A Regioselective Synthesis of Novel Functionalized Organochalcogen Compounds by Chalcogenocyclofunctionalization Reactions Based on Chalcogen Halides and Natural Products
Abstract
:1. Introduction
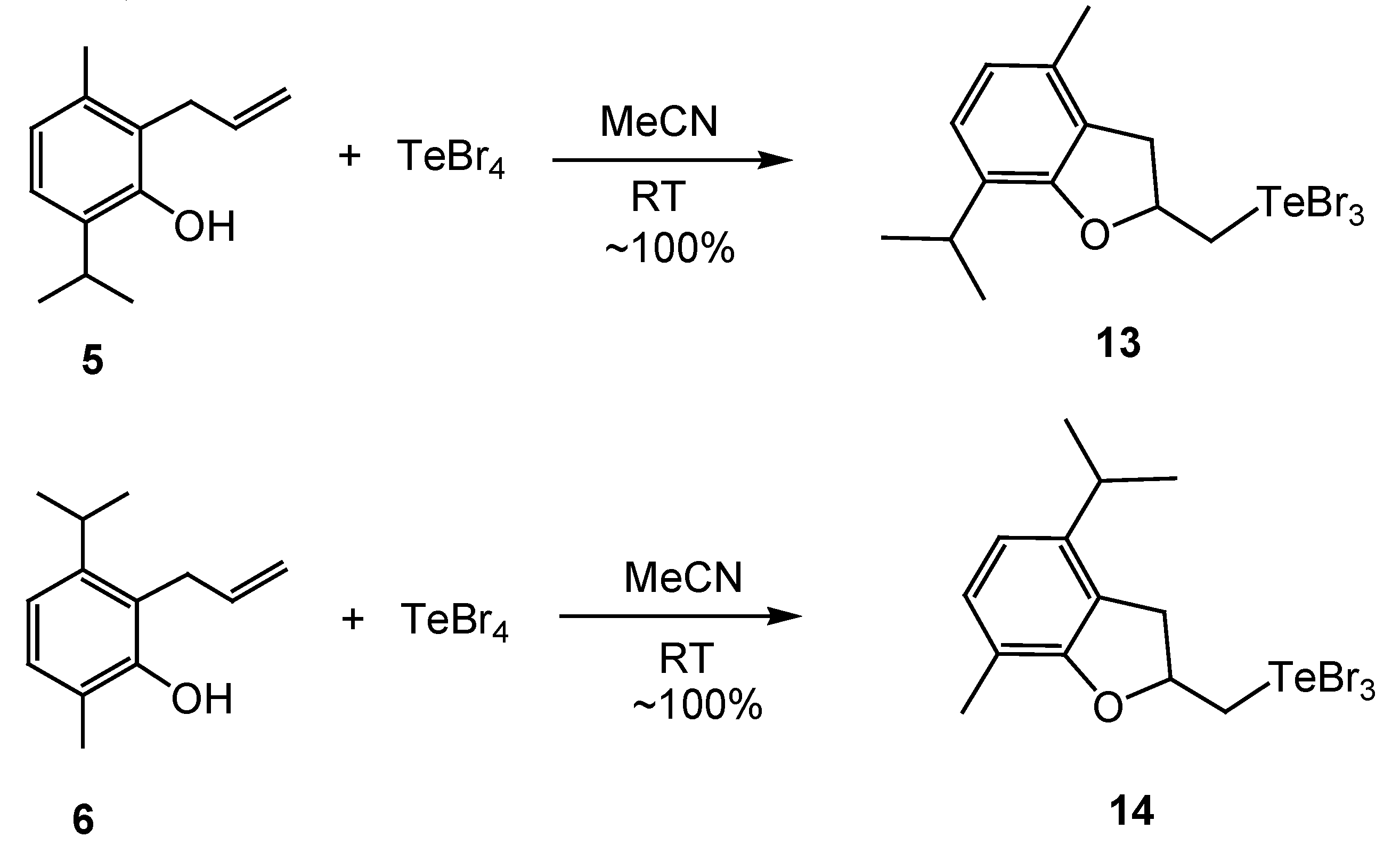
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Synthesis of Starting Compounds 5 and 6
3.3. Synthesis of Products 7–16
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [Green Version]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, B.K.; Lavoie, S.; Sweeney-Jones, A.M.; Kubanek, J. Recent trends in the structural revision of natural products. Nat. Prod. Rep. 2018, 35, 514–531. [Google Scholar] [CrossRef]
- Beghyn, T.; Deprez-Poulain, R.; Willand, N.; Folleas, B.; Deprez, B. Natural compounds: Leads or ideas? Bioinspired molecules for drug discovery. Chem. Biol. Drug Design 2008, 72, 3–15. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, J. Ancient Herbs in the J. Paul Getty Museum Gardens; Getty Publications: Malibu, CA, USA, 1982; p. 84. [Google Scholar]
- Tilford, G.L. Edible and Medicinal Plants of the West; Mountain Press Publishing: Missoula, MT, USA, 1997; p. 240. [Google Scholar]
- Nieto, G. Biological Activities of Three Essential Oils of the Lamiaceae Family. Medicines 2017, 4, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and Antifungal Activities of Thymol: A Brief Review of the Literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Krepker, M.; Shemesh, R.; Danin Poleg, Y.; Kashi, Y.; Vaxman, A.; Segal, E. Active Food Packaging Films with Synergistic Antimicrobial Activity. Food Control 2017, 76, 117–126. [Google Scholar] [CrossRef]
- Mezzoug, N.; Elhadri, A.; Dallouh, A.; Amkiss, S.; Skali, N.S.; Abrini, J.; Zhiri, A.; Baudoux, D.; Diallo, B.; El Jaziri, M.; et al. Investigation of the Mutagenic and Antimutagenic Effects of Origanum Compactum Essential Oil and Some of Its Constituents. Mutat. Res. Toxicol. Environ. Mutagen. 2007, 629, 100–110. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, Y.; Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Quek, S.Y.; Yao, W. Antifungal Effects of Thymol and Salicylic Acid on Cell Membrane and Mitochondria of Rhizopus Stolonifer and Their Application in Postharvest Preservation of Tomatoes. Food Chem. 2019, 285, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Morais, S.M.; Vila-Nova, N.S.; Bevilaqua, C.M.L.; Rondon, F.C.; Lobo, C.H.; Moura, A.D.A.A.N.; Sales, A.D.; Rodrigues, A.P.R.; de Figuereido, J.R.; Campello, C.C.; et al. Thymol and Eugenol Derivatives as Potential Antileishmanial Agents. Bioorg. Med. Chem. 2014, 22, 6250–6255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llana-Ruiz-Cabello, M.; Pichardo, S.; Maisanaba, S.; Puerto, M.; Prieto, A.I.; Gutiérrez-Praena, D.; Jos, A.; Cameán, A.M. In Vitro Toxicological Evaluation of Essential Oils and Their Main Compounds Used in Active Food Packaging: A Review. Food Chem. Toxicol. 2015, 81, 9–27. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Moreno, F.J.; Bermúdez, J.M.; Aucejo, S.; Cameán, A.M. Cytotoxicity and Morphological Effects Induced by Carvacrol and Thymol on the Human Cell Line Caco-2. Food Chem. Toxicol. 2014, 64, 281–290. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Maisanaba, S.; Puerto, M.; Prieto, A.I.; Pichardo, S.; Jos, Á.; Cameán, A.M. Evaluation of the Mutagenicity and Genotoxic Potential of Carvacrol and Thymol Using the Ames Salmonella Test and Alkaline, Endo III- and FPG-Modified Comet Assays with the Human Cell Line Caco-2. Food Chem. Toxicol. 2014, 72, 122–128. [Google Scholar] [CrossRef]
- Dheer, D.; Singh, D.; Kumar, G.; Karnatak, M.; Chandra, S.; Verma, V.P.; Shankar, R. Thymol Chemistry: A Medicinal Toolbox. Curr. Bioact. Compd. 2019, 15, 454–474. [Google Scholar] [CrossRef]
- Rajput, J.D.; Bagul, S.D.; Bendre, R.S. Design, synthesis, biological screenings and docking simulations of novel carvacrol and thymol derivatives containing acetohydrazone linkage. Res. Chem. Intermed. 2017, 43, 4893–4906. [Google Scholar] [CrossRef]
- Narkhede, H.P.; More, U.B.; Dalal, D.S.; Mahulikar, P.P. Solid-Supported Synthesis of Bio-active Carvacrol Compounds Using Microwaves. Synth. Commun. 2008, 38, 2413–2418. [Google Scholar] [CrossRef]
- Bkhaitan, M.M.; Alarjah, M.; Mirza, A.Z.; Abdalla, A.N.; El-Said, H.M.; Faidah, H.S. Preparation and biological evaluation of metronidazole derivatives with monoterpenes and eugenol. Chem. Biol. Drug Design. 2018, 92, 1954–1962. [Google Scholar] [CrossRef]
