; Mnif, W. Identification of a new chemotye of the plant Mentha aquatica grown in Tunisia: Chemical composition, antioxidant and biological activities of its essential oil. J. Essent. Oil-Bear. Plants 2011, 14, 320–328. [Google Scholar] [CrossRef]
- Esmaeili, A.; Rustaiyan, A.; Masoudi, S.; Nadji, K. Composition of the essential oils of Mentha aquatica L. and Nepeta meyeri Benth. from Iran. J. Essent. Oil Res. 2006, 18, 263–265. [Google Scholar] [CrossRef]
- Guetat, A.; Al-Ghamdi, F.A.; Osman, A.K. 1,8-Cineole, α-Pinene and Verbenone chemotype of essential oil of species Rosmarinus officinalis L. from Saudi Arabia. Int. J. Herb. Med. 2014, 2, 137–141. [Google Scholar]
- Rivett, D.E.A. Isolation of marrubiin from Leonotis leonurus. J. Chem. Soc. 1964, 1857–1858. [Google Scholar]
- Dellar, J.E.; Cole-Davies, M.; Waterman, P.G. Unusual antimicrobial compounds from Aeollanthus buchnerianus. Experientia 1996, 52, 175–179. [Google Scholar] [CrossRef]
- Chen, Y.S.; Yu, H.M.; Shie, J.J.; Cheng, T.J.R.; Wu, C.Y.; Fang, J.M.; Wong, C.H. Chemical constituents of Plectranthus amboinicus and the synthetic analogs possessing anti-inflammatory activity. Bioorg. Med. Chem. 2014, 22, 1766–1772. [Google Scholar] [CrossRef]
- Teixeira, A.P.; Batista, O.; Fátima Simões, M.; Nascimento, J.; Duarte, A.; De La Torre, M.C.; Rodríguez, B. Abietane diterpenoids from Plectranthus grandidentatus. Phytochemistry 1997, 44, 325–327. [Google Scholar] [CrossRef] [Green Version]
- Hanson, J.R.; Rivett, D.E.A.; Ley, S.V.; Williams, D.J. The X-ray structure and absolute configuration of insect antifeedant clerodane diterpenoids from Teucrium africanum. J. Chem. Soc. Perkin Trans. 1 1982, 1005–1008. [Google Scholar] [CrossRef]
- Iyambo, N.K.; Kibuule, D.; Ilonga, K.S. Antipseudomonal potential of Colophospermum mopane and Acrotome inflata, medicinal plants indigenous to Namibia. Afr. J. Pharm. Pharmacol. 2017, 11, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Davies-Coleman, M.T.; Rivett, D.E.A. Structure of the 5,6-dihydro-α-pyrone, umuravumbolide. Phytochemistry 1995, 38, 791–792. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; De Kimpe, N. Tetradenolide, an α-pyrone from Tetradenia riparia. Phytochemistry 1998, 49, 1157–1158. [Google Scholar] [CrossRef]
- Collett, L.A.; Davies-Coleman, M.T.; Rivett, D.E.A.; Drewes, S.E.; Horn, M.M. Absolute configuration of α-pyrones from Cryptocarya latifolia and Syncolostemon densiflorus. Phytochemistry 1997, 44, 935–938. [Google Scholar] [CrossRef]
- Ladan, Z.; Amupitan, J.O.; Oyewale, O.A.; Ayo, R.G.; Temple, E.; Ladan, E.O. Phytochemical screening of the leaf extracts of Hyptis spicigera plant. Afr. J. Pure Appl. Chem. 2014, 8, 83–88. [Google Scholar] [CrossRef]
- Matias, D.; Nicolai, M.; Fernandes, A.S.; Saraiva, N.; Almeida, J.; Saraiva, L.; Faustino, C.; Díaz-Lanza, A.M.; Reis, C.P.; Rijo, P. Comparison study of different extracts of Plectranthus madagascariensis, P. neochilus and the rare P. porcatus (Lamiaceae): Chemical characterization, antioxidant, antimicrobial and cytotoxic activities. Biomolecules 2019, 9, 179. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A.; Passacantilli, P.; Righi, G.; Nicoletti, M.; Serafini, M. 10-Deoxymelittoside, an iridoid diglucoside, and other iridoids from Lamiastrum galeobdolon. Phytochemistry 1986, 25, 1981–1983. [Google Scholar] [CrossRef]
- Nafuka, S.N. In Vitro Antiplasmodial Activity and Phytochemicals Screening of Ethnomedicinal Plants Used to Treat Malaria Associated Symptoms. Ph.D. Thesis, University of Namibia, Windhoek, Namibia, 2014. [Google Scholar]
- Grayer, R.J.; Eckert, M.R.; Veitch, N.C.; Kite, G.C.; Marin, P.D.; Kokubun, T.; Simmonds, M.S.J.; Paton, A.J. The chemotaxonomic significance of two bioactive caffeic acid esters, Nepetoidins A and B, in the Lamiaceae. Phytochemistry 2003, 64, 519–528. [Google Scholar] [CrossRef]
- Chisowa, E.H.; Mumba, P.; Hall, D.R.; Farman, D.I. Composition of the essential oil of Aeollanthus parvifolius Benth. J. Essent. Oil Res. 1998. [Google Scholar] [CrossRef]
- Cui, H.X.; Qiu, Y.; Ge, W.C.; Cheng, F.R.; Yuan, K. Biological activity and phytochemical composition of the volatile oils from Basilicum polystachyon. J. Chem. Soc. Pak. 2017, 39, 43–49. [Google Scholar]
- Tan, Y.P.; Xue, Y.; Savchenko, A.I.; Houston, S.D.; Modhiran, N.; McMillan, C.L.D.; Boyle, G.M.; Bernhardt, P.V.; Young, P.R.; Watterson, D.; et al. Basimarols A, B, and C, highly oxygenated Pimarane Diterpenoids from Basilicum polystachyon. J. Nat. Prod. 2019, 82, 2828–2834. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.P.; Houston, S.D.; Modhiran, N.; Savchenko, A.I.; Boyle, G.M.; Young, P.R.; Watterson, D.; Williams, C.M. Stachyonic Acid: A Dengue Virus inhibitor from Basilicum polystachyon. Chem. A Eur. J. 2019, 25, 5664–5667. [Google Scholar] [CrossRef] [PubMed]
- Touani, F.K.; Seukep, A.J.; Djeussi, D.E.; Fankam, A.G.; Noumedem, J.A.K.; Kuete, V. Antibiotic-potentiation activities of four Cameroonian dietary plants against multidrug-resistant Gram-negative bacteria expressing efflux pumps. BMC Complement. Altern. Med. 2014, 14, 258. [Google Scholar] [CrossRef] [Green Version]
- Koba, K.; Raynaud, C.; Millet, J.; Chaumont, J.-P.; Sanda, K. Chemical composition of Hyptis pectinata L., H. lanceolata Poit, H. suaveolens (L) Poit and H. spicigera Lam. essential oils from Togo. J. Essent. Oil-Bear. Plants 2007, 10, 357–364. [Google Scholar] [CrossRef]
- Aycard, J.-P.; Kini, F.; Kam, B.; Gaydou, E.M.; Faure, R. Isolation and Identification of Spicigera Lactone: Complete 1H and 13C assignments using Two-Dimensional NMR Experiments. J. Nat. Prod. 1993, 56, 1171–1173. [Google Scholar] [CrossRef]
- Kini, F.; Kam, B.; Aycard, J.P.; Gaydou, E.M.; Bombarda, I. Chemical composition of the essential oil of Hyptis spicigera Lam. from Burkina Faso. J. Essent. Oil Res. 1993, 5, 219–221. [Google Scholar] [CrossRef]
- Fragoso-Serrano, M.; González-Chimeo, E.; Pereda-Miranda, R. Novel labdane diterpenes from the insecticidal plant Hyptis spicigera. J. Nat. Prod. 1999, 62, 45–50. [Google Scholar] [CrossRef]
- Bailac, P.; Duschatzky, C.; Ponzi, M.; Firpo, N. Essential oil of Hyptis mutabilis (Rich.) Briq. Grown in San Luis, Argentina. J. Essent. Oil Res. 1999, 11, 217–219. [Google Scholar] [CrossRef]
- Pereda-Miranda, R.; Gascon-Figueroa, M. Chemistry of Hyptis mutabilis: New pentacyclic triterpenoids. J. Nat. Prod. 1988, 51, 996. [Google Scholar] [CrossRef]
- McNeil, M.; Facey, P.; Porter, R. Essential oils from the Hyptis genus—A review (1909–2009). Nat. Prod. Commun. 2011, 6, 1775–1796. [Google Scholar] [CrossRef] [Green Version]
- Velasco-Negueruela, A.; Perez-Alonso, M.J.; Esteban, J.L.; Guzman, C.A.; Zygadlo, J.A.; Espinar, L.A. Volatile constituents of Hyptis mutabilis (Rich.) Briq. J. Essent. Oil Res. 1995, 7, 81. [Google Scholar] [CrossRef]
- López-Garcia, R.E.; Hernandez-Perez, M.; Rabanal, R.M.; Darias, V.; Martin-Herrera, D.; Arias, A.; Sanz, J. Essential oils and antimicrobial activity of two varieties of Cedronella canariensis (L.) W. et B. J. Ethnopharmacol. 1992, 36, 207. [Google Scholar] [CrossRef]
- Carreiras, M.E.; Rodríguez, B.; López-Garcia, R.E.; Rabanal, R.M. A dimer of D-Pinocarvone from Cedronella canariensis. Phytochemistry 1987, 26, 3351–3353. [Google Scholar] [CrossRef]
- Abdel-Mogib, M.; Albar, H.A.; Batterjee, S.M. Chemistry of the genus Plectranthus. Molecules 2002, 7, 271–301. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, G.; Sinniah, U.R.; Swamy, M.K.; Lynch, P.T. Micropropagation and essential oil characterization of Plectranthus amboinicus (Lour.) Sprengel, an aromatic medicinal plant. In Vitro Cell. Dev. Biol. Plant 2020, 56, 491–503. [Google Scholar] [CrossRef]
- Janakiraman, D.; Parameswari, C.S. Active phenolic constituents and brine shrimp lethality assay of hydroalcoholic extract of Plectranthus amboinicus. Asian J. Pharm. Clin. Res. 2017, 10, 186–189. [Google Scholar] [CrossRef] [Green Version]
- Ashaari, N.S.; Rahim, M.H.A.; Sabri, S.; Lai, K.S.; Song, A.A.L.; Rahim, R.A.; Wan Abdullah, W.M.A.N.; Abdullah, J.O. Functional characterization of a new terpene synthase from Plectranthus amboinicus. PLoS ONE 2020, 15, e0235416. [Google Scholar] [CrossRef]
- Aguiar, J.J.S.; Sousa, C.P.B.; Araruna, M.K.A.; Silva, M.K.N.; Portelo, A.C.; Lopes, J.C.; Carvalho, V.R.A.; Figueredo, F.G.; Bitu, V.C.N.; Coutinho, H.D.M.; et al. Antibacterial and modifying-antibiotic activities of the essential oils of Ocimum gratissimum L. and Plectranthus amboinicus L. Eur. J. Integr. Med. 2015, 7, 151–156. [Google Scholar] [CrossRef]
- Wibisono, K.; Aisyah, S.I.; Suhesti, S.; Nurcholis, W.; Science, N.; Biopharmaca, T. Optimization of total flavonoids extraction and A-glucosidase inhibitory activity from Plectranthus amboinicus (Lour.) Spreng. leaves using the simplex-centroid design. Molekul 2019, 14, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, R.d.C.d.F.; Neto, F.B.d.O.; da Silva, F.F.M.; Bertini, L.M.; Alves, L.A. Seasonal effect in essential oil composition and antioxidant activity of Plectranthus amboinicus leaves. Biosci. J. 2017, 1608–1616. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.Q.; Minh, L.V.; Trieu, L.H.; Bui, L.M.; Lam, T.D.; Hieu, V.Q.; Khang, T.V.; Trung, L.N.Y. Evaluation of total polyphenol content, total flavonoid content, and antioxidant activity of Plectranthus amboinicus leaves. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736. [Google Scholar] [CrossRef]
- Bugayong, A.M.; Cruz, P.; Padilla, P.I. Antibacterial activity and chemical composition of essential oils from leaves of some aromatic plants of Philippines. J. Essent. Oil Bear. Plants 2019, 22, 932–946. [Google Scholar] [CrossRef]
- Hsu, K.P.; Ho, C.L. Antimildew effects of Plectranthus amboinicus leaf essential oil on paper. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Murthy, P.S.; Ramalakshmi, K.; Srinivas, P. Fungitoxic activity of Indian borage (Plectranthus amboinicus) volatiles. Food Chem. 2009, 114, 1014–1018. [Google Scholar] [CrossRef]
- Gelmini, F.; Squillace, P.; Testa, C.; Sparacino, A.C.; Angioletti, S.; Beretta, G. GC-MS characterisation and biological activity of essential oils from different vegetative organs of Plectranthus barbatus and Plectranthus caninus cultivated in north Italy. Nat. Prod. Res. 2015, 29, 993–998. [Google Scholar] [CrossRef]
- Alasbahi, R.H.; Melzig, M.F. Plectranthus barbatus: A review of phytochemistry, ethnobotanical uses and pharmacology—Part 1. Planta Med. 2010, 76, 653–661. [Google Scholar] [CrossRef] [Green Version]
- Falé, P.L.; Borges, C.; Madeira, P.J.A.; Ascensão, L.; Araújo, M.E.M.; Florêncio, M.H.; Serralheiro, M.L.M. Rosmarinic acid, scutellarein 4′-methyl ether 7-O-glucuronide and (16S)-coleon E are the main compounds responsible for the antiacetylcholinesterase and antioxidant activity in herbal tea of Plectranthus barbatus (“falso boldo”). Food Chem. 2009, 114, 798–805. [Google Scholar] [CrossRef]
- Kelecom, A.; Dos Santos, T.C. Cariocal, a new Seco-Abietane Diterpene from the Labiate Coleus barbatus. Tetrahedron Lett. 1985, 26, 3659–3662. [Google Scholar] [CrossRef]
- Kelecom, A.; Dos Santos, T.C.; Medeiros, W.L.B. Secoabitane Diterpenes from Coleus barbatus. Phytochemistry 1987, 26, 2337–2340. [Google Scholar] [CrossRef]
- Kelecom, A. Isolation, structure determination, and absolute configuration of barbatusol, a new bioactive diterpene with a rearranged abietane skeleton from the Labiate Coleus barbatus. Tetrahedron 1983, 39, 3603–3608. [Google Scholar] [CrossRef]
- Kelecom, A. An abietane Diterpene from the Labiate Coleus barbatus. Phytochemistry 1984, 23, 1677–1679. [Google Scholar] [CrossRef]
- Musayeib, N.M.; Al Amina, M.; Al-hamoud, G.A.; Mohamed, G.A.; Ibrahim, S.R.M.; Shabana, S. Plectrabarbene, a new abietane diterpene from Plectranthus barbatus aerial parts. Molecules 2020, 25, 2365. [Google Scholar] [CrossRef]
- Schultz, C.; Bossolani, M.P.; Torres, L.M.B.; Lima-Landman, M.T.R.; Lapa, A.J.; Souccar, C. Inhibition of the gastric H+,K+-ATPase by plectrinone A, a diterpenoid isolated from Plectranthus barbatus Andrews. J. Ethnopharmacol. 2007, 111, 1–7. [Google Scholar] [CrossRef]
- Cuthbertson, D.J.; Johnson, S.R.; Piljac-Žegarac, J.; Kappel, J.; Schäfer, S.; Wüst, M.; Ketchum, R.E.B.; Croteau, R.B.; Marques, J.V.; Davin, L.B.; et al. Accurate mass-time tag library for LC/MS-based metabolite profiling of medicinal plants. Phytochemistry 2013, 91, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Banthorpe, D.V.; Bilyard, H.J.; Brown, G.D. Enol esters of caffeic acid in several genera of the Labiatae. Phytochemistry 1989, 28, 2109–2113. [Google Scholar] [CrossRef]
- Mota, L.; Figueiredo, A.C.; Pedro, L.G.; Barroso, J.G.; Miguel, G.M.; Faleiro, M.L.; Ascensão, L. Volatile-oils composition, and bioactivity of the essential oils of Plectranthus barbatus, P. neochilus, and P. ornatus grown in Portugal. Chem. Biodivers. 2014, 11, 719–732. [Google Scholar] [CrossRef]
- Mesquita, L.S.F.; Matos, T.S.; Do Nascimento Ávila, F.; Da Silva Batista, A.; Moura, A.F.; De Moraes, M.O.; Da Silva, M.C.M.; Ferreira, T.L.A.; Nascimento, N.R.F.; Monteiro, N.K.V.; et al. Diterpenoids from leaves of cultivated Plectranthus ornatus. Planta Med. 2020. [Google Scholar] [CrossRef]
- Passinho-Soares, H.C.; Meira, P.R.; David, J.P.; Mesquita, P.R.R.; Do Vale, A.E.; De Rodrigues, F.M.; De Pereira, P.A.P.; De Santana, J.R.F.; De Oliveira, F.S.; De Andrade, J.B.; et al. Volatile organic compounds obtained by in vitro callus cultivation of Plectranthus ornatus Codd. (Lamiaceae). Molecules 2013, 18, 10320–10333. [Google Scholar] [CrossRef] [Green Version]
- Rijo, P.; Rodriguez, B.; Duarte, A.; Fatima Simoes, M. Antimicrobial Properties of Plectranthus ornatus Extracts, 11-acetoxyhalima-5, 13-dien-15-oic Acid Metabolite and its Derivatives. Nat. Prod. J. 2011, 1, 57–64. [Google Scholar] [CrossRef]
- Rijo, P.; Gaspar-Marques, C.; Simões, M.F.; Duarte, A.; del Apreda-Rojas, M.C.; Cano, F.H.; Rodríguez, B. Neoclerodane and labdane diterpenoids from Plectranthus ornatus. J. Nat. Prod. 2002, 65, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Medrado, H.H.; Dos Santos, E.O.; Ribeiro, E.M.O.; David, J.M.; David, J.P.; Araújo, J.F.; Do Vale, A.E.; Bellintani, M.C.; Brandão, H.N.; Meira, P.R. Rosmarinic and cinnamic acid derivatives of in vitro tissue culture of Plectranthus ornatus: Overproduction and correlation with antioxidant activities. J. Braz. Chem. Soc. 2017, 28, 505–511. [Google Scholar] [CrossRef]
- Ávila, F.N.; Pinto, F.C.L.; Sousa, T.S.; Torres, M.C.M.; Costa-Lotufo, L.V.; Rocha, D.D.; De Vasconcelos, M.A.; Cardoso-Sá, N.; Teixeira, E.H.; Albuquerque, M.R.J.R.; et al. Miscellaneous diterpenes from the aerial parts of Plectranthus ornatus Codd. J. Braz. Chem. Soc. 2017, 28, 1014–1022. [Google Scholar] [CrossRef]
- Rijo, P.; Gaspar-Marques, C.; Simões, M.F.; Jimeno, M.L.; Rodríguez, B. Further diterpenoids from Plectranthus ornatus and P. grandidentatus. Biochem. Syst. Ecol. 2007, 35, 215–221. [Google Scholar] [CrossRef]
- Otaifah, Y.N.; Bouyahya, A.; Talbaoui, A.; Harhar, H.; El Hajjaji, S. Chemical composition of Yemeni medicinal plants essentials oils and their antibacterial and antioxidant activities. Phytothérapie 2018. [Google Scholar] [CrossRef]
- Amina, M.; Al Musayeib, N.M.; Mohamed, G.A.; Ibrahim, S.R.M. Plectraterpene, a new ursane-type triterpene ester and other steroids from the aerial parts of Plectranthus montanus. Rev. Bras. Farmacogn. 2017, 27, 698–701. [Google Scholar] [CrossRef]
- Grayer, R.J.; Eckert, M.R.; Lever, A.; Veitch, N.C.; Kite, G.C.; Paton, A.J. Distribution of exudate flavonoids in the genus Plectranthus. Biochem. Syst. Ecol. 2010, 38, 335–341. [Google Scholar] [CrossRef]
- Maistry, K. The Antimicrobial Properties and Chemical Composition of Leaf Extracts and Essential Oils of Indigenous Plectranthus Species. Ph.D. Thesis, University of the Witwatersand, Johannesburg, South Africa, 2007. [Google Scholar]
- Uchida, M.; Miyase, T.; Yoshizaki, F.; Bieri, J.H.; Riiedi, P.; Eugster, C.H. 4-Hydroxytaxodion als Hauptditerpen in Plectranthus grandidentatus Gurke. Helv. Chim. Acta 1981, 64, 2227–2250. [Google Scholar] [CrossRef]
- Ibrahim, M.E.; Ahmed, S.S.; Hussein, M.S. Chemical investigations and the antimicrobial activity of Ocimum hadiensis (Forssk) plant grown wild in Egypt. J. Mater. Environ. Sci. 2019, 2508, 457–462. [Google Scholar]
- Dukhea, S. The Isolation, Structure Elucidation and Biological Testing of Compounds from Plectranthus hadiensis. Ph.D. Thesis, University of KwaZulu-Natal, Durban, South Africa, 2010. [Google Scholar]
- Sripathi, R.; Ravi, S. Chemical composition and antibacterial activity of the essential oil from the seeds of Plectranthus hadiensis. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 637–639. [Google Scholar] [CrossRef]
- Gurlal, P. Testing for Microbiologically Active Compounds Extracted from Members of the Family Lamiaceae and Other Indigenous Plants. Ph.D. Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2005. [Google Scholar]
- Van Zyl, R.L.; Khan, F.; Edwards, T.J.; Drewes, S.E. Antiplasmodial activities of some abietane diterpenes from the leaves of five Plectranthus species. S. Afr. J. Sci. 2008, 104, 62–64. [Google Scholar]
- Batista, O.; Duarte, A.; Nascimento, J.; Simōes, M.F.; de la Torre, M.C.; Rodríguez, B. Structure and antimicrobial activity of diterpenes from the roots of Plectranthus hereroensis. J. Nat. Prod. 1994, 57, 858–861. [Google Scholar] [CrossRef]
- Batista, O.; Fátima Simões, M.; Duarte, A.; Luisa Valdeira, M.; de la Torre, M.C.; Rodríguez, B. An antimicrobial abietane from the root of Plectranthus hereroensis. Phytochemistry 1995, 38, 167–169. [Google Scholar] [CrossRef]
- Rodriguez, B.; Maria , C.; Simoes, F.; Batsta, O.; Nascimento, J.; Duartet, A.; Mayer, R. Revision of the structure of an Aristolane Sesquiterpene Aldehyde isolated from the root of Plectranthus hereroensis and Aristolochia debilis. Phytochemistry 1995, 38, 905–907. [Google Scholar] [CrossRef]
- Ascensao, L.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Schripsema, J.; Deans, S.G.; Scheffer, J.J.C. Plectranthus madagascariensis: Morphology of the glandular trichomes, essential oil composition, and its biological activity. Int. J. Plant Sci. 1998, 159, 31–38. [Google Scholar] [CrossRef]
- Kubinova, R.; Porizkova, R.; Navratilova, A.; Farsa, O.; Hanakova, Z.; Bacinska, A.; Cizek, A.; Valentova, M. Antimicrobial and enzyme inhibitory activities of the constituents of Plectranthus madagascariensis (Pers.) Benth. J. Enzyme Inhib. Med. Chem. 2014, 29, 749–752. [Google Scholar] [CrossRef]
- Matias, D.; Nicolai, M.; Saraiva, L.; Pinheiro, R.; Faustino, C.; Diaz Lanza, A.; Pinto Reis, C.; Stankovic, T.; Dinic, J.; Pesic, M.; et al. Cytotoxic activity of royleanone diterpenes from Plectranthus madagascariensis Benth. ACS Omega 2019, 4, 8094–8103. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, G.P.; Lima, K.A.; Severiano, M.E.; Groppo, M.; Ambrosio, S.R.; Crevelin, E.J. Antifungal activity of the essential oils of Plectranthus neochilus (Lamiaceae) and Tagetes erecta (Asteraceae) cultivated in Brazil. Int. J. Complement. Altern. Med. 2018, 11, 1–5. [Google Scholar] [CrossRef] [Green Version]
- El-Sakhawy, F.S.; Kassem, H.A.; El-Gayed, S.H.; Mostafa, M.M. Headspace Solid Phase Microextraction Analysis of volatile compounds of the aerial parts and flowers of Plectranthus neochilus Schltr. and Salvia farinacea Benth. J. Essent. Oil-Bear. Plants 2018, 21, 674–686. [Google Scholar] [CrossRef]
- Lambrechts, I.A.; Lall, N. Plectranthus neochilus. In Underexplored Medicinal Plants from Sub-Saharan Africa; Lall, N., Ed.; Academic Press: London, UK, 2020; pp. 235–240. ISBN 9780128168141. [Google Scholar]
- Fanela, T.L.M.; Baldin, E.L.L.; Pannuti, L.E.R.; Cruz, P.L.; Crotti, A.E.M.; Takeara, R.; Kato, M.J. Lethal and inhibitory activities of plant-derived essential oils against Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) Biotype B in tomato. Neotrop. Entomol. 2016, 45, 201–210. [Google Scholar] [CrossRef]
- Da Cruz Marçalo de Andrade, J.E. Unravelling New Ethnopharmacological Roles of Plectranthus Species: Biological Activity Screening. Ph.D. Thesis, University of Lisbon, Lisbon, Portugal, 2016. [Google Scholar]
- Rijo, P. Phytochemical Study and Biological Activities of Diterpenes and Derivatives from Plectranthus Species. Ph.D. Thesis, University of Lisbon, Lisbon, Portugal, 2010. [Google Scholar]
- Manikandan, S.; Alagu Lakshmanan, G.; Chandran, C.; Alagu Lakshmanan, C.G. Phytochemical screening and evaluation of tuber extract of Plectranthus rotundifolius Spreng. by GC-MS and FT-IR spectrum analysis. Int. J. Herb. Med. 2016, 36, 36–40. [Google Scholar]
- Maree, J.E.; Khondkar, P.; Kwapong, A.A.; Oyedemi, B.M.; Aljarba, T.M.; Stapleton, P.; Viljoen, A.M.; Gibbons, S. Bioactive acetophenones from Plectranthus venteri. Phytochem. Lett. 2014, 10. [Google Scholar] [CrossRef]
- Chagonda, L.S.; Chalchat, J.-C. The essential oil of wild and cultivated Hoslundia opposita Vahl. from Zimbabwe. Flavour Fragr. J. 2005, 20, 193–195. [Google Scholar] [CrossRef]
- Achenbach, H.; Waibel, R.; Nkunya, M.H.H.; Weenen, H. Antimalarial compounds from Hoslundia opposita. Phytochemistry 1992, 31, 3781–3784. [Google Scholar] [CrossRef]
- Ngadjui, B.T.; Ayafor, J.F.; Sondengam, B.L.; Connolly, J.D.; Rycroft, D.S. Hoslundin, hoslundal, and hoslunddiol: Three new flavonoids from the twigs of Hoslundia opposita (Lamiaceae). Tetrahedron 1991, 47, 3555. [Google Scholar] [CrossRef]
- Usman, L.A.; Zubair, M.F.; Adebayo, S.A.; Oladosu, I.A.; Muhammad, N.O.; Akolade, J.O. Chemical composition of leaf and fruit essential oils of Hoslundia opposita Vahl grown in Nigeria. Am. J. Agric. Environ. Sci. 2010, 8, 40–43. [Google Scholar]
- Ngadjui, B.T.; Ayafor, J.F.; Sondengam, B.L.; Connolly, J.D.; Rycroft, D.S.; Tillequin, F. Oppositin and 5-O-Methylhoslundin, pyrone-substituted flavonoids of Hoslundia opposita. Phytochemistry 1993, 32, 1313–1315. [Google Scholar] [CrossRef]
- Ngadjui, B.T.; Tsopmo, A.; Ayafor, J.F.; Connolly, J.D.; Tamboue, H. Hosloppin, a new pyrone-substituted flavonoid from Hoslundia opposita. J. Nat. Prod. 1995, 58, 109. [Google Scholar] [CrossRef]
- Sajjadi, S.E.; Ghannadi, A. Analysis of the essential oil of Lamium amplexicaule L. from Northeastern Iran. J. Essent. Oil Bear. Plants 2012, 15, 577–581. [Google Scholar] [CrossRef]
- Yalçin, F.N.; Kaya, D. Ethnobotany, pharmacology and phytochemistry of the genus Lamium (Lamiaceae). Fabad J. Pharm. Sci. 2006, 31, 43–53. [Google Scholar]
- Nickavar, B.; Mojab, F.; Bamasian, S. Volatile components from aerial parts of Lamium amplexicaule from Iran. J. Essent. Oil Bear. Plants 2008, 11, 36–40. [Google Scholar] [CrossRef]
- Alipieva, K.; Kokubun, T.; Taskova, R.; Evstatieva, L.; Handjieva, N. LC-ESI-MS analysis of iridoid glucosides in Lamium species. Biochem. Syst. Ecol. 2007, 35, 17–22. [Google Scholar] [CrossRef]
- Alipieva, K.I.; Taskova, R.M.; Evstatieva, L.N.; Handjieva, N.V.; Popov, S.S. Benzoxazinoids and iridoid glucosides from four Lamium species. Phytochemistry 2003, 64, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- El-Hela, A.A.; Abdel-Hady, N.M.; Dawoud, G.T.; Ghoneim, M.M. HPTLC fingerprint profile of triterpenes of Lamium amplexicaule Benth. and Ajuga iva L. (Lamiaceae) monitored with screening of their anti-inflammatory effect. J. Pharmacogn. Phytochem. 2016, 5, 176–181. [Google Scholar]
- Guiso, M.; Martino, C. 6-Deoxylamioside, a new iridoid glucoside from Lamium amplexicaule. J. Nat. Prod. 1983, 46, 157–160. [Google Scholar] [CrossRef]
- Kikuchi, M.; Onoguchi, R.; Yaoita, Y. Three new monoterpene glucosides from Lamium amplexicaule. Helv. Chim. Acta 2009, 92, 2063–2070. [Google Scholar] [CrossRef]
- Alipieva, K.I.; Evstatieva, L.; Handjieva, N.; Popov, S. Comparative analysis of the composition of flower volatiles from Lamium L. species and Lamiastrum galeobdolon Heist. ex Fabr. Z. Naturforsch. Sect. C J. Biosci. 2003, 58, 779–782. [Google Scholar] [CrossRef]
- Nsuala, B.N.; Kamatou, G.P.P.; Sandasi, M.; Enslin, G.; Viljoen, A. Variation in essential oil composition of Leonotis leonurus, an important medicinal plant in South Africa. Biochem. Syst. Ecol. 2017, 70, 155–161. [Google Scholar] [CrossRef]
- He, F.; Lindqvist, C.; Harding, W.W. Leonurenones A-C: Labdane diterpenes from Leonotis leonurus. Phytochemistry 2012, 83, 168–172. [Google Scholar] [CrossRef] [Green Version]
- Oyedeji, O.A.; Afolayan, A.J. Comparative study of the essential oil composition and antimicrobial activity of Leonotis leonurus and L. ocymifolia in the Eastern Cape, South Africa. S. Afr. J. Bot. 2005, 71, 114–116. [Google Scholar] [CrossRef] [Green Version]
- Naidoo, D.; Maharaj, V.; Crouch, N.R.; Ngwane, A. New labdane-type diterpenoids from Leonotis leonurus support circumscription of Lamiaceae s.l. Biochem. Syst. Ecol. 2011, 39, 216–219. [Google Scholar] [CrossRef]
- Vallabh, J.B. The Antimicrobial Properties and Chemical Composition of Leaf Essential Oils of Selected Lamiaceae Species in South Africa. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2003. [Google Scholar]
- Wu, H.; Li, J.; Fronczek, F.R.; Ferreira, D.; Burandt, C.L.; Setola, V.; Roth, B.L.; Zjawiony, J.K. Labdane diterpenoids from Leonotis leonurus. Phytochemistry 2013, 91, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Pedro, L.G.; Barroso, J.G.; Marques, N.T.; Ascensao, L.; Pais, M.S.S.; Scheffer, J.J.C. Composition of the essential oil from sepals of Leonotis leonurus R. Br. J. Essent. Oil Res. 1991, 3, 451. [Google Scholar] [CrossRef]
- Vos, W.T. A Systematic Study of Leonotis (Pers.) R.Br. (Lamiaceae) in Southern Africa. Ph.D. Thesis, University of Natal, Pietermaritzburg, South Africa, 1995. [Google Scholar]
- Agnihotri, V.K.; ElSohly, H.N.; Smillie, T.J.; Khan, I.A.; Walker, L.A. Constituents of Leonotis leonurus flowering tops. Phytochem. Lett. 2009, 2, 103–105. [Google Scholar] [CrossRef]
- Cragg, G.M.L.; Little, G.E. The extractives of Leonotis leonurus. J. S. Afr. Chem. Inst. 1962, 15, 29–30. [Google Scholar]
- El-Ansari, M.A.; Aboutabl, E.A.; Farrag, A.R.H.; Sharaf, M.; Hawas, U.W.; Soliman, G.M.; El-Seed, G.S. Phytochemical and pharmacological studies on Leonotis leonurus. Pharm. Biol. 2009, 47, 894–902. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, E.R.; Rivett, D.E.A. Structures of compounds X and Y, two labdane diterpenoids, from Leonotis leonurus. J. Chem. Soc. C Org. 1968, 262–266. [Google Scholar] [CrossRef]
- Kuchta, K.; Volk, R.B.; Rauwald, H.W. Stachydrine in Leonurus cardiaca, Leonurus japonicus, Leonotis leonurus: Detection and quantification by instrumental HPTLC and 1H-qNMR analyses. Pharmazie 2013, 68, 534–540. [Google Scholar] [CrossRef]
- Mazimba, O. Leonotis leonurus: A herbal medicine review. J. Pharmacogn. Phytochem. 2015, 3, 74–82. [Google Scholar]
- McKenzie, J.M.; Green, I.R.; Mugabo, P. Leonurun, a novel labdane diterpenoid from Leonotis leonurus. S. Afr. J. Chem. 2006, 59, 114–116. [Google Scholar]
- Naidu, K. An Investigation of Compounds Occuring in Leonotis Species. Ph.D. Thesis, Rhodes University, Grahamstown, South Africa, 1970. [Google Scholar]
- Narukawa, Y.; Komori, M.; Niimura, A.; Noguchi, H.; Kiuchi, F. Two new diterpenoids from Leonotis leonurus R. Br. J. Nat. Med. 2015, 69, 130–134. [Google Scholar] [CrossRef]
- Tonisi, S.; Okaiyeto, K.; Hoppe, H.; Mabinya, L.V.; Nwodo, U.U.; Okoh, A.I. Chemical constituents, antioxidant and cytotoxicity properties of Leonotis leonurus used in the folklore management of neurological disorders in the Eastern Cape, South Africa. 3 Biotech 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Damasceno, L.M.O.; Silva, A.L.N.; dos Santos, R.F.; Feitosa, T.A.; Viana, L.G.F.; de Oliveira, R.G.; e Silva, M.G.; Rolim, L.A.; Araújo, C.S.; Araújo, E.C.C.; et al. Cytotoxic activity of chemical constituents and essential oil from the leaves of Leonotis nepetifolia (Lamiaceae). Rev. Virtual Quim. 2019, 11, 517–528. [Google Scholar] [CrossRef]
- Li, J.; Fronczek, F.R.; Ferreira, D.; Burandt, C.L.; Setola, V.; Roth, B.L.; Zjawiony, J.K. Bis-spirolabdane diterpenoids from Leonotis nepetaefolia. J. Nat. Prod. 2012, 75, 728–734. [Google Scholar] [CrossRef] [Green Version]
- Muhayimana, A.; Chalchat, J.C.; Garry, R.-P. Chemical composition of essential oils of some medicinal plants from Rwanda. J. Essent. Oil Res. 1998, 10, 251–259. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Melo, F.G.; Balogun, S.O.; Flach, A.; De Souza, E.C.A.; De Souza, G.P.; Rocha, I.D.N.A.; Da Costa, L.A.M.A.; Soares, I.M.; Da Silva, L.I.; et al. Antibacterial mode of action of the hydroethanolic extract of Leonotis nepetifolia (L.) R. Br. involves bacterial membrane perturbations. J. Ethnopharmacol. 2015, 172, 356–363. [Google Scholar] [CrossRef]
- Ueda, F.; Iizuka, K.; Tago, K.; Narukawa, Y.; Kiuchi, F.; Kasahara, T.; Tamura, H.; Funakoshi-Tago, M. Nepetaefuran and leonotinin isolated from Leonotis nepetaefolia R. Br. potently inhibit the LPS signaling pathway by suppressing the transactivation of NF-κB. Int. Immunopharmacol. 2015, 28, 967–976. [Google Scholar] [CrossRef]
- Nibret, E.; Wink, M. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. Phytomedicine 2010, 17, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Kruger, G.J.; Rivett, D.E.A. Diterpenoids of Leonotis species. Part 6.1 The crystal and molecular structure of leonitin, a 9,13-epoxylabdane. S. Afr. J. Chem. 1979, 32, 61–62. [Google Scholar] [CrossRef]
- Vagionas, K.; Graikou, K.; Chinou, I.B.; Runyoro, D.; Ngassapa, O. Chemical analysis and antimicrobial activity of essential oils from the aromatic plants Artemisia afra Jacq. and Leonotis ocymifolia (Burm. F.) Iwarsson var. raineriana (Vision1) Iwarsson growing in Tanzania. J. Essent. Oil Res. 2007, 19, 396–400. [Google Scholar] [CrossRef]
- Habtemariam, S.; Gray, A.I.; Waterman, P.G. Diterpenes from the leaves of Leonotis ocymifolia var. raineriana. J. Nat. Prod. 1994, 57, 1570–1574. [Google Scholar] [CrossRef]
- Rivett, D.E.A. The structure of lasiocoryin, a Grindelane Diterpenoid from Lasiocorys capensis Benth. (Labiate). S. Afr. J. Chem. 1975, 28, 305–311. [Google Scholar]
- Vagionas, K.; Ngassapa, O.; Runyoro, D.; Graikou, K.; Gortzi, O.; Chinou, I. Chemical analysis of edible aromatic plants growing in Tanzania. Food Chem. 2007, 105, 1711–1717. [Google Scholar] [CrossRef]
- Eze, U.A.; Bello, S.O.; Etuk, E.U.; Ameh, G.I.; Ugwah, O.M.; Ugwah-oguejiofor, C.J. Phytochemical and preliminary toxicological studies of the aqueous leave extract of Leucas martinicensis in wistar rats. Int. J. Med. Plants Res. 2013, 2, 166–169. [Google Scholar]
- Kadri, A.; Zarai, Z.; Ben Chobba, I.; Bekir, A.; Gharsallah, N.; Damak, M.; Gdoura, R. Chemical composition and antioxidant activity of Marrubium vulgare L. essential oil from Tunisia. Afr. J. Biotechnol. 2011, 10, 3908–3914. [Google Scholar]
- Ahmed, B.; Masoodi, M.H.; Siddique, A.H.; Khan, S. A new monoterpene acid from Marrubium vulgare with potential antihepatotoxic activity. Nat. Prod. Res. 2010, 24, 1671–1680. [Google Scholar] [CrossRef]
- Zarai, Z.; Kadri, A.; Ben Chobba, I.; Ben Mansour, R.; Bekir, A.; Mejdoub, H.; Gharsallah, N. The in vitro evaluation of antibacterial, antifungal and cytotoxic properties of Marrubium vulgare L. essential oil grown in Tunisia. Lipids Health Dis. 2011, 10, 161. [Google Scholar] [CrossRef] [Green Version]
- Amessis-Ouchemoukh, N.; Abu-Reidah, I.M.; Quirantes-Piné, R.; Madani, K.; Segura-Carretero, A. Phytochemical profiling, in vitro evaluation of total phenolic contents and antioxidant properties of Marrubium vulgare (horehound) leaves of plants growing in Algeria. Ind. Crops Prod. 2014, 61, 120–129. [Google Scholar] [CrossRef]
- Nagy, M.; Svajdlenka, E. Comparison of essential oils from Marrubium vulgare L. and M. peregrinum L. J. Essent. Oil Res. 1998, 10, 585–587. [Google Scholar] [CrossRef]
- Amri, B.; Martino, E.; Vitulo, F.; Corana, F.; Ben-Kaâb, L.B.; Rui, M.; Rossi, D.; Mori, M.; Rossi, S.; Collina, S. Marrubium vulgare L. leave extract: Phytochemical composition, antioxidant and wound healing properties. Molecules 2017, 22, 1851. [Google Scholar] [CrossRef] [Green Version]
- Khanavi, M.; Ghasemian, L.; Motlagh, E.H.; Hadjiakhoondi, A.; Shafiee, A. Chemical composition of the essential oils of Marrubium parviflorum Fisch. and C. A. Mey. and Marrubium vulgare L. from Iran. Flavour Fragr. J. 2005, 20, 324–326. [Google Scholar] [CrossRef]
- Aćimović, M.; Jeremić, K.; Salaj, N.; Gavarić, N.; Kiprovski, B.; Sikora, V.; Zeremski, T. Marrubium vulgare L.: A phytochemical and pharmacological overview. Molecules 2020, 25, 2898. [Google Scholar] [CrossRef] [PubMed]
- Chaker, A.N.; Boukhebti, H.; Sahraoui, R.; Ramdhani, M. Essential oils and morphological study of Mentha aquatica. Pharmacogn. Commun. 2014, 4, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Benabdallah, A.; Rahmoune, C.; Boumendjel, M.; Aissi, O.; Messaoud, C. Total phenolic content and antioxidant activity of six wild Mentha species (Lamiaceae) from northeast of Algeria. Asian Pac. J. Trop. Biomed. 2016, 6, 760–766. [Google Scholar] [CrossRef] [Green Version]
- Safaiee, P.; Taghipour, A.; Vahdatkhoram, F.; Movagharnejad, K. Extraction of phenolic compounds from Mentha aquatica: The effects of sonication time, temperature and drying method. Chem. Pap. 2019, 73, 3067–3073. [Google Scholar] [CrossRef]
- Stagos, D.; Portesis, N.; Spanou, C.; Mossialos, D.; Aligiannis, N.; Chaita, E.; Panagoulis, C.; Reri, E.; Skaltsounis, L.; Tsatsakis, A.M.; et al. Correlation of total polyphenolic content with antioxidant and antibacterial activity of 24 extracts from Greek domestic Lamiaceae species. Food Chem. Toxicol. 2012, 50, 4115–4124. [Google Scholar] [CrossRef]
- Senatore, F.; D’Alessio, A.; Formisano, C.; Özcan, M. Chemical composition and antibacterial activity of the essential oil of a 1,8-cineole chemotype of Mentha aquatica L. growing wild in Turkey. J. Essent. Oil Bear. Plants 2005, 8, 148–153. [Google Scholar] [CrossRef]
- Ferhat, M.; Kabouche, Z.; Fujimoto, Y.; Araya, H. Two new triterpenes and other compounds from Mentha aquatica (Lamiaceae). Nat. Prod. Commun. 2017, 12, 483–486. [Google Scholar] [CrossRef] [Green Version]
- Malingré, T.M.; Maarse, H. The composition of the essential oil of Mentha aquatica. Phytochemistry 1974, 13, 1531–1535. [Google Scholar] [CrossRef]
- Mentha aquatica L. In Natural Compound—Flavonoids; Azimova, S.S.; Vinogradova, V.I. (Eds.) Springer Science+Business Media: New York, NY, USA, 2013; ISBN 9781461405351. [Google Scholar]
- Morteza-Semnani, K.; Saeedi, M.; Akbarzadeh, M. The essential oil composition of Mentha aquatica L. J. Essent. Oil Bear. Plants 2006, 9, 283–286. [Google Scholar] [CrossRef]
- Burzanska-Hermann, Z.; Rzadkowska-Bodalska, H.; Olechnowicz-Stepien, W. Isolation and identification of flavonoid compounds of Mentha aquatica L. herb. Rocz. Chem. 1977, 51, 701. [Google Scholar]
- Voirin, B.; Bayet, C.; Faure, O.; Jullien, F. Free flavonoid aglycones as markers of parentage in Mentha aquatica, M. citrata, M. spicata and M. × piperita. Phytochemistry 1999, 50, 1189–1193. [Google Scholar] [CrossRef]
- Shimizu, S.; Karasawa, D.; Ikeda, N. A new Mint (variety of Mentha aquatica L.) conatining (-)-isopinocamphone as a major constituent of essential oil. Agric. Biol. Chem. 1966, 30, 200–201. [Google Scholar]
- Mkaddem, M.; Bouajila, J.; Ennaiar, M.; Lebrihi, A.; Mathieu, F.; Romdhane, M. Chemical composition and antimicrobial and antioxidant activities of Mentha (longifolia L. and viridis) essential oils. J. Food Sci. 2009, 74, M358. [Google Scholar] [CrossRef]
- Bourwieg, D.; Pohl, R. Flavonoids from Mentha longifolia. Planta Med. 1973, 24, 304. [Google Scholar] [CrossRef] [PubMed]
- Monfared, A.; Nabid, M.R.; Rustaiyan, A. Composition of a carvone chemotype of Mentha longifolia (L.) Huds. from Iran. J. Essent. Oil Res. 2002, 14, 51–52. [Google Scholar] [CrossRef]
- Ghoulami, S.; Il Idrissi, A.; Fkih-Tetouani, S. Phytochemical study of Mentha longifolia of Morocco. Fitoterapia 2001, 72, 596–598. [Google Scholar] [CrossRef]
- Salman, M.; Abdel-Hameed, E.-S.S.; Bazaid, S.A.; Dabi, M.M. Chemical composition for hydrodistillation essential oil of Mentha longifolia by gas chromatography-mass spectrometry from north regions in Kingdom of Saudi Arabia. Pharma Chem. 2015, 7, 34–40. [Google Scholar]
- Sharaf, M.; El-Ansari, M.A.; Saleh, N.A.M. Flavone glycosides from Mentha longifolia. Fitoterapia 1999, 70, 478–483. [Google Scholar] [CrossRef]
- Okoh, O.O.; Afolayan, A.J. The effects of hydrodistillation and solvent free microwave extraction methods on the chemical composition and toxicity of essential oils from the leaves of Mentha longifolia L. subsp. capensis. Afr. J. Pharm. Pharmacol. 2011, 5, 2474–2478. [Google Scholar] [CrossRef]
- Oyedeji, A.O.; Afolayan, A.J. Chemical composition and antibacterial activity of the essential oil isolated from South African Mentha longifolia (L.) L. subsp. capensis (Thunb.). Briq. J. Essent. Oil Res. 2006, 18, 57–59. [Google Scholar] [CrossRef]
- Pektar, S.Y. The Composition, Geographical Variation and Antimicrobial Activity of Mentha longifolia subsp. polyadena (Lamiaceae) Leaf Essential Oils. Ph.D. Thesis, University of the Witswatersrand, Johannesburg, South Africa, 2006. [Google Scholar]
- Viljoen, A.M.; Petkar, S.; Van Vuuren, S.F.; Figueiredo, A.C.; Pedro, L.G.; Barroso, J.G. The chemo-geographical variation in essential oil composition and the antimicrobial properties of “wild mint”—Mentha longifolia subsp. polyadena (Lamiaceae) in Southern Africa. J. Essent. Oil Res. 2006, 18, 60–65. [Google Scholar] [CrossRef]
- Mahboubi, M.; Haghi, G. Antimicrobial activity and chemical composition of Mentha pulegium L. essential oil. J. Ethnopharmacol. 2008, 119, 325–327. [Google Scholar] [CrossRef]
- Fatiha, B.; Didier, H.; Naima, G.; Khodir, M.; Martin, K.; Léocadie, K.; Caroline, S.; Mohamed, C.; Pierre, D. Phenolic composition, in vitro antioxidant effects and tyrosinase inhibitory activity of three Algerian Mentha species: M. spicata (L.), M. pulegium (L.) and M. rotundifolia (L.) Huds (Lamiaceae). Ind. Crops Prod. 2015, 74, 722–730. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Batista, I.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. European pennyroyal (Mentha pulegium) from Portugal: Chemical composition of essential oil and antioxidant and antimicrobial properties of extracts and essential oil. Ind. Crops Prod. 2012, 36, 81–87. [Google Scholar] [CrossRef]
- Gülçin, İ.; Gören, A.C.; Taslimi, P.; Alwasel, S.H.; Kılıc, O.; Bursal, E. Anticholinergic, antidiabetic and antioxidant activities of Anatolian pennyroyal (Mentha pulegium)-analysis of its polyphenol contents by LC-MS/MS. Biocatal. Agric. Biotechnol. 2020, 23. [Google Scholar] [CrossRef]
- Mallavarapu, G.R.; Ramesh, S.; Subrahmanyam, K. Composition of the essential oil of Micromeria biflora. J. Essent. Oil Res. 1997, 9, 23–26. [Google Scholar] [CrossRef]
- Moghadam, S.E.; Ebrahimi, S.N.; Gafner, F.; Ochola, J.B.; Marubu, R.M.; Lwande, W.; Haller, B.F.; Salehi, P.; Hamburger, M. Metabolite profiling for caffeic acid oligomers in Satureja biflora. Ind. Crops Prod. 2015, 76, 892–899. [Google Scholar] [CrossRef]
- Matasyoha, J.C.; Kiplimoa, J.J.; Karubiub, N.M.; Hailstorksc, T.P. Chemical composition and antimicrobial activity of Satureja biflora (Lamiaceae). Bull. Chem. Soc. Ethiop. 2007, 21, 249–254. [Google Scholar] [CrossRef]
- Chowdhury, T.; Mandal, A.; Roy, S.C.; De Sarker, D. Diversity of the genus Ocimum (Lamiaceae) through morpho-molecular (RAPD) and chemical (GC–MS) analysis. J. Genet. Eng. Biotechnol. 2017, 15, 275–286. [Google Scholar] [CrossRef]
- Thappa, R.K.; Bhatia, M.S.; Aggarwal, S.G.; Dhar, K.L.; Atal, C.K. Ocimin, a novel neolignan from Ocimum americanum. Phytochemistry 1979, 18, 9422. [Google Scholar] [CrossRef]
- Djibo, A.K.; Samate, A.D.; Nacro, M. Chemical composition of the essential oil of Ocimum americanum Linn., syn. Ocimum. canum Sims from Burkina Faso. Comptes Rendus Chim. 2004, 7, 1033–1037. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Misra, L.N.; Singh, G. Sesquiterpene alcohols of the copane series from essential oil of Ocimum americanum. Phytochemistry 1991, 30, 691–693. [Google Scholar] [CrossRef]
- Matasyoh, J.C.; Bendera, M.M.; Ogendo, J.O.; Omollo, E.O.; Deng, A.L. Volatile leaf oil constituents of Ocimum americanum L. occurring in western Kenya. Bull. Chem. Soc. Ethiop. 2006, 20, 177–180. [Google Scholar] [CrossRef] [Green Version]
- Vieira, R.F.; Grayer, R.J.; Paton, A.J. Chemical profiling of Ocimum americanum using external flavonoids. Phytochemistry 2003, 63, 555–567. [Google Scholar] [CrossRef]
- Shadia, E.; El-Aziz, A.; Omer, E.A.; Sabra, A.S. Chemical composition of Ocimum americanum essential oil and its biological effects against Agrotis ipsilon, (Lepidoptera: Noctuidae). Res. J. Agric. Biol. Sci. 2007, 3, 740–747. [Google Scholar]
- Sishu, R.; Taddesse, S.; Bucar, F.; Asres, K. Chemical composition and antioxidant activity of the essential oils of Ocimum americanum and Ocimum basillicum var. thyrsiflorum. Int. J. Essent. Oil Ther. 2010, 4, 64–68. [Google Scholar]
- Padalia, R.C.; Verma, R.S.; Chauhan, A. Diurnal variations in aroma profile of Ocimum basilicum L., O. gratissimum L., O. americanum L., and O. kilimandscharicum Guerke. J. Essent. Oil Res. 2017, 29, 248–261. [Google Scholar] [CrossRef]
- Vieira, P.R.N.; De Morais, S.M.; Bezerra, F.H.Q.; Travassos, P.A.; Oliveira, Í.R.; Silva, M.G.V. Chemical composition and antifungal activity of essential oils from Ocimum species. Ind. Crops Prod. 2014, 55, 267–271. [Google Scholar] [CrossRef]
- Hussein, A.A.; Meyer, J.J.M.; Jimeno, M.L.; Rodríguez, B. Bioactive diterpenes from Orthosiphon labiatus and Salvia africana-lutea. J. Nat. Prod. 2007, 70, 293–295. [Google Scholar] [CrossRef]
- Kapewangolo, P.; Omolo, J.J.; Fonteh, P.; Kandawa-Schulz, M.; Meyer, D. Triterpenoids from Ocimum labiatum activates latent HIV-1 expression in vitro: Potential for use in adjuvant therapy. Molecules 2017, 22, 1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidoo, Y.; Sadashiva, C.T.; Kasim, N.; Nicholas, A.; Naidoo, G. Chemical Composition and Antimicrobial Activity of the Essential Oil of Ocimum obovatum E. Mey. Ex Benth. (Lamiaceae). J. Essent. Oil Bear. Plants 2014, 17, 142–147. [Google Scholar] [CrossRef]
- Burger, I.; Burger, B.V.; Albrecht, C.F.; Spies, H.S.C.; Sandor, P. Triterpenoid saponins from Becium grandiflorum var. obovatum. Phytochemistry 1998, 49, 2087–2095. [Google Scholar] [CrossRef]
- Sundarammal, S.; Thirugnanasampandan, R.; Selvi, M.T. Chemical composition analysis and antioxidant activity evaluation of essential oil from Orthosiphon thymiflorus (Roth) Sleesen. Asian Pac. J. Trop. Biomed. 2012, 2, S112–S115. [Google Scholar] [CrossRef]
- François, T.; Michel, J.D.P.; Vyry, W.N.A.; Fabrice, F.B.; Lambert, S.M.; Henri, A.Z.P.; Chantal, M. Composition and antifungal properties of essential oils from five plants growing in the mountainous area of the West Cameroon. J. Essent. Oil Bear. Plants 2013, 16, 679–688. [Google Scholar] [CrossRef]
- Hussein, A.A. Chemistry of South African Lamiaceae: Structures and biological activity of terpenoids. In Terpenes and Terpenoids; Perveen, S., Al-Taweel, A., Eds.; IntechOpen: London, UK, 2018; pp. 13–38. [Google Scholar]
- Ngassapa, O.D.; Runyoro, D.K.B.; Vagionas, K.; Graikou, K.; Chinou, I.B. Chemical composition and antimicrobial activity of Geniosporum rotundifolium Briq and Haumaniastrum villosum (Bene) AJ Paton (Lamiaceae) essential oils from Tanzania. Trop. J. Pharm. Res. 2016, 15, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Ruedi, P. Phyllocladanes (13B-Kauranes) from Plectranthus ambiguus. Phytochemistry 1996, 41, 1563–1568. [Google Scholar]
- Viljoen, A.M.; Demirci, B.; Başer, K.H.C.; Potgieter, C.J.; Edwards, T.J. Microdistillation and essential oil chemistry—A useful tool for detecting hybridisation in Plectranthus (Lamiaceae). S. Afr. J. Bot. 2006, 72, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Stavri, M.; Paton, A.; Skelton, B.W.; Gibbons, S. Antibacterial diterpenes from Plectranthus ernstii. J. Nat. Prod. 2009, 72, 1191–1194. [Google Scholar] [CrossRef]
- Fournier, G.; Paris, M.; Dumitresco, S.M.; Pages, N.; Boudene, C. Contribution to the study of Plectranthus fruticosus leaf essential oil. Planta Med. 1986, 52, 486–488. [Google Scholar] [CrossRef]
- Gaspar-Marques, C.; Simões, M.F.; Duarte, A.; Rodríguez, B. Labdane and kaurane diterpenoids from Plectranthus fruticosus. J. Nat. Prod. 2003, 66, 491–496. [Google Scholar] [CrossRef]
- Katti, S.B.; Rueedi, P.; Eugster, C.H. Diterpenoid quinomethans, vinylogous quinones and a phyllocladene derivative from Plectranthus purpuratus Harv. (Labiatae). Helv. Chim. Acta 1982, 65, 2189. [Google Scholar] [CrossRef]
- Simões, M.F.; Rijo, P.; Duarte, A.; Barbosa, D.; Matias, D.; Delgado, J.; Cirilo, N.; Rodríguez, B. Two new diterpenoids from Plectranthus species. Phytochem. Lett. 2010, 3, 221–225. [Google Scholar] [CrossRef]
- Wellsow, J.; Grayer, R.J.; Veitch, N.C.; Kokubun, T.; Lelli, R.; Kite, G.C.; Simmonds, M.S.J. Insect-antifeedant and antibacterial activity of diterpenoids from species of Plectranthus. Phytochemistry 2006, 67, 1818–1825. [Google Scholar] [CrossRef]
- Mota, A.H.; Sousa, A.; Figueira, M.; Amaral, M.; Sousa, B.; Rocha, J.; Fattal, E.; Almeida, A.J.; Reis, C.P. Chapter 19—Natural-based consumer health nanoproducts: Medicines, cosmetics, and food supplements. In Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 527–578. ISBN 978-0-12-816787-8. [Google Scholar]
- Brito, E.; Gomes, E.; Falé, P.L.; Borges, C.; Pacheco, R.; Teixeira, V.; Machuqueiro, M.; Ascensão, L.; Serralheiro, M.L.M. Bioactivities of decoctions from Plectranthus species related to their traditional use on the treatment of digestive problems and alcohol intoxication. J. Ethnopharmacol. 2018, 220, 147–154. [Google Scholar] [CrossRef]
- Narukawa, Y.; Shimizu, N.; Shimotohno, K.; Takeda, T. Two new diterpenoids from Plectranthus nummularius Briq. Chem. Pharm. Bull. 2001, 49, 1182–1184. [Google Scholar] [CrossRef] [Green Version]
- Lamiaceae, L.; Lawson, S.K.; Sharp, L.G.; Satyal, P.; Setzer, W.N. Volatile components of the aerial parts of Prunella vulgaris L. (Lamiaceae). Am. J. Essent. Oils Nat. Prod. 2020, 8, 17–19. [Google Scholar]
- Gu, X.; Li, Y.; Mu, J.; Zhang, Y. Chemical constituents of Prunella vulgaris. J. Environ. Sci. 2013, 25, S161–S163. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Q.; Zhu, Z.; Zhang, L.; Dai, X. Comparative analysis of the essential oil of flowers, leaves and stems of Prunella vulgaris L. J. Essent. Oil Bear. Plants 2012, 15, 662–666. [Google Scholar] [CrossRef]
- Kojima, H.; Ogura, H. Triterpenoids from Prunella vulgaris. Phytochemistry 1986, 25, 729–733. [Google Scholar] [CrossRef]
- Kojima, H.; Tominaga, H.; Sato, S.; Takayanagi, H.; Ogura, H. Two novel hexacyclic triterpenoids from Prunella vulgaris. Phytochemistry 1988, 27, 2921–2925. [Google Scholar] [CrossRef]
- Lou, H.Y.; Jin, L.; Huang, T.; Wang, D.P.; Liang, G.Y.; Pan, W.D. Vulgarisins B–D, three novel diterpenoids with a rare skeleton isolated from Prunella vulgaris Linn. Tetrahedron Lett. 2017, 58, 401–404. [Google Scholar] [CrossRef]
- Wang, S.-J.; Wang, X.-H.; Dai, Y.-Y.; Ma, M.-H.; Rahman, K.; Nian, H.; Zhang, H. Prunella vulgaris: A comprehensive review of chemical constituents, pharmacological effects and clinical applications. Curr. Pharm. Des. 2019. [Google Scholar] [CrossRef]
- Davies-Coleman, M.T.; Rivett, D.E.A. Transformation of Hispanolone from Ballota africana into 15,16-epoxy-9-hydroxylabda-13(16),14-diene. S. Afr. J. Chem. 1990, 43, 117–119. [Google Scholar]
- Davies-Coleman, M.T. Extractives from Six Species of Lamiaceae. Ph.D. Thesis, Rhodes University, Grahamstown, South Africa, 1987. [Google Scholar]
- Gray, C.A.; Rivett, D.E.A.; Davies-Coleman, M.T. The absolute stereochemistry of a diterpene from Ballota aucheri. Phytochemistry 2003, 63, 409–413. [Google Scholar] [CrossRef]
- Bashwira, S.; Hootele, C.; Tourwe, D.; Pepermans, H.; Laus, G.; Van Binst, G. Cleromyrine I, a new cyclohexapeptide from Clerodendrum myricoides. Tetrahedron 1989, 45, 5845. [Google Scholar] [CrossRef]
- Msonthi, J.; Hostettmann, K.; Maillard, M. Phytochemical studies of medicinal plants from Malawi. In Chemistry, Biological and Pharmacological Properties of African Medicinal Plants; Hostettmann, K., Chinyanganya, F., Maillard, M., Wolfender, J.-L., Eds.; University of Zimbabwe Publications: Harare, Zimbabwe, 1996. [Google Scholar]
- Toyota, M.; Msonthi, J.D.; Hostettmann, K. A molluscicidal and antifungal triterpenoid saponin from the roots of Clerodendrum wildii. Phytochemistry 1990, 29, 2849–2851. [Google Scholar] [CrossRef]
- De Martino, L.; Roscigno, G.; Mancini, E.; De Falco, E.; De Feo, V. Chemical composition and antigerminative activity of the essential oils from five Salvia species. Molecules 2010, 15, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, R.C.F.; Cardoso, S.M. Phytochemical composition and bioactive effects of Salvia africana, Salvia officinalis “Icterina” and Salvia mexicana aqueous Extracts. Molecules 2019, 24, 4327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, V.L. Indigenous Salvia Species—An Investigation of the Antimicrobial Activity, Antioxidant Activity and Chemical Composition of Leaf Extracts. Ph.D. Thesis, University of the Witswatersrand, Johannesburg, South Africa, 2005. [Google Scholar]
- Kamatou, G.P.P.; Van Zyl, R.L.; Van Vuuren, S.F.; Viljoen, A.M.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Tilney, P.M. Chemical composition, leaf trichome types and biological activities of the essential oils of four related Salvia species indigenous to Southern Africa. J. Essent. Oil Res. 2006, 18, 72–79. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M.; Steenkamp, P. Antioxidant, antiinflammatory activities and HPLC analysis of South African Salvia species. Food Chem. 2010, 119, 684–688. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Van Zyl, R.L.; Van Vuuren, S.F.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Viljoen, A.M. Seasonal variation in essential oil composition, oil toxicity and the biological activity of solvent extracts of three South African Salvia species. S. Afr. J. Bot. 2008, 74, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Kamatou, G.P.P.; Viljoen, A.M.; Figueiredo, A.C.; Tilney, P.M.; Van Zyl, R.L.; Barroso, J.G.; Pedro, L.G.; Van Vuuren, S.F. Trichomes, essential oil composition and biological activities of Salvia albicaulis Benth. and S. dolomitica Codd, two species from the Cape region of South Africa. S. Afr. J. Bot. 2007, 73, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Arief, M.M.H.; Abdel, A.; Hussein, F.; Mohammed, A.; Elmwafy, H.M. Chemical and Bioactivity Studies on Salvia africana-lutea: Cytotoxicity and Apoptosis Induction by Abietane Diterpenes Isolated from Salvia africana-lutea. J. Basic Environ. Sci. 2018, 5, 72–79. [Google Scholar]
- Glyphis, J.P.; Puttick, G.M. Phenolics in some southern African Mediterranean shrubland plants. Phytochemistry 1988, 27, 743–751. [Google Scholar] [CrossRef]
- Lim Ah Tock, M.J.; Kamatou, G.P.P.; Combrinck, S.; Sandasi, M.; Viljoen, A.M. A chemometric assessment of essential oil variation of three Salvia species indigenous to South Africa. Phytochemistry 2020, 172, 112249. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Van Vuuren, S.F.; Van Heerden, F.R.; Seaman, T.; Viljoen, A.M. Antibacterial and antimycobacterial activities of South African Salvia species and isolated compounds from S. chamelaeagnea. S. Afr. J. Bot. 2007, 73, 552–557. [Google Scholar] [CrossRef] [Green Version]
- Onayade, O.A.; Scheffer, J.J.C.; Svendsen, A.B. Polynuclear aromatic compounds and other constituents of the herb essential oil of Salvia coccinea Juss. ex Murr. Flavour Fragr. J. 1991, 6, 281–289. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Salachna, P.; Meller, E. Changes in photosynthetic pigments, total phenolic content, and antioxidant activity of Salvia coccinea Buc’hoz Ex Etl. induced by exogenous salicylic acid and soil salinity. Molecules 2018, 23, 1296. [Google Scholar] [CrossRef] [Green Version]
- Abreu, M.E.; Muller, M.; Alegre, L.; Munne-Bosch, S. Phenolic diterpene and α-tocopherol contents in leaf extracts of 60 Salvia species. J. Sci. Food Agric. 2008, 88, 2648–2653. [Google Scholar] [CrossRef]
- Bassolino, L.; Giacomelli, E.; Giovanelli, S.; Pistelli, L.; Cassetti, A.; Damonte, G.; Bisio, A.; Ruffoni, B. Tissue culture and aromatic profile in Salvia dolomitica Codd. Plant Cell. Tissue Organ Cult. 2015, 121, 83–95. [Google Scholar] [CrossRef]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Pistelli, L.; Pistelli, L.; Scariot, V. Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind. Crops Prod. 2019, 129, 85–96. [Google Scholar] [CrossRef]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Giovanelli, S.; Ruffoni, B.; D’Ascenzi, C.; Pistelli, L.; Mancianti, F. Activity of Salvia dolomitica and Salvia somalensis essential oils against bacteria, molds and yeasts. Molecules 2018, 23, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamatou, G.P.P.; Van Zyl, R.L.; Van Vuuren, S.F.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Biological activities and composition of Salvia muirii L. Bol. essential oil. J. Essent. Oil Res. 2006, 18, 48–51. [Google Scholar] [CrossRef]
- Grierson, D.S.; Afolayan, A.J. Antibacterial activity of the extracts and the essential oil from the shoots of Salvia namaensis Schinz. S. Afr. J. Sci. 2005, 101, 507–510. [Google Scholar]
- Gono-Bwalya, A. The Chemotaxonomy and Biological Activity of Salvia stenophylla (Lamiaceae) and Related Taxa. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2003. [Google Scholar]
- Kamatou, G.P.P.; Viljoen, A.M.; Gono-Bwalya, A.B.; Van Zyl, R.L.; Van Vuuren, S.F.; Lourens, A.C.U.; Başer, K.H.C.; Demirci, B.; Lindsey, K.L.; Van Staden, J.; et al. The in vitro pharmacological activities and a chemical investigation of three South African Salvia species. J. Ethnopharmacol. 2005, 102, 382–390. [Google Scholar] [CrossRef]
- Mokoka, T.A.; Peter, X.K.; Fouche, G.; Moodley, N.; Adams, M.; Hamburger, M.; Kaiser, M.; Brun, R.; Maharaj, V.; Koorbanally, N. Antileishmanial activity of 12-methoxycarnosic acid from Salvia repens Burch. ex Benth. (Lamiaceae). S. Afr. J. Bot. 2014, 90, 93–95. [Google Scholar] [CrossRef] [Green Version]
- Viljoen, A.M.; Gono-Bwalya, A.; Kamatou, G.P.P.; Başer, K.H.C.; Demirci, B. The essential oil composition and chemotaxonomy of Salvia stenophylla and its allies S. repens and S. runcinata. J. Essent. Oil Res. 2006, 18, 37–45. [Google Scholar] [CrossRef]
- Mokoka, T.A. The Discovery and Characterization of Antiprotozoal Compounds from South African Medicinal Plants by a HPLC-Based Activity Profiling Technique. Ph.D. Thesis, University of KwaZulu-Natal, Durban, South Africa, 2013. [Google Scholar]
- Figlan, S. Generation of Clonal Microplants and Hairt Root Cultures of the Aromatic Medicinal Plant Salvia runcinata L.f. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2012. [Google Scholar]
- Sandasi, M.; Kamatou, G.P.P.; Viljoen, A.M. An untargeted metabolomic approach in the chemotaxonomic assessment of two Salvia species as a potential source of α-Bisabolol. Phytochemistry 2012, 84, 94–101. [Google Scholar] [CrossRef]
- Wollenweber, E.; Dörr, M.; Rustaiyan, A.; Roitman, J.N.; Graven, E.H. Exudate flavonoids of some Salvia and a Trichostema species. Zeitschrift Naturforsch. Sect. C J. Biosci. 1992, 47, 782–784. [Google Scholar] [CrossRef] [Green Version]
- Jequier, C.; Nicollier, G.; Tabacchi, R.; Garnero, J. Constituents of the essential oil of Salvia stenophylla. Phytochemistry 1980, 19, 461–462. [Google Scholar] [CrossRef]
- Canzoneri, M.; Bruno, M.; Rosselli, S.; Russo, A.; Cardile, V.; Formisano, C.; Rigano, D.; Senatore, F. Chemical composition and biological activity of Salvia verbenaca essential oil. Nat. Prod. Commun. 2011, 6, 1023–1026. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.; Cardile, V.; Graziano, A.C.E.; Formisano, C.; Rigano, D.; Canzoneri, M.; Bruno, M.; Senatore, F. Comparison of essential oil components and in vitro anticancer activity in wild and cultivated Salvia verbenaca. Nat. Prod. Res. 2015, 29, 1630–1640. [Google Scholar] [CrossRef]
- Kostić, M.; Zlatković, B.; Miladinović, B.; Živanović, S.; Mihajilov-Krstev, T.; Pavlović, D.; Kitić, D. Rosmarinic acid levels, phenolic contents, antioxidant and antimicrobial activities of the extracts from Salvia verbenaca L. obtained with different solvents and procedures. J. Food Biochem. 2015, 39, 199–208. [Google Scholar] [CrossRef]
- Pitarokili, D.; Tzakou, O.; Loukis, A. Essential oil composition of Salvia verticillata, S. verbenaca, S. glutinosa and S. candidissima growing wild in Greece. Flavour Fragr. J. 2006, 21, 670–673. [Google Scholar] [CrossRef]
- Chorianopoulos, N.; Evergetis, E.; Mallouchos, A.; Kalpoutzakis, E.; Nychas, G.J.; Haroutounian, S.A. Characterization of the essential oil volatiles of Satureja thymbra and Satureja parnassica: Influence of harvesting time and antimicrobial activity. J. Agric. Food Chem. 2006, 54, 3139–3145. [Google Scholar] [CrossRef]
- Skendi, A.; Irakli, M.; Chatzopoulou, P. Analysis of phenolic compounds in Greek plants of Lamiaceae family by HPLC. J. Appl. Res. Med. Aromat. Plants 2017, 6, 62–69. [Google Scholar] [CrossRef]
- Scott, G.; Springfield, E.P.; Coldrey, N. A pharmacognostical study of 26 South African plant species used as traditional medicines. Pharm. Biol. 2004, 42, 186–213. [Google Scholar] [CrossRef]
- Collett, L.A.; Davies-Coleman, M.T.; Rivett, D.E.A. 5,6-Dihydro-Pyrones From Syncolostemon argenteus. Phytochemistry 1998, 48, 651. [Google Scholar] [CrossRef]
- Marthe, D.C.Z.; Fidèle, M.A.; Fernand, G.; Mansourou, M. Chemical composition and in vitro investigation of biological activities of Hemizygia bracteosa (Benth.) Briq leaves. J. Pharmacogn. Phyther. 2018, 10, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Davies-Coleman, M.T.; Rivett, D.E.A. An α-pyrone from Syncolostemon densiflorus. Phytochemistry 1994, 35, 1590–1592. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Birkett, M.A.; Blande, J.; Hooper, A.M.; Martin, J.L.; Khambay, B.; Prosser, I.; Smart, L.E.; Wadhams, L.J. Response of economically important aphids to components of Hemizygia petiolata essential oil. Pest Manag. Sci. 2005, 61, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Davies-Coleman, M.T.; Rivett, D.E.A. Stereochemical studies on boronolide, an α-pyrone from Tetradenia barberae. Phytochemistry 1987, 26, 3047–3050. [Google Scholar] [CrossRef]
- Baldin, V.P.; de Scodro, R.B.L.; Lopes-Ortiz, M.A.; de Almeida, A.L.; Gazim, Z.C.; Ferarrese, L.; Faiões, V.d.S.; Torres-Santos, E.C.; Pires, C.T.A.; Caleffi-Ferracioli, K.R.; et al. Anti-Mycobacterium tuberculosis activity of essential oil and 6,7-dehydroroyleanone isolated from leaves of Tetradenia riparia (Hochst.) Codd (Lamiaceae). Phytomedicine 2018, 47, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Blythe, E.K.; Tabanca, N.; Demirci, B.; Kendra, P.E. Chemical composition of essential oil from Tetradenia riparia and its attractant activity for Mediterranean fruit fly, Ceratitis capitata. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- Godoy, R.L.O.; Koketsu, M.; Goncalves, S.L.; Lopes, D.; Sa Sobrinho, A.F.; Torquilho, H.S. Essential oil of Moschosma riparium Hochst. (Lamiaceae) from Manaus, Amazonas, Brazil. J. Essent. Oil Res. 1999, 11, 321–323. [Google Scholar] [CrossRef]
- Milato, J.V.; Silva, R.S.F.; Figueiredo, F.d.S.; Azevedo, D.d.A.; Ribeiro, C.A.B.; Leitão, G.G. Use of counter-current chromatography as a selective extractor for the diterpenequinone 7α-hydroxyroyleanone from Tetradenia riparia. J. Chromatogr. A 2018, 1537, 135–140. [Google Scholar] [CrossRef]
- Hannweg, K.; Visser, G.; de Jager, K.; Bertling, I. In vitro-induced polyploidy and its effect on horticultural characteristics, essential oil composition and bioactivity of Tetradenia riparia. S. Afr. J. Bot. 2016, 106, 186–191. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; Dube, S.; Uwimana, E.; Uwera, C.; Dommisse, R.A.; Esmans, E.L.; Van Schoor, O.; Vlietinck, A.J. New α-pyrones from Iboza riparia. Phytochemistry 1979, 18, 1215–1218. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; De Kimpe, N.; Dubé, S.; Chagnon-Dubé, M.; Boily, Y.; Borremans, F.; Schamp, N.; Anteunis, M.J.O. 1′,2′-Didecacetylboronolide, an α-Pyrone from Iboza riparia. Phytochemistry 1981, 20, 2753–2755. [Google Scholar] [CrossRef]
- Tokuda, M.; Kurogome, Y.; Katoh, R.; Nohara, Y.; Hattori, Y.; Makabe, H. Synthesis of four diastereomers and structural revision of tetradenolide. Tetrahedron Lett. 2014, 55, 4189–4192. [Google Scholar] [CrossRef] [Green Version]
- Van Puyvelde, L.; Nyirankuriza, S.; Panaebianco, R.; Boily, Y.; Geizer, I.; Sebikali, B.; De Kimpe, N.; Schamp, N. Active principles of Tetradenia riparia. I. Antimicrobial activity of 8(14),15-Sandracopimaradiene-7α,18-diol. J. Ethnopharmacol. 1986, 17, 269–275. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; Liu, M.; Veryser, C.; De Borggraeve, W.M.; Mungarulire, J.; Mukazayire, M.J.; Luyten, W. Active principles of Tetradenia riparia. IV. Anthelmintic activity of 8(14),15-sandaracopimaradiene-7α,18-diol. J. Ethnopharmacol. 2018, 216, 229–232. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; De Kimpe, N.; Borremans, F.; Zhang, W.; Schamp, N. 8(14),15-Sandaracopimaradiene-2α,18-diol, a minor constituent of the Rwandese medicinal plant Tetradenia riparia. Phytochemistry 1987, 26, 493. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; Lefebvre, R.; Mugabo, P.; De Kimpe, N.; Schamp, N. Active principles of Tetradenia riparia; II. Antispasmodic activity of 8(14),15-sandaracopimaradiene-7α,18-diol. Planta Med. 1987, 53, 156. [Google Scholar] [CrossRef] [Green Version]
- Zelnik, R.; Rabenhorst, E.; Matida, A.K.; Gottlier, H.E.; Lavie, D.; Panizza, S. Ibozol, a new Diterpenoid from Iboza riparia. Phytochemistry 1978, 17, 1795–1797. [Google Scholar] [CrossRef]
- Ruiters, A.K.; Tilney, P.M.; Van Vuuren, S.F.; Viljoen, A.M.; Kamatou, G.P.P.; Van Wyk, B.-E. The anatomy, ethnobotany, antimicrobial activity and essential oil composition of southern African species of Teucrium (Lamiaceae). S. Afr. J. Bot. 2016, 102, 175–185. [Google Scholar] [CrossRef]
- Davies-Coleman, M.T.; Hanson, J.R.; Rivett, D.E.A. Teutrifidin, A neo-clerodane diterpenoid from Teucrium trifidum. Phytochemistry 1994, 36, 1549–1550. [Google Scholar] [CrossRef]
- Cabral, C.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L.; Antunes, T.; Sevinate-Pinto, I.; Sales, F. Vitex ferruginea Schumach. Et. Thonn subsp. amboniensis (Gürke) Verdc: Glandular trichomes micromorphology, composition and antifungal activity of the essential oils. J. Essent. Oil Res. 2008, 20, 86–90. [Google Scholar] [CrossRef]
- Nyiligira, E.; Viljoen, A.M.; Başer, K.H.C.; Ózek, T.; Van Vuuren, S.F. Essential oil composition and in vitro antimicrobial and anti-inflammatory activity of South African Vitex species. S. Afr. J. Bot. 2004, 70, 611–617. [Google Scholar] [CrossRef] [Green Version]
- Suksamrarn, A.; Werawattanametin, K.; Brophy, J.J. Variation of essential oil constituents in Vitex trifolia species. Flavour Fragr. J. 1991, 6, 97–99. [Google Scholar] [CrossRef]
- Nair, A.G.R.; Ramesh, P.; Subramanian, S.S. Two unusual flavones (artemetin and 7-desmethyl artemetin) from the leaves of Vitex trifolia. Curr. Sci. 1975, 44, 214–216. [Google Scholar]
- Ono, M.; Sawamura, H.; Ito, Y.; Mizuki, K.; Nohara, T. Diterpenoids from the fruits of Vitex trifolia. Phytochemistry 2000, 55, 873–877. [Google Scholar] [CrossRef]
- Masevhe, N.A.; Awouafack, M.D.; Ahmed, A.S.; McGaw, L.J.; Eloff, J.N. Clerodendrumic Acid, a new triterpenoid from Clerodendrum glabrum (Verbenaceae), and antimicrobial activities of fractions and constituents. ChemInform 2014, 45. [Google Scholar] [CrossRef]
- Teclegeorgish, Z.W.; Mokgalaka, N.S.; Vukea, N.; de la Mare, J.A.; Tembu, V.J. Cytotoxicity of triterpenoids from Clerodendrum glabrum against triple negative breast cancer cells in vitro. S. Afr. J. Bot. 2020, 133, 144–150. [Google Scholar] [CrossRef]
- Wahba, H.M.; Abouzid, S.F.; Sleem, A.A.; Apers, S.; Pieters, L.; Shahat, A.A. Chemical and biological investigation of some Clerodendrum species cultivated in Egypt. Pharm. Biol. 2011, 49, 66–72. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.E. 1.25—Alkaloids. In Comprehensive Natural Products II; Liu, H.-W., Mander, L.B.T.-C.N.P.I.I., Eds.; Elsevier: Oxford, UK, 2010; pp. 977–1007. ISBN 978-0-08-045382-8. [Google Scholar]
- Wansi, J.D.; Devkota, K.P.; Tshikalange, E.; Kuete, V. Alkaloids from the Medicinal Plants of Africa; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9780124059276. [Google Scholar]
- Harborne, J.B. Methods of Plant Analysis BT—Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; Harborne, J.B., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 1984; ISBN 978-94-009-5570-7. [Google Scholar]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Yordi Molina, E.; Guardado, E. Coumarins—An Important Class of Phytochemicals. In Phytochemicals—Isolation, Characterisation and Role in Human Health; Rao, V., Rao, L., Eds.; IntechOpen: London, UK, 2015; ISBN 9781626239777. [Google Scholar]
- Faridi Esfanjani, A.; Assadpour, E.; Jafari, S.M. Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Karakaya, S. Bioavailability of phenolic compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464. [Google Scholar] [CrossRef]
- Shahidi, F.; Varatharajan, V.; Oh, W.Y.; Peng, H. Phenolic compounds in agri-food by-products, their bioavailability and health effects. J. Food Bioact. 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Peng, H. Bioaccessibility and bioavailability of phenolic compounds. J. Food Bioact. 2018, 4, 11–68. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Panchon, M.S.; Villano, D.; Troncoso, A.M.; Garcia-Parrilla, M.C. Antioxidant activity of phenolic compounds: From in vitro results to in vivo evidence. Crit. Rev. Food Sci. Nutr. 2008, 48, 649–671. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Zhao, Y.Y.; Luo, C.X.; Li, J.; Gao, Y.Q. Total phenolic contents of 33 fruits and their antioxidant capacities before and after in vitro digestion. Ind. Crops Prod. 2014, 57, 150–157. [Google Scholar] [CrossRef]
- Lukhoba, C.W.; Simmonds, M.S.J.; Paton, A.J. Plectranthus: A review of ethnobotanical uses. J. Ethnopharmacol. 2006, 103, 1–24. [Google Scholar] [CrossRef]
- Salame, R.; Cheikh-Ali, Z.; Bories, C.; Adiko, M.; Poupon, E.; Champy, P. Pyrone and unusually furanone-substituted flavones from the leaves of Hoslundia opposita. Planta Med. 2012, 78, 1777–1779. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Kosar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J. Agric. Food Chem. 2003, 51, 4563–4569. [Google Scholar] [CrossRef]
- Kumar, T.V.; Reddy, G.V.; Babu, K.S.; Rao, J.M. Stereoselective synthesis of umuravumbolide. Tetrahedron Asymmetry 2013, 24, 594–598. [Google Scholar] [CrossRef]
- Demetzos, C.; Dimas, K.S. Labdane-type diterpenes: Chemistry and biological activity. In Bioactive Natural Products (Part F); Elsevier: Amsterdam, The Netherlands, 2001; Volume 25, pp. 235–292. ISBN 1572-5995. [Google Scholar]
- Manse, Y.; Ninomiya, K.; Nishi, R.; Hashimoto, Y.; Chaipech, S.; Muraoka, O.; Morikawa, T. Labdane-type diterpenes, Galangalditerpenes A–C, with melanogenesis inhibitory activity from the fruit of Alpinia galanga. Molecules 2017, 22, 2279. [Google Scholar] [CrossRef] [Green Version]
- Said, S.A. Antimalarial effect and other properties of Hoslundia opposita—A review. Glob. J. Pharm. Pharm. Sci. 2017, 4, 1–6. [Google Scholar] [CrossRef]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Pia Gallo, M.; Maffei, M.E.; Bovolin, P. Protective effects of (E)-β-caryophyllene (BCP) in chronic inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef]
- Adlof, C.C. How Does Harvesting Impact White Sage (Salvia apiana) as a Cultural Resource in Southern California? Ph.D. Thesis, California State University, Northridge, CA, USA, 2015. [Google Scholar]
- Cohen, M.M. Tulsi—Ocimum sanctum: A herb for all reasons. J. Ayurveda Integr. Med. 2014, 5, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Khoury, M.; Stien, D.; Eparvier, V.; Ouaini, N.; El Beyrouthy, M. Report on the medicinal use of eleven Lamiaceae species in Lebanon and rationalization of their antimicrobial potential by examination of the chemical composition and antimicrobial activity of their essential oils. Evid. Based Complement. Altern. Med. 2016, 2016, 2547169. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Li, Q.; Zhang, C.; Zhang, N.; Cui, Z.; Huang, L.; Xiao, P. An ethnopharmacological investigation of medicinal Salvia plants (Lamiaceae) in China. Acta Pharm. Sin. B 2013, 3, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Lin, C.; Xu, W.; Liu, Y.; Long, C. Ethnobotanical study on medicinal plants used by Mulam people in Guangxi, China. J. Ethnobiol. Ethnomed. 2020, 16, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Perrino, E.V.; Perrino, P. Crop Wild Relatives: Know How Past and Present to Improve Future Research, Conservation and Utilization Strategies, Especially in Italy: A Review. Genet. Resour. Crop Evol. 2020, 67, 1067–1105. [Google Scholar] [CrossRef]
- Asong, J.A.; Ndhlovu, P.T.; Khosana, N.S.; Aremu, A.O.; Otang-Mbeng, W. Medicinal plants used for skin-related diseases among the Batswanas in Ngaka Modiri Molema District Municipality, South Africa. S. Afr. J. Bot. 2019, 126, 11–20. [Google Scholar] [CrossRef]
- Nafuka, S.N.; Mumbengegwi, D.R. Phytochemical analysis and in vitro anti-plasmodial activity of selected ethnomedicinal plants used to treat malaria associated Symptoms in Northern Namibia. Int. Sci. Technol. J. Namibia 2013, 2, 78–93. [Google Scholar]
- Jäger, A.K.; Mohoto, S.P.; Van Heerden, F.R.; Viljoen, A.M. Activity of a traditional South African epilepsy remedy in the GABA-benzodiazepine receptor assay. J. Ethnopharmacol. 2005, 96, 603–606. [Google Scholar] [CrossRef]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa, 2nd ed.; Livingstone: London, UK, 1962; ISBN 0608142948. [Google Scholar]
- Moteetee, A.; Moffett, R.O.; Seleteng-Kose, L. A review of the ethnobotany of the Basotho of Lesotho and the Free State Province of South Africa (South Sotho). S. Afr. J. Bot. 2019, 122, 21–56. [Google Scholar] [CrossRef]
- Leffers, A. Gemsbok Bean and Kalahari Truffle. Traditional Plant Use by Jul’hoansi in North-Eastern Namibia; Macmillan Education: Windhoek, Namibia, 2003; ISBN 999160491X. [Google Scholar]
- Semenya, S.S.; Maroyi, A. Ethnobotanical survey of plants used by Bapedi traditional healers to treat tuberculosis and its opportunistic infections in the Limpopo Province, South Africa. S. Afr. J. Bot. 2019, 122, 401–421. [Google Scholar] [CrossRef]
- Pooley, E. A Field Guide to Wildflowers: KwaZulu-Natal and the Eastern Region; Natal Flora Publications Trust: Durban, South Africa, 1999; ISBN 0620215003. [Google Scholar]
- Sahrial, I.; Solfaine, R. Coleus amboinicus extract increases transforming growth factor-1β expression in Wistar rats with cisplatin-induced nephropathy. Vet. World 2019, 12, 1346–1351. [Google Scholar] [CrossRef]
- Thanaseelungkoon, N.; Julsrigival, J.; Phannachet, K.; Chansakaow, S. Chemical compositions and biological activities of essential oils obtained from some Apiaceous and Lamiaceous plants collected in Thailand. Asian Pac. J. Trop. Med. 2018, 11, 486–494. [Google Scholar] [CrossRef]
- Musila, F.M.; Nguta, J.; Lukhoba, C.W.; Dossaji, S.F. Antibacterial and antifungal activities of 10 Kenyan Plectranthus species in the Coleus clade. Artic. J. Pharm. Res. 2017, 11, 1–7. [Google Scholar]
- Haryani, R.; Harahap, U.; Masfria, M.; Satria, D. Cytoprotective activity of ethanol fraction of Coleus amboinicus Lour. leaves against vero cells induced by H2O2. Asian J. Pharm. Clin. Res. 2018, 11, 28–30. [Google Scholar] [CrossRef] [Green Version]
- Tarigan, M.H.; Harahap, U.; Dalimunthe, A.; Nerdy, N. Antioxidant activity and cardioprotective activity of bangun-bangun leaves (Plectranthus amboinicus Lour.) ethanolic extract. Asian J. Pharm. Clin. Res. 2018, 11, 165–168. [Google Scholar] [CrossRef]
- Lipin Dev, D.S.; Menon, D.B. Essential oil extracted from Plectranthus amboinicus induces apoptosis in the lung cancer cells via mitochondrial pathway. Int. J. Pharm. Sci. Drug Res. 2017, 9, 83–89. [Google Scholar] [CrossRef]
- Hutchings, A.; Lewis, A.H.; Cunningham, A.B. Zulu Medicinal Plants: An Inventory; University of Natal Press: Pietermaritzburg, South Africa, 1996; ISBN 0869808931. [Google Scholar]
- Menon, D.B.; Sasikumar, J.M.; Latha, K. Anti inflammtory and cytotoxic activity of methanolic extract of Plectranthus hadiensis stem. Pharmacologyonline 2011, 3, 275–282. [Google Scholar]
- Mogale, M.M.P. The Ethnobotany of Central Sekhukhuneland, South Africa. Master’s Thesis, University of Johannesburg, Johannesburg, South Africa, 2018. [Google Scholar]
- Mogale, M.M.P.; Raimondo, D.C.; Van Wyk, B.-E. The ethnobotany of Central Sekhukhuneland, South Africa. S. Afr. J. Bot. 2019, 122, 90–119. [Google Scholar] [CrossRef]
- Magwede, K. A Quantitative Survey of Traditional Plant Use of the Vhavenḓa, Limpopo Province, South Africa. Ph.D. Thesis, University of Johannesburg, Johannesburg, South Africa, 2018. [Google Scholar]
- Palmer, E. The South African Herbal; Tafelberg: Cape Town, South Africa, 1985; ISBN 0624021777. [Google Scholar]
- Maharaj, R.; Maharaj, V.; Crouch, N.R.; Bhagwandin, N.; Folb, P.I.; Pillay, P.; Gayaram, R. Evaluation of selected South African ethnomedicinal plants as mosquito repellents against the Anopheles arabiensis mosquito in a rodent model. Malar. J. 2010, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Von Koenen, E. Heil-und Giftplanzen in Sudwestafrika; Akademischer Verlag: Windhoek, Namibia, 1977; ISBN 0620028874. [Google Scholar]
- Oloyede, A.M.; Akindele, S.K. A preliminary investigation of the acute toxicity of the ethanolic extract of Hoslundia opposita in Mus musculus (Swiss Mice). Herb. Med. J. 2019, 4, 163–170. [Google Scholar] [CrossRef]
- Olajide, O.A.; Awe, S.O.; Makinde, J.M. Central nervous system depressant effect of Hoslundia opposita Vahl. Phyther. Res. 1999, 13, 425–426. [Google Scholar] [CrossRef]
- Risa, J.; Risa, A.; Adsersen, A.; Gauguin, B.; Stafford, G.I.; Van Staden, J.; Jäger, A.K. Screening of plants used in southern Africa for epilepsy and convulsions in the GABAA-benzodiazepine receptor assay. J. Ethnopharmacol. 2004, 93, 177–182. [Google Scholar] [CrossRef]
- Afolayan, A.J.; Mbaebie, B.O. Ethnobotanical study of medicinal plants used as anti-obesity remedies in Nkonkobe Municipality of South Africa. Pharmacogn. J. 2010, 2, 368–373. [Google Scholar] [CrossRef] [Green Version]
- Davids, D.; Gibson, D.; Johnson, Q. Ethnobotanical survey of medicinal plants used to manage High Blood Pressure and Type 2 Diabetes Mellitus in Bitterfontein, Western Cape Province, South Africa. J. Ethnopharmacol. 2016, 194, 755–766. [Google Scholar] [CrossRef] [Green Version]
- Hulley, I.M.; Van Wyk, B.-E. Quantitative medicinal ethnobotany of Kannaland (western Little Karoo, South Africa): Non-homogeneity amongst villages. S. Afr. J. Bot. 2019, 122, 225–265. [Google Scholar] [CrossRef]
- Klos, M.; van de Venter, M.; Milne, P.J.; Traore, H.N.; Meyer, D.; Oosthuizen, V. In vitro anti-HIV activity of five selected South African medicinal plant extracts. J. Ethnopharmacol. 2009, 124, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Maphosa, V.; Masika, P.J.; Bizimenyera, E.S.; Eloff, J.N. In vitro anthelminthic activity of crude aqueous extracts of Aloe ferox, Leonotis leonurus and Elephantorrhiza elephantina against Haemonchus contortus. Trop. Anim. Health Prod. 2010, 42, 301–307. [Google Scholar] [CrossRef]
- Maphosa, V.; Masika, P.J. In vivo validation of Aloe ferox (Mill), Elephantorrhiza elephantina Bruch. Skeels. and Leonotis leonurus (L) R.Br. as potential anthelminthics and antiprotozoals against mixed infections of gastrointestinal nematodes in goats. Parasitol. Res. 2012, 110, 103–108. [Google Scholar] [CrossRef]
- Odei-Addo, F.; Shegokar, R.; Müller, R.H.; Levendal, R.A.; Frost, C. Nanoformulation of Leonotis leonurus to improve its bioavailability as a potential antidiabetic drug. 3 Biotech 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Mnonopi, N.; Levendal, R.A.; Davies-Coleman, M.T.; Frost, C.L. The cardioprotective effects of marrubiin, a diterpenoid found in Leonotis leonurus extracts. J. Ethnopharmacol. 2011, 138, 67–75. [Google Scholar] [CrossRef]
- Mnonopi, N.; Levendal, R.A.; Mzilikazi, N.; Frost, C.L. Marrubiin, a constituent of Leonotis leonurus, alleviates diabetic symptoms. Phytomedicine 2012, 19, 488–493. [Google Scholar] [CrossRef]
- Popoola, O.K.; Elbagory, A.M.; Ameer, F.; Hussein, A.A. Marrubiin. Molecules 2013, 18, 9049–9069. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M. Indigenous Healing Plants; Southern Book Publishers: Halfway House, South Africa, 1992; ISBN 1875093826. [Google Scholar]
- Rood, B. Uit Die Veld-Apteek; Tafelberg: Cape Town, South Africa, 1994; ISBN 1869192745. [Google Scholar]
- Batten, A.; Bokelman, H. Wildflowers of the Eastern Cape; Books of Africa: Cape Town, South Africa, 1996; ISBN B0006BSHIA. [Google Scholar]
- De Oliveira, D.P.; De Almeida, L.; Marques, M.J.; De Carvalho, R.R.; Dias, A.L.T.; Da Silva, G.A.; De Pádua, R.M.; Braga, F.C.; Da Silva, M.A. Exploring the bioactivity potential of Leonotis nepetifolia: Phytochemical composition, antimicrobial and antileishmanial activities of extracts from different anatomical parts. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Hulley, I.M. Medicinal Ethnobotany of the Little Karoo, South Africa. Ph.D. Thesis, University of Johannesburg, Johannesburg, South Africa, 2018. [Google Scholar]
- Mhlongo, L.S.; Van Wyk, B.-E. Zulu medicinal ethnobotany: New records from the Amandawe area of KwaZulu-Natal, South Africa. S. Afr. J. Bot. 2019, 122, 266–290. [Google Scholar] [CrossRef]
- Rodin, R.J. The Ethnobotany of the Kwanyama Ovambos. Monographs in Systematic Botany 9; Missouri Botanical Garden: St. Louis, MO, USA, 1985; ISBN 999516843X. [Google Scholar]
- Borah, M.; Das, S. Antidiabetic, antihyperlipidaemic and antioxidant activities of leaves of Leucas linifolia Spreng In Streptozocin induced diabetic rats. Eur. J. Biomed. Pharm. Sci. 2015, 2, 538–551. [Google Scholar]
- Chandrashekar, K.S.; Prasanna, K.S.; Joshi, A.B. Hepatoprotective activity of the Leucas lavandulaefolia on d(+)galactosamine-induced hepatic injury in rats. Fitoterapia 2007, 78, 440–442. [Google Scholar] [CrossRef]
- Ramalingam, R.; Nath, A.R.; Madhavi, B.B.; Nagulu, M. In vitro free radical scavenging, cytotoxic and acetylcholinesterase inhibitory activities of Leucas martinicensis. Int. J. Chem. Anal. Sci. 2013, 4, 91–95. [Google Scholar] [CrossRef]
- Palmer, E. A Gardener’s Year; Tafelberg: Cape Town, South Africa, 1995; ISBN 0624034062. [Google Scholar]
- Thibane, V.S.; Ndhlala, A.R.; Abdelgadir, H.A.; Finnie, J.F.; Van Staden, J. The cosmetic potential of plants from the Eastern Cape Province traditionally used for skincare and beauty. S. Afr. J. Bot. 2019, 122, 475–483. [Google Scholar] [CrossRef]
- Lodhi, S.; Vadnere, G.P.; Sharma, V.K.; Usman, M.R. Marrubium vulgare L.: A review on phytochemical and pharmacological aspects. J. Intercult. Ethnopharmacol. 2017, 6, 429–452. [Google Scholar] [CrossRef]
- Yabrir, B. Essential oil of Marrubium vulgare: Chemical composition and biological activities. A review. Nat. Prod. Sci. 2019, 25, 81–91. [Google Scholar] [CrossRef] [Green Version]
- De Beer, J.J.J.; Van Wyk, B.-E. An ethnobotanical survey of the Agter-Hantam, Northern Cape Province, South Africa. S. Afr. J. Bot. 2011, 77, 741–754. [Google Scholar] [CrossRef] [Green Version]
- Thring, T.S.A.; Weitz, F.M. Medicinal plant use in the Bredasdorp/Elim region of the Southern Overberg in the Western Cape Province of South Africa. J. Ethnopharmacol. 2006, 103, 261–275. [Google Scholar] [CrossRef]
- McGaw, L.J.; Jäger, A.K.; Van Staden, J. Prostaglandin synthesis inhibitory activity in Zulu, Xhosa and Sotho medicinal plants. Phyther. Res. 1997, 11, 113–117. [Google Scholar] [CrossRef]
- Al-Bayati, F.A. Isolation and identification of antimicrobial compound from Mentha longifolia L. leaves grown wild in Iraq. Ann. Clin. Microbiol. Antimicrob. 2009, 8. [Google Scholar] [CrossRef] [Green Version]
- Akroum, S.; Bendjeddou, D.; Satta, D.; Lalaoui, K. Antibacterial activity and acute toxicity effect of flavonoids extracted from Mentha longifolia. Am. J. Sci. Res. 2009, 4, 93–96. [Google Scholar]
- Smith, C.A. Common Names of South African Plants. Memoirs of the Botanical Survey of South Africa No. 35; Department of Agriculture and Technical Services, Pretoria: Pretoria, South Africa, 1966; ISBN 5016872-1963-64-2,500. [Google Scholar]
- Van den Eynden, V.; Vernemmen, P.; Van Damme, P. The Ethnobotany of the Topnaar; Universiteit Gent: Gent, Belgium, 1992; ISBN B00J2HBINK. [Google Scholar]
- Carović-Stanko, K.; Orlić, S.; Politeo, O.; Strikić, F.; Kolak, I.; Milos, M.; Satovic, Z. Composition and antibacterial activities of essential oils of seven Ocimum taxa. Food Chem. 2010, 119, 196–201. [Google Scholar] [CrossRef]
- Cavalcanti, E.S.B.; de Morais, S.M.; Lima, M.A.A.; Santana, E.W.P. Larvicidal activity of essential oils from Brazilian plants against Aedes aegypti L. Mem. Inst. Oswaldo Cruz 2004, 99, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Fawole, O.A.; Finnie, J.F.; Van Staden, J. Antimicrobial activity and mutagenic effects of twelve traditional medicinal plants used to treat ailments related to the gastro-intestinal tract in South Africa. S. Afr. J. Bot. 2009, 75, 356–362. [Google Scholar] [CrossRef] [Green Version]
- Mhlongo, L.S. The Medicinal Ethnobotany of the Amandawe Area in KwaCele, KwaZulu-Natal, South Africa. Master’s Thesis, University of Johannesburg, Johannesburg, South Africa, 2019. [Google Scholar]
- Figueiredo, N.L.; de Aguiar, S.R.M.M.; Falé, P.L.; Ascensão, L.; Serralheiro, M.L.M.; Lino, A.R.L. The inhibitory effect of Plectranthus barbatus and Plectranthus ecklonii leaves on the viability, glucosyltransferase activity and biofilm formation of Streptococcus sobrinus and Streptococcus mutans. Food Chem. 2010, 119, 664–668. [Google Scholar] [CrossRef]
- Boon, R. Pooley’s Trees of Eastern South Africa. A Complete Guide, 2nd ed.; Natal Flora Publications Trust: Durban, South Africa, 2010; ISBN 9780620460194. [Google Scholar]
- Njeru, S.N.; Obonyo, M.; Nyambai, S.; Ngari, S.; Mwakubambanya, R.; Mavura, H. Antimicrobial and cytotoxicity properties of the organic solvent fractions of Clerodendrum myricoides (Hochst.) R. Br. ex Vatke: Kenyan traditional medicinal plant. J. Intercult. Ethnopharmacol. 2016, 5, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Chege, B.M.; Waweru, M.P.; Frederick, B.; Nyaga, N.M. The freeze-dried extracts of Rotheca myricoides (Hochst.) Steane and Mabb possess hypoglycemic, hypolipidemic and hypoinsulinemic on type 2 diabetes rat model. J. Ethnopharmacol. 2019, 244, 112077. [Google Scholar] [CrossRef]
- Hayelom, K.; Mekbeb, A.; Eyasu, M.; Wondwossen, E.; Kelbesa, U. Methanolic effect of Clerodendrum myricoides root extract on blood, liver and kidney tissues of mice. Afr. Health Sci. 2012, 12, 489–497. [Google Scholar] [CrossRef] [Green Version]
- Van Wyk, B.-E. A review of Khoi-San and Cape Dutch medical ethnobotany. J. Ethnopharmacol. 2008, 119, 331–341. [Google Scholar] [CrossRef]
- Motlhatlego, K.E. Evaluation of Plants Used in African Traditional Medicine for Asthma and Related Conditions. Ph.D. Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2014. [Google Scholar]
- Cock, I.E.; Van Vuuren, S.F. The potential of selected South African plants with anti-Klebsiella activity for the treatment and prevention of Ankylosing spondylitis. Inflammopharmacology 2014, 23, 21–35. [Google Scholar] [CrossRef] [Green Version]
- Jäger, A.K.; Van Staden, J. Salvia in southern Africa. In Sage: The genus Salvia; Kintzios, S.E., Ed.; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000; ISBN 0203304551. [Google Scholar]
- Nortje, J.M.; Van Wyk, B.-E. Useful plants of Namaqualand, South Africa: A checklist and analysis. S. Afr. J. Bot. 2019, 122, 120–135. [Google Scholar] [CrossRef]
- Mössmer, N.; David, L. Traditional Herbal Remedies; Montagu Museum: Montagu, South Africa, 2019. [Google Scholar]
- Smith, A. A Contribution to South African Materia Medica; Juta: Cape Town, South Africa, 1895; ISBN 978-0-86810-469-0. [Google Scholar]
- Shearing, D. Karoo. South African wild flower guide 6; Botanical Scociety of South Africa: Kirstenbosch, Cape Town, South Africa, 1994; ISBN 9781874999041. [Google Scholar]
- Elgorashi, E.E.; Taylor, J.L.S.; Maes, A.; Van Staden, J.; De Kimpe, N.; Verschaeve, L. Screening of medicinal plants used in South African traditional medicine for genotoxic effects. Toxicol. Lett. 2003, 143, 195–207. [Google Scholar] [CrossRef]
- Coopoosamy, R.M.; Naidoo, K.K. Assessing the potential of Tetradenia riparia in treatment of common skin conditions in rural communities of South Africa. African J. Microbiol. Res. 2011, 5, 2942–2945. [Google Scholar] [CrossRef] [Green Version]
- Akhalwaya, S.; Van Vuuren, S.; Patel, M. An in vitro investigation of indigenous South African medicinal plants used to treat oral infections. J. Ethnopharmacol. 2018, 210, 359–371. [Google Scholar] [CrossRef]
- Endo, E.H.; Costa, G.M.; Nakamura, T.U.; Nakamura, C.V.; Dias Filho, B.P. Antidermatophytic activity of hydroalcoholic extracts from Rosmarinus officinalis and Tetradenia riparia. J. Mycol. Med. 2015, 25, 274–279. [Google Scholar] [CrossRef]
- Endo, E.H.; Costa, G.M.; Makimori, R.Y.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P. Anti-biofilm activity of Rosmarinus officinalis, Punica granatum and Tetradenia riparia against methicillin-resistant Staphylococcus aureus (MRSA) and synergic interaction with penicillin. J. Herb. Med. 2018, 14, 48–54. [Google Scholar] [CrossRef]
- Ghuman, S.; Ncube, B.; Finnie, J.F.; McGaw, L.J.; Coopoosamy, R.M.; Van Staden, J. Antimicrobial activity, phenolic content, and cytotoxicity of medicinal plant extracts used for treating dermatological diseases and wound healing in KwaZulu-Natal, South Africa. Front. Pharmacol. 2016, 7, 320. [Google Scholar] [CrossRef] [Green Version]
- Campbell, W.E.; Gammon, D.W.; Smith, P.; Abrahams, M.; Purves, T.D. Composition and antimalarial activity in vitro of the essential oil of Tetradenia riparia. Planta Med. 1997, 63, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Jarić, S.; Mitrović, M.; Pavlović, P. Ethnobotanical features of Teucrium species. In Teucrium Species: Biology and Applications; Stanković, M., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 111–142. ISBN 978-3-030-52159-2. [Google Scholar]
- McGaw, L.J.; Eloff, J.N. Ethnoveterinary use of southern African plants and scientific evaluation of their medicinal properties. J. Ethnopharmacol. 2008, 119, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Stefanović, O. Antibacterial and antifungal activity of secondary metabolites of Teucrium species. In Teucrium Species: Biology and Applications; Stanković, M., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 319–354. ISBN 978-3-030-52159-2. [Google Scholar]
- Coats Palgrave, K. Trees of Southern Africa; Struik Publishers: Cape Town, South Africa, 1977; ISBN 1868251713. [Google Scholar]
- McGaw, L.J.; Jäger, A.K.; Staden, J. Van Antibacterial, anthelmintic and anti-amoebic activity in South African medicinal plants. J. Ethnopharmacol. 2000, 72, 247–263. [Google Scholar] [CrossRef]
- Adamu, M.; Naidoo, V.; Eloff, J.N. Some southern African plant species used to treat helminth infections in ethnoveterinary medicine have excellent antifungal activities. BMC Complement. Altern. Med. 2012, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waller, S.B.; Cleff, M.B.; Serra, E.F.; Silva, A.L.; dos Gomes, A.R.; de Mello, J.R.B.; de Faria, R.O.; Meireles, M.C.A. Plants from Lamiaceae family as source of antifungal molecules in humane and veterinary medicine. Microb. Pathog. 2017, 104, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Bapela, M.J.; Meyer, J.J.M.; Kaiser, M. In vitro antiplasmodial screening of ethnopharmacologically selected South African plant species used for the treatment of malaria. J. Ethnopharmacol. 2014, 156, 370–373. [Google Scholar] [CrossRef] [Green Version]
- Bapela, M.J.; Kaiser, M.; Meyer, J.J.M. Antileishmanial activity of selected South African plant species. S. Afr. J. Bot. 2017, 108, 342–345. [Google Scholar] [CrossRef]
- Kunkel, G. Plants Africa gave to the world. Bothalia 1983, 14, 465–469. [Google Scholar] [CrossRef]
- Asfaw, Z.; Tadesse, M. Prospects for sustainable use and development of wild food plants in Ethiopia. Econ. Bot. 2001, 55, 47–62. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Gericke, N. People’s Plants: A Guide to Useful Plants of Southern Africa, 2nd ed.; Briza Publications: Pretoria, South Africa, 2017; ISBN 9781920217716. [Google Scholar]
- Crouch, N.R.; Styles, D.G.A. Rediscovery in South Africa of the Neglected African Vegetable Plectranthus Esculentus. Bothalia 2010, 40, 65–67. [Google Scholar] [CrossRef]
- Rice, L.J.; Brits, G.J.; Potgieter, C.J.; Van Staden, J. Plectranthus: A plant for the future? S. Afr. J. Bot. 2011, 77, 947–959. [Google Scholar] [CrossRef] [Green Version]
- Welcome, A.K.; Van Wyk, B.-E. An inventory and analysis of the food plants of southern Africa. S. Afr. J. Bot. 2019, 122, 136–179. [Google Scholar] [CrossRef]
- Magwede, K.; Van Wyk, B.-E.; Van Wyk, A.E. An inventory of Vhavenḓa useful plants. S. Afr. J. Bot. 2019, 122, 57–89. [Google Scholar] [CrossRef]
- Welcome, A.K. Food Plants of Southern Africa. Ph.D. Thesis, University of Johannesburg, Johannesburg, South Africa, 2019. [Google Scholar]
- Nortje, J.M. Ethnobotany of Namaqualand, South Africa. Ph.D. Thesis, University of Johannesburg, Johannesburg, South Africa, 2019. [Google Scholar]
- Malan, J.S.; Owen-Smith, G.L. The ethnobotany of Kaoland. In Proceedings of the Cimbeasia. 3.2.5.; Indiana University: Bloomington, IN, USA, 1974; pp. 131–178. [Google Scholar]
- Archer, F.M. Planning with people—Ethnobotany and African uses of plants in Namaqualand (South Africa). In Proceedings of the 12th Plenary Meeting of AEFAT, Hamburg, Germany, 4–10 September 1988; pp. 959–972. [Google Scholar]
- Archer, F.M. Ethnobotany of Namaqualand: The Richtersveld. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 1994. [Google Scholar]
- Van Breda, P.A.B.; Barnard, S.A. 100 Veld Plants of the Winter Rainfall Region; Department of Agricultural Development: Elsenburg, South Africa, 1991; ISBN 0-621-13470-8. [Google Scholar]
- Viljoen, A.M.; Van Vuuren, S.; Ernst, E.; Klepser, M.; Demirci, B.; Başer, H.; Van Wyk, B.-E. Osmitopsis asteriscoides (Asteraceae)—The antimicrobial activity and essential oil composition of a Cape-Dutch remedy. J. Ethnopharmacol. 2003, 88, 137–143. [Google Scholar] [CrossRef]

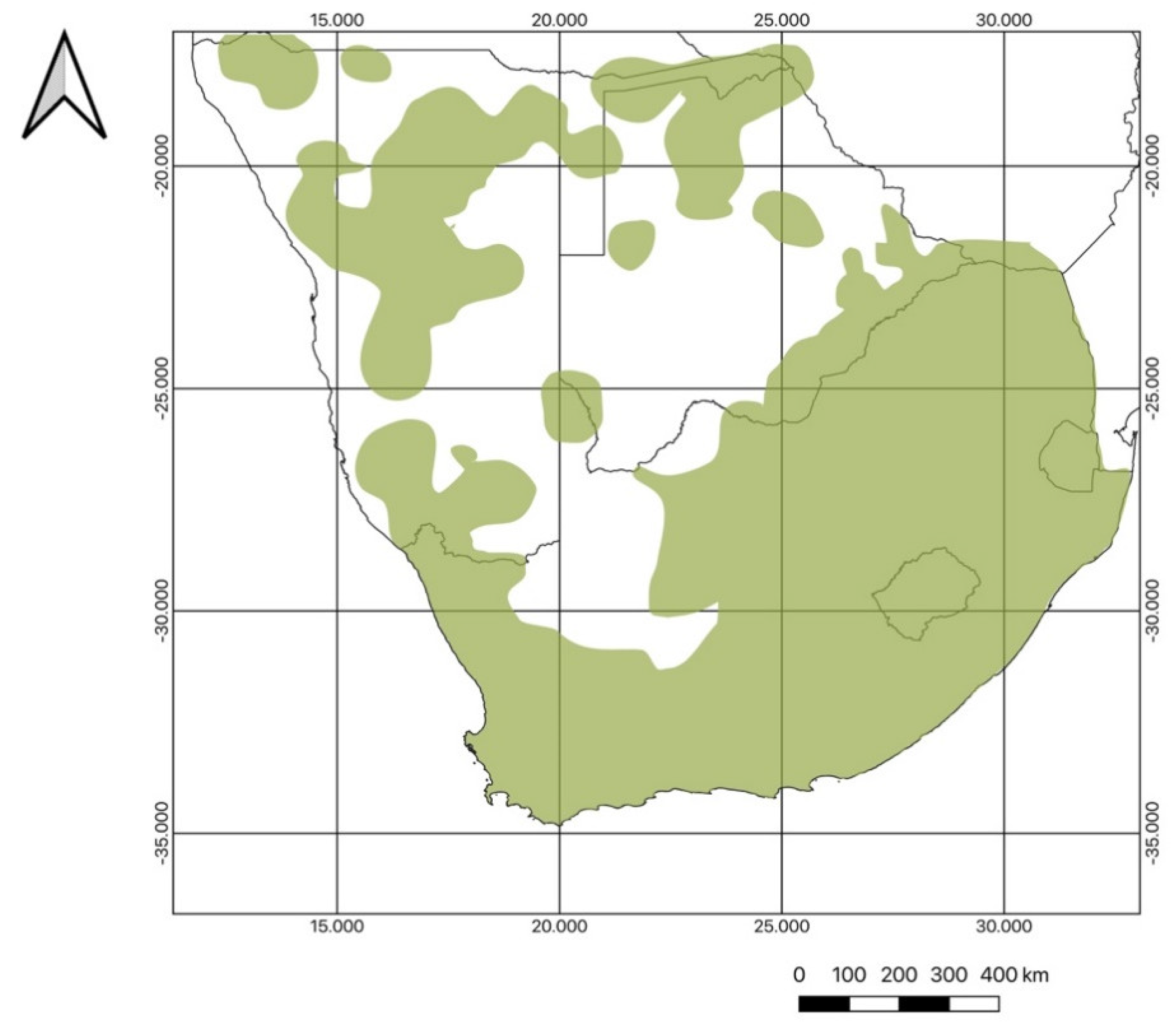





| Genus | Species | Main Compounds in Essential Oil ^ | Number of Samples Analyzed | Number of Compounds Reported | Reference | Quality of Study | Other Phytochemical Compounds | Reference | Level of Study |
|---|---|---|---|---|---|---|---|---|---|
| Acrotome | A. inflata | - | - | - | - | - | Coumarins Flavonoids Tannins | [34] | ★ |
| Terpenoids | [41] | ★ | |||||||
| Aeollanthus | A. buchnerianus | - | - | - | - | - | Caffeic acid esters | [42] | ★★ |
| Diterpenoids | [30] | ★★★ | |||||||
| A. parvifolius | α-Muurolol | 1 a | 26 | [43] | ★★ | - | - | - | |
| Basilicum | B. polystachyon | Epiglobulol Ylangene | 1 a | 64 | [44] | ★★★ | Diterpenoids | [45] | ★★★ |
| Stachyonic acid A | [46] | ★★★ | |||||||
| Alkaloids Coumarins Flavonoids Phenols Tannins Triterpenes Sterols Saponins | [47] | ★ | |||||||
| Cantinoa * | C. americana * | α-Bergamotene β-Caryophyllene Germacrene-A | 1 b | 30 | [48] | ★★ | Spicigera Lactone | [49] | ★★ |
| δ-3-Carene β-Caryophyllene | 1 a | 34 | [50] | ★★ | Labdane diterpenes | [51] | ★★★ | ||
| Alkaloids | [38] | ★ | |||||||
| - | - | - | - | - | Carbohydrates Cardiac glycosides Coumarins Flavonoids | [38] | ★ | ||
| Cantinoa * (Cont.) | C. americana * (Cont.) | - | - | - | - | - | Quinones Resins Saponins Steroids Terpenoids Vitannins | [38] | ★ |
| C. mutabilis * | Bicyclogermacrene β-Caryophyllene Curzerene Germacrene-D | 2 c | 24 | [52] | ★★ | Triterpenoids | [53] | ★★★ | |
| 1,8-Cineole Limonene Spathulenol | 12 # | 105 | [54] | ★★★ | |||||
| Camphor cis-Dihydrocarvone trans-Dihydrocarvone | 1 a | 31 | [55] | ★★ | |||||
| Cedronella * | C. canariensis * | β-Pinene Pinocarvone p-Allyl anisole | 2 a - | 27 - | [56] - | ★★ - | Terpenoids | [57] | ★★★ |
| Caffeic acid esters | [42] | ★★ | |||||||
| Coleus | C. aliciae | - | - | - | - | - | Diterpenoids | [58] | # |
| C. amboinicus | Carvacrol p-Cymene γ-Terpinene | 2 b | 12 | [59] | ★★ | Phenolics | [60] | ★★ | |
| Coleus (Cont.) | C. amboinicus (Cont.) | Carvacrol Caryophyllene α-Bergamotene | 1 b | 9 | [61] | ★★ | Terpenoids | [31] | ★★★ |
| (E)-Caryophyllene Germacrene-D Zingiberene | 1 a | 8 | [62] | ★★ | Flavonoids | [63] | ★★ | ||
| Carvacrol Caryophyllene α-Bergamotene | 8 a | 32 | [64] | ★★ | Flavonoids | [65] | ★ | ||
| Carvacrol Caryophyllene β-Cymene γ-Terpinene | 1 a | 27 | [66] | ★★ | - | - | - | ||
| Carvacrol β-Caryophyllene γ-Terpinene | 1 a | 43 | [67] | ★★★ | |||||
| Carvacrol | 1 a | 13 | [68] | ★★ | |||||
| C. barbatus * | Caryophyllene β-Phellandrene β-Linalool | 1 a,b | 137 | [69] | ★★★ | Diterpenoids | [58] | # | |
| Diterpenoids | [70] | # | |||||||
| Phenolics | [71] | ★★ | |||||||
| Diterpenoids | [72] | ★★ | |||||||
| Diterpenoids | [73] | ★★ | |||||||
| Diterpenoids | [74] | ★★ | |||||||
| Diterpenoids | [75] | ★★ | |||||||
| Diterpenoids | [76] | ★★ | |||||||
| Diterpenoids | [77] | ★★ | |||||||
| Coleus (Cont.) | C. caninus | β-Caryophyllene β-Pinene Terpinyl acetate | 2 a,b | 131 | [69] | ★★★ | Phenylpropanoids Terpenoids | [78] | ★★★ |
| Caffeic acid esters | [79] | ★★ | |||||||
| Caffeic acid esters | [42] | ★★ | |||||||
| C. comosus * | α-Pinene Sabinene β-Pinene | 3 b,d | 77 | [80] | ★★★ | Diterpenoids | [81] | ★★★ | |
| α-Thujene β-Caryophyllene | 11 b | 33 | [82] | ★★ | Carboxylic acid | [83] | ★★★ | ||
| - | - | - | - | - | Diterpenoids | [84] | ★★★ | ||
| Phenolic acids | [85] | ★★ | |||||||
| Diterpenoids | [86] | ★★★ | |||||||
| Diterpenoids | [87] | ★★★ | |||||||
| C. cylindraceus | α-Thujene β-Maaliene | 1 a | 23 | [88] | ★★ | Triterpene ester Steroids | [89] | ★★ | |
| Flavonoids | [90] | ★★ | |||||||
| C. grandidentatus | Camphor τ-Cadinol | 1 a | 62 | [91] | ★★★ | Diterpenoids | [32] | ★★ | |
| Diterpenoids | [92] | ★★★ | |||||||
| Diterpenoids | [87] | ★★★ | |||||||
| C. hadiensis | Methyl eugenol | 1 a | 22 | [93] | ★★ | Terpenoids | [94] | ★★★ | |
| Pipertone oxide | 1 a | 25 | [95] | ★★ | Diterpenoids | [96] | ★★ | ||
| - | - | - | - | - | Diterpenoids | [97] | ★★ | ||
| Coumarins Flavonoids Tannins Terpenes Sterols | [93] | ★ | |||||||
| Flavonoids | [90] | ★★ | |||||||
| Coleus (Cont.) | C. hereroensis | - | - | - | - | - | Diterpenoids | [98] | ★★ |
| Diterpenoids | [99] | ★★ | |||||||
| Aristolane sesquiterpene aldehyde | [100] | ★★ | |||||||
| C. madagascariensis | α-Fenchyl acetate β-Caryophyllene | 3 d | 23 | [101] | ★★ | Diterpenoids Phenolic acid | [102] | ★★ | |
| Diterpenoids Phenolics | [39] | ★★★ | |||||||
| Diterpenoids Phenolic acid | [103] | ★★ | |||||||
| C. neochilus | Caryophyllene oxide β-Caryophyllene α-Pinene | 1 a | 31 | [104] | ★★ | Flavonoid glycosides Polyphenols | [39] | ★★★ | |
| Aromadendrene Selina-3, 7(11)-diene | 1 a | 80 | [105] | ★★ | Phenolic acids | [106] | # | ||
| β-Caryophyllene α-Pinene α-Thujene | 1 a | 17 | [107] | ★★ | - | - | - | ||
| C. porcatus | - | - | - | - | - | Diterpenoids Phenolic acid | [108] | ★★ | |
| Diterpenoids | [109] | ★★★ | |||||||
| C. rotundifolius | - | - | - | - | - | Alcohols Aldehydes Alkanes Alkyne Amines Aromatics Carboxylic acid Chloro compounds | [110] | ★★★ | |
| Coleus (Cont.) | C. rotundifolius (Cont.) | - | - | - | - | - | Isocynate Isocyanides Ketones Phenols Primary alcohols Tertiary alcohols | [110] | ★★★ |
| C. venteri | - | - | - | - | - | Acetophenones | [111] | ★★ | |
| Endostemon | E. obtusifolius | - | - | - | - | - | Caffeic acid esters | [42] | ★★ |
| Equilabium | E. petiolare | - | - | - | - | - | Flavonoids | [90] | ★★ |
| Hoslundia | H. opposita | Eugenol | 5 d | 88 | [112] | ★★★ | Abietane-type esters | [113] | ★★★ |
| Flavonoids | [114] | ★★ | |||||||
| Camphor 1,8-Cineole | 1 a | 37 | [115] | ★★ | Flavonoids | [116] | ★★ | ||
| Flavonoids | [117] | ★★ | |||||||
| Lamium * | L. amplexicaule * | - | 1 a | 33 | [118] | ★★ | Iridoid glucosides | [119] | # |
| Camphor Germacrene-D | 1 a | 48 | [120] | ★★ | Iridoid glucosides | [121] | ★ ★ ★ | ||
| - | - | - | - | - | Iridoid glucosides | [122] | ★ ★ ★ | ||
| Terpenoids | [123] | ★ ★ | |||||||
| Iridoid glucosides | [124] | ★ ★ | |||||||
| Terpenoids | [125] | ★ ★ ★ | |||||||
| L. galeobdolon * | - | 1 a | 21 | [126] | ★★ | Benzoxazinoids Benzoxazinones | [122] | ★★★ | |
| Iridoid glucosides | [40] | ★★ | |||||||
| Leonotis | L. leonurus | Bourbonene cis-β-Ocimene Germacrene-D Limonene α-Humulene β-Caryophyllene | 3 d | 25 | [127] | ★★ | Diterpenes | [128] | ★★ |
| Germacrene-D Limonene β-Caryophyllene | 1 a | 30 | [129] | ★★ | Diterpenes | [130] | ★★ | ||
| β-Caryophyllene Germacrene-D | 1 a | 56 | [131] | ★★★ | Diterpenes | [132] | ★★★ | ||
| α-Pinene β-Caryophyllene | 1 a | 33 | [133] | ★★ | Alkaloids Dicarboxylic acid Diterpene esters Flavonoids Iridoid glycoside | [10] | # | ||
| Caryophyllene Germacrene-D | 1 a | 21 | [134] | ★★ | Diterpene ester | [135] | ★★★ | ||
| Unknown compounds | [136] | ★ | |||||||
| Flavonoids | [137] | ★★★ | |||||||
| Labdane diterpenoids | [138] | ★ | |||||||
| Diterpenoid | [139] | ★★★ | |||||||
| Flavonoids Labdane diterpenoids Phenolics | [140] | # | |||||||
| Labdane diterpenoids | [141] | ★★★ | |||||||
| Labdane diterpenoids | [142] | ★ | |||||||
| Diterpenoids | [143] | ★★★ | |||||||
| Labdane diterpenoid | [29] | ★ | |||||||
| Leonotis (Cont.) | L. leonurus (Cont.) | - | - | - | - | - | Alkaloids Flavonoids Phenolics | [144] | ★★★ |
| L. nepetifolia | E-Ocimene | 2 c | 31 | [145] | ★★ | Diterpenoids | [146] | ★★★ | |
| β-Caryophyllene Germacrene-D δ-Selinene | 1 a | 43 | [147] | ★★ | Carotenoids Flavonoids Phenolics | [148] | ★★ | ||
| Germacrene-D | - | 3 | [134] | ★★ | Diterpenoids | [149] | ★★ | ||
| L. ocymifolia | Caryophyllene oxide | 1 a | 68 | [150] | ★★★ | Diterpenoids | [151] | ★★ | |
| Caryophyllene Germacrene | 1 a | 21 | [134] | ★★ | |||||
| (Z)-β-Ocimene β-Caryophyllene Germacrene-D | 1 a | 26 | [129] | ★★ | |||||
| L. ocymifolia var. ocymifolia | Caryophyllene Germacrene-D | 1 a | 10 | [134] | ★★ | - | - | ||
| L. ocymifolia var. raineriana | Germacrene-D | 1 a | 27 | [152] | ★★ | Diterpenoids | [153] | ★★ | |
| L. ocymifolia var. schinzii | Caryophyllene Germacrene-D | 1 a | 16 | [134] | ★★ | - | - | ||
| Leucas | L. capensis | - | - | - | - | - | Diterpenoids | [154] | ★★★ |
| L. glabrata | Isomenthone Piperitone Pulegone | 1 a | 37 | [155] | ★★ | - | - | - | |
| L. martinicensis | Germacrene-D | 1 a | 39 | [147] | ★★ | Alkaloids Flavonoids Glycosides Saponins | [156] | ★ | |
| Marrubium * | M. vulgare * | γ-Eudesmol | 1 a | 34 | [157] | ★★ | Monoterpene acid | [158] | ★★★ |
| γ-Eudesmol | 1 b | 34 | [159] | ★★ | Flavonoids | [160] | ★★★ | ||
| Marrubium * (Cont.) | M. vulgare * (Cont.) | - | - | - | - | - | Phenylethanoid Terpenoids | [160] | ★★★ |
| β-Caryophyllene Germacrene-D | 1 a | 12 | [161] | ★★ | Diterpenoids | [162] | ★★★ | ||
| β-Caryophyllene β-Bisabolene | 1 a | 33 | [163] | ★★ | Coumarins Flavonoids Phenolic acids Phenylpropanoid acids Phenylpropanoid esters Phenylpropanoid glycosides Terpenoids | [164] | # | ||
| Mentha | M. aquatica | Linalool Linalyl acetate α-Pinene | 1 a | 42 | [165] | ★★ | Phenolics | [166] | ★★ |
| L-Menthone Pulegone | 1 a | 18 | [26] | ★★ | Phenolics | [167] | ★★ | ||
| 1,8-Cineole Piperitenone β-Caryophyllene | 1 a | 29 | [27] | ★★ | Phenolics | [168] | ★★ | ||
| 1,8-Cineole | 1 a | 31 | [169] | ★★ | Diterpenes | [170] | ★★ | ||
| Limonene β-Caryophyllene Germacrene-D | 1 b | 34 | [171] | ★★ | Flavonoids | [172] | ★★ | ||
| 1,8-Cineole Methanofuran β-Caryophyllene | 1 a | 29 | [173] | ★★ | Flavonoids | [174] | ★★ | ||
| - | - | - | - | - | Flavonoid glycones | [175] | ★★★ | ||
| Monoterpene ketones | [176] | ★★★ | |||||||
| M. longifolia | Isomenthone Pulegone | 1 a | 36 | [177] | ★★ | Flavonoids | [178] | ★★★ | |
| Mentha (Cont.) | M. longifolia (Cont.) | Carvone Limonene | 1 a | 23 | [179] | ★★ | Flavonoids | [180] | ★★★ |
| 1,8-Cineole Menthone Pulegone | 4 d | 34 | [181] | ★★ | Flavonoids | [182] | ★★★ | ||
| 1,8-Cineole Isomenthone Pulegone | 1 a | 30 | [131] | ★★ | - | - | - | ||
| M. longifolia subsp. capensis | Menthone Pulegone | 2 a | 21 | [183] | ★★ | - | - | - | |
| Menthone Pulegone | 1 b | 31 | [184] | ★★ | |||||
| M. longifolia subsp. polyadenia | Methanofuran cis-Piperitone oxide Piperitone oxide | 8 d | 59 | [185] | ★★★ | - | - | - | |
| Methanofuran cis-Piperitone oxide Piperitone oxide | 8 d | 52 | [186] | ★★★ | |||||
| M. pulegium * | Piperitone Piperitenone | 1 a | 16 | [187] | ★★ | Phenolics | [188] | ★★★ | |
| Menthone Pulegone | 1 a | 53 | [189] | ★★★ | Phenolics | [190] | ★★★ | ||
| Micromeria | M. biflora | Geranial Neral | 2 c | 55 | [191] | ★★★ | Caffeic acid | [192] | ★★★ |
| Germacrene-D Linalool | 1 a | 40 | [193] | ★★ | - | - | - | ||
| Ocimum | O. x africanum | - | 1 a | 19 | [194] | ★★★ | - | - | - |
| Ocimum (Cont.) | O. americanum | - | 1 a | 27 | [194] | ★★ | Neolignan | [195] | ★★★ |
| 1,8-Cineole Camphor | 3 d | 32 | [196] | ★★ | Sesquiterpene alcohols | [197] | ★★★ | ||
| 1,8-Cineole Terpinen-4-ol | 2 d | 36 | [198] | ★★ | Flavones | [199] | ★★★ | ||
| Eugenol Methyl carvacrol Terpineol | 4 b,c | 17 | [200] | ★★ | - | - | - | ||
| cis-β-Ocimene Estragol β-Bisabolene | 1 a | 22 | [201] | ★★ | |||||
| Camphor | 1 c | 51 | [202] | ★★★ | |||||
| Carvone Elemol α-Humulene | 1 a | 41 | [131] | ★★ | |||||
| 1,8-Cineole (Z)-Methyl cinnamate | 1 a | 14 | [203] | ★★ | |||||
| O. gratissimum | Eugenol Germacrene-D | 1 c | 35 | [202] | ★★ | Caffeic acid esters | [42] | ★ | |
| O. labiatum | - | - | - | - | - | Diterpenoids | [204] | ★★ | |
| Terpenoids | [205] | ★★★ | |||||||
| O. obovatum | - | 1 a | 43 | [206] | ★★ | Terpenoid saponins | [207] | ★★★ | |
| Orthosiphon | O. thymiflorus | 2-isopropyl-5-methyl-9-methylene-bicyclo-1-decene(4.4.0) | 1 a | 33 | [208] | ★★ | - | - | - |
| Platostoma | P. rotundifolium | Germacrene-D β-Caryophyllene β-Gurjunene | 1 d | 24 | [209] | ★★ | Terpenoids | [210] | # |
| Spathulenol | 1 a | 59 | [211] | ★★★ | |||||
| Plectranthus | P. ambiguus | - | - | - | - | - | Terpenoids | [58] | # |
| Caffeic acid esters | [42] | ★ | |||||||
| Flavonoids | [90] | ★★★ | |||||||
| Phyllocladanes | [212] | ★★★ | |||||||
| P. cilliatus | Bicyclogermacrene Spathulenol | 2 d | 61 | [91] | ★★★ | Terpenoids | [58] | # | |
| Spathulenol Bicyclogermacrene δ-Cadinine | 2 d | 106 | [213] | ★★★ | |||||
| P. ecklonii | - | - | - | - | - | Terpenoids | [58] | # | |
| Flavonoids | [90] | ★★★ | |||||||
| P. ernestii | - | - | - | - | - | Terpenoids | [212] | # | |
| Terpenoids | [214] | ★★★ | |||||||
| P. fruticosus | Sabinyl acetate | 1 c | 52 | [215] | ★★★ | Terpenoids | [216] | ★★★ | |
| Terpenoids | [210] | # | |||||||
| P. lucidus | - | - | - | - | - | Terpenoids | [96] | ★★★ | |
| Terpenoids | [97] | ★★ | |||||||
| P. praetermissus | - | - | - | - | - | Terpenoids | [96] | ★★★ | |
| P. purpuratus | - | - | - | - | - | Diterpenoid quinomethans Vinylogous quinones Phyllocladene derivative | [217] | ★★★ | |
| P. purpuratus subsp. purpuratus | - | - | - | - | - | Terpenoids | [97] | ★★ | |
| Plectranthus (Cont.) | P. saccatus | - | - | - | - | - | Terpenoids | [218] | ★★ |
| Terpenoids | [219] | ★★★ | |||||||
| P. strigosus | - | - | - | - | - | Terpenoids | [58] | # | |
| Terpenoids | [210] | # | |||||||
| Terpenoids | [220] | # | |||||||
| P. verticillatus | - | - | - | - | - | Terpenoids | [221] | ★★ | |
| Terpenoids | [222] | ★★★ | |||||||
| P. zuluensis | - | - | - | - | - | Terpenoids | [221] | ★★ | |
| Terpenoids | [96] | ★★★ | |||||||
| Terpenoids | [213] | ★★★ | |||||||
| Terpenoids | [219] | ★★★ | |||||||
| Prunella * | P. vulgaris * | Germacrene-D | 1 a | 28 | [223] | ★★ | Pentacyclic triterpenoid Flavonoids Flavonoid glycosides Phytosterols | [224] | ★★ |
| Aromadendrene | 1 c | 28 | [225] | ★★ | Terpenoids | [226] | ★★★ | ||
| - | - | - | - | - | Terpenoids | [227] | ★★★ | ||
| Terpenoids | [228] | ★★★ | |||||||
| - | - | - | - | - | Triterpenoids Saponins Sterols Flavonoids Coumarins Phenylpropanoids | [229] | # | ||
| Pseudodictamnus | P. africanus | - | - | - | - | - | Terpenes | [230] | ★★★ |
| Terpenes Phenolics | [231] | ★★★ | |||||||
| Terpenes | [232] | ★★★ | |||||||
| Rotheca | R. myricoides | - | - | - | - | - | Cyclohexapeptide | [233] | ★★★ |
| R. wildii | - | - | - | - | - | Saponins | [234] | # | |
| Triterpenoid Saponins | [235] | # | |||||||
| Salvia | S. africana | p-Cymene α-Eudesmol γ-Terpinene | 1 b | 53 | [236] | ★★★ | Diterpenoids | [237] | ★★ |
| Caryophyllene oxide β-Caryophyllene | 1 a | 56 | [238] | ★★★ | Terpenoids | [204] | ★★ | ||
| Caryophyllene oxide Spathulenol | 1 a | 45 | [239] | ★★ | Phenolics | [240] | ★★★ | ||
| Limonene Viridiflorol | 12 d | 46 | [241] | ★★ | |||||
| S. albicaulis | Viridiflorol | 1 a | 38 | [242] | ★★ | Phenolics | [240] | ★★★ | |
| S. aurea | Caryophyllene oxide α-Eudesmol β-Eudesmol | 1 a | 48 | [238] | ★★ | Terpenoids | [243] | ★★ | |
| Myrcene | 1 a | 43 | [239] | ★★ | Phenolics | [244] | ★★ | ||
| Terpinene-4-ol + β-Caryophyllene α-Eudesmol β-Eudesmol | 6 d | 20 | [245] | ★★ | Phenolics | [240] | ★★★ | ||
| Limonene α-Humulene β-Caryophyllene + Terpinen-4-ol τ-Cadinol | 1 a | 43 | [131] | ★★ | - | - | - | ||
| S. aurita | - | - | - | - | - | Phenolics | [240] | ★★★ | |
| Salvia (Cont.) | S. chamelaeagnea | 1,8-Cineole α-Pinene | 1 a | 43 | [239] | ★★ | Terpenoids | [210] | # |
| Viridiflorol | 5 d | 18 | [245] | ★★ | Phenolics | [240] | ★★★ | ||
| Terpenoids | [246] | ★★★ | |||||||
| S. coccinea | 2,5-Dimethoxy-p-cymene Acenaphthene Aromadendrene Globulol | 3 c | 21 | [247] | ★★★ | Phenolics | [248] | ★★ | |
| S. dentata | - | - | - | - | - | Phenolic diterpene | [249] | ★ | |
| S. disermas | Linalyl acetate Shyobunone | 3 d | 28 | [238] | ★★ | Phenolic diterpene | [249] | ★ | |
| Linalool Linalyl acetate | 1 a | 42 | [131] | ★★ | Phenolics | [240] | ★★★ | ||
| S. dolomitica | 1,8-Cineole Borneol β-Caryophyllene | 3 b,d | 110 | [250] | ★★★ | Phenolics | [240] | ★★★ | |
| β-Caryophyllene Limonene Germacrene-D | 3 b | 37 | [251] | ★★ | |||||
| 1,8-Cineole β-Caryophyllene | 1 a,b | 46 | [252] | ★★ | |||||
| 1,8-Cineole β-Caryophyllene | 12 d | 66 | [238] | ★★★ | |||||
| Linalool | 1 a | 34 | [242] | ★★ | - | - | - | ||
| S. garipensis | - | - | - | - | - | Phenolics | [240] | ★★★ | |
| S. lanceolata | Sabinene Spathulenol | 1 a | 41 | [238] | ★★ | Phenolic diterpene | [249] | ★ | |
| Salvia (Cont.) | S. lanceolata (Cont.) | Caryophyllene oxide Spathulenol | 1 a | 43 | [239] | ★★ | Phenolics | [240] | ★★★ |
| β-Caryophyllene | 12 d | 48 | [241] | ★★ | - | - | - | ||
| Bicyclogermacrene Terpinene-4-ol + β-Caryophyllene | 5 d | 15 | [245] | ★★ | |||||
| S. muirii | 1,8-Cineole Limonene α-Pinene | 1 a | 39 | [253] | ★★ | Phenolics | [240] | ★★★ | |
| S. namaensis | Camphene Camphor | 1 a | 55 | [238] | ★★★ | Phenolic diterpene | [249] | ★ | |
| 1,8-Cineole Camphene Camphor α-Pinene | 1 a | 20 | [254] | ★★ | Phenolics | [240] | ★★★ | ||
| Camphor | 1 a | 64 | [131] | ★★★ | |||||
| S. radula | - | - | - | - | - | Phenolics | [240] | ★★★ | |
| S. repens | Ledol α-Bisabolol β-Phellandrene δ-3-Carene E-Nerolidol | 6 d | 106 | [255] | ★★★ | Phenolic diterpene | [249] | ★ | |
| β-Phellandrene β-Caryophyllene | 1 a | 55 | [256] | ★★★ | Terpenoids | [257] | ★★★ | ||
| Ledol α-Bisabolol β-Caryophyllene β-Phellandrene E-Nerolidol | 4 d | 90 | [258] | ★★★ | Terpenoids | [259] | ★★★ | ||
| Phenolics | [240] | ★★★ | |||||||
| Salvia (Cont.) | S. runcinata | Caryophyllene Ledol β-Bisabolone | 2 b | 26 | [260] | ★★ | Phenolic diterpene | [249] | ★ |
| α-Bisabolol | 1 a | 24 | [238] | ★★ | Phenolics | [240] | ★★★ | ||
| α-Pinene β-Caryophyllene E-Nerolidol | 15 d | 157 | [255] | ★★★ | |||||
| α-Bisabolol β-Caryophyllene | 1 a | 73 | [256] | ★★★ | |||||
| Limonene Nerolidol α-Bisabolol β-Caryophyllene β-Eudesmol δ-3-Carene | 20 d | 11 (x > 5%) | [261] | ★★ | |||||
| Viridiflorol β-Caryophyllene | 1 a | 44 | [131] | ★★ | |||||
| α-Bisabolol β-Caryophyllene E-Nerolidol | 12 d | 118 | [258] | ★★★ | |||||
| S. scabra | - | - | - | - | - | Phenolic diterpene | [249] | ★ | |
| S. schlechteri | - | - | - | - | - | Phenolics | [240] | ★★★ | |
| S. stenophylla | Myrcene α-Bisabolol | 1 a | 34 | [238] | ★★ | Phenolic diterpene | [249] | ★ | |
| α-Bisabolol δ-3-Carene | 10 d | 128 | [255] | ★★★ | Flavonoids | [262] | ★★ | ||
| (+)-α-Bisabolol α-Phellandrene | 1 a | 31 | [263] | ★★ | Phenolics | [240] | ★★★ | ||
| δ-3-Carene Manool | 1 a | 59 | [256] | ★★★ | |||||
| Salvia (Cont.) | S. stenophylla (Cont.) | δ-3-Carene Mycrene Limonene β-Phellandrene γ-Terpinene p-Cymene Nerolidiol α-Bisabolol | 27 d | 12 (x > 5%) | [261] | ★★ | - | - | - |
| δ-3-Carene p-Cymene (E)-nerolidiol α-Bisabolol | 12 d | 128 | [258] | ★★★ | |||||
| S. verbenaca * | - | 1 a | 76 | [264] | ★★★ | Terpenoids | [210] | # | |
| - | 2 a,b | 63 | [265] | ★★★ | Phenolics | [266] | ★★ | ||
| - | 1 a | 18 | [267] | ★★ | - | - | - | ||
| Satureja * | S. thymbra * | p-Cymene γ-Terpinene Thymol Carvacrol | 7 c | 40 | [268] | ★★ | Phenolics | [269] | ★★ |
| Stachys | S. aethiopica | - | - | - | - | - | Saponins Tannins | [270] | ★★ |
| Syncolostemon | S. argenteus | - | - | - | - | - | Pyrones | [271] | ★★★ |
| S. bracteosus | - | - | - | - | - | Alcohols Acids Esters Lactones Phenolics Sesquiterpenes | [272] | ★★ | |
| Syncolostemon (Cont.) | S. densiflorus | - | - | - | - | - | Pyrones | [37] | ★★★ |
| Pyrones | [273] | ★★★ | |||||||
| S. modestus | p-Cymene Spathulenol Viridiflorol γ-Terpinene | 1 a | 10 | [131] | ★★ | - | - | - | |
| S. parviflorus | - | - | - | - | - | Pyrones | [231] | ★★★ | |
| S. petiolatus | (+)-Linalool (E)-β-farnesene (+)-Bicyclogermacrene (−)-Germacrene D | 1 a | N/A | [274] | ★★ | - | - | - | |
| S. pretoriae | β-Caryophyllene | 1 a | 6 | [131] | ★★ | - | - | - | |
| S. rotundifolius | - | - | - | - | - | Pyrones | [231] | ★★★ | |
| Tetradenia | T. barberae | - | - | - | - | - | Pyrones | [275] | ★★★ |
| T. riparia | 14-Hydroxy-9-epi-(E)-Caryophyllene 6,7-Dehydroroyleanone α-Cadinol | 2 c | 51 | [276] | ★★★ | Pyrones | [35] | ★★★ | |
| Fenchone δ-Cadinene | 1 a | 49 | [277] | ★★ | Pyrones | [278] | ★★★ | ||
| 14-Hydroxy-9-epi-β-Caryophyllene Fenchone | 1 a | 64 | [278] | ★★★ | Pyrones | [279] | ★★★ | ||
| Fenchone | 2 b | 27 | [280] | ★★ | Pyrones | [281] | ★★★ | ||
| - | - | - | - | - | Pyrones | [282] | ★★★ | ||
| Terpenoids | [283] | ★★★ | |||||||
| Tetradenia (Cont.) | T. riparia (Cont.) | - | - | - | - | - | Terpenoids | [284] | ★★★ |
| Terpenoids | [285] | ★★★ | |||||||
| Terpenoids | [286] | ★★★ | |||||||
| Terpenoids | [287] | ★★★ | |||||||
| Terpenoids | [288] | ★★★ | |||||||
| Teucrium | T. africanum | α-Cubebene β-Cubebene | 3 d | 29 | [289] | ★★ | Terpenoids | [33] | ★★★ |
| Terpenoids | [210] | # | |||||||
| T. sessiliflorum | α-Cubebene β-Cubebene | 3 d | 29 | [289] | ★★ | Terpenoids | [290] | ★★★ | |
| Vitex | V. ferruginea | Germacrene-D | 2 d | 53 | [291] | - | - | - | - |
| V. obovata | 1,8-Cineole α-Copaene | 1 a | 92 | [292] | ★★★ | Terpenoids | [210] | # | |
| V. pooara | Limonene Cryptone β-Selinene | 1 a | 61 | [292] | ★★★ | Terpenoids | [210] | # | |
| V. rehmannii | Caryophyllene oxide Spathulenol | 1 a | 60 | [292] | ★★★ | Terpenoids | [210] | # | |
| V. trifolia * | α-Pinene 1,8-Cineole Terpinyl acetate | 1 a | 30 | [293] | ★★ | Flavones | [294] | ★★ | |
| Terpenoids | [295] | ★★★ | |||||||
| V. zeyheri | 1,8-Cineole | 1 a | 58 | [292] | ★★★ | Terpenoids | [210] | # | |
| Volkameria | V. glabra | - | - | - | - | - | Terpenoids | [210] | # |
| Terpenoids | [296] | ★★★ | |||||||
| Terpenoids | [297] | ★★ | |||||||
| Terpenoids | [298] | ★★ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rattray, R.D.; Van Wyk, B.-E. The Botanical, Chemical and Ethnobotanical Diversity of Southern African Lamiaceae. Molecules 2021, 26, 3712. https://doi.org/10.3390/molecules26123712
Rattray RD, Van Wyk B-E. The Botanical, Chemical and Ethnobotanical Diversity of Southern African Lamiaceae. Molecules. 2021; 26(12):3712. https://doi.org/10.3390/molecules26123712
Chicago/Turabian StyleRattray, Ryan D., and Ben-Erik Van Wyk. 2021. "The Botanical, Chemical and Ethnobotanical Diversity of Southern African Lamiaceae" Molecules 26, no. 12: 3712. https://doi.org/10.3390/molecules26123712
APA StyleRattray, R. D., & Van Wyk, B.-E. (2021). The Botanical, Chemical and Ethnobotanical Diversity of Southern African Lamiaceae. Molecules, 26(12), 3712. https://doi.org/10.3390/molecules26123712