- Nostro, A.; Blanco, A.R.; Cannatelli, M.A.; Enea, V.; Flamini, G.; Morelli, I.; Sudano Roccaro, A.; Alonzo, V. Susceptibility of methicillin-resistant staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiol. Lett. 2004, 230, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Li, Y.; Liu, Q.; Gao, K. Antimicrobial activities of some thymol derivatives from the roots of Inula hupehensis. Food Chem. 2010, 120, 512–516. [Google Scholar] [CrossRef]
- El-Miligy, M.M.M.; Hazzaa, A.A.; El-Zemity, S.R.; Al-Kubeisi, A.K. Synthesis of Thymol Derivatives as Potential Non-Irritant Antimicrobial and Insecticidal Agents. Curr. Bioactive Comp. 2019, 15, 125–137. [Google Scholar] [CrossRef]
- Mastelic, J.; Jerkovic, I.; Blazevic, I.; Poljak-Blazi, M.; Borovic, S.; Ivancic-Bace, I.; Smrecki, V.; Zarkovic, N.; Brcic-Kostic, K.; Vikic-Topic, D. Comparative Study on the Antioxidant and Biological Activities of Carvacrol, Thymol, and Eugenol Derivatives. J. Agricult. Food Chem. 2008, 56, 3989–3996. [Google Scholar] [CrossRef]
- Piombino, C.; Lange, H.; Sabuzi, F.; Galloni, P.; Conte, V.; Crestini, C. Lignosulfonate microcapsules for delivery and controlled release of thymol and derivatives. Molecules 2020, 25, 866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagul, S.D.; Rajput, J.D.; Tadavi, S.K.; Bendre, R.S. Design, synthesis and biological activities of novel 5-isopropyl-2-methylphenolhydrazide-based sulfonamide derivatives. Res. Chem. Intermed. 2017, 43, 2241–2252. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, S.; Pu, T.; Fan, L.; Su, F.; Ye, M. Antifungal activity of phenolic monoterpenes and structure-related compounds against plant pathogenic fungi. Nat. Prod. Res. 2019, 33, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Pricopie, A.-I.; Ionu, I.; Marc, G.; Arseniu, A.-M.; Vlase, L.; Grozav, A.; Gaina, L.I.; Vodnar, D.C.; Pirnau, A.; Tiperciuc, B. Design and synthesis of novel 1,3-thiazole and 2-hydrazinyl-1,3-thiazole derivatives as anti-Candida agents: In vitro antifungal screening, molecular docking study and spectroscopic investigation of their binding interaction with bovine serum albumin. Molecules 2019, 24, 3435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koshti, S.M.; Patil, P.A.; Patil, C.B.; Patil, A.S. Synthesis and characterization of prodrugs of sulfonamides as an azo derivatives of carvacrol. Pharma. Chem. 2018, 10, 1–15. [Google Scholar]
- Sobotta, L.; Lijewski, S.; Dlugaszewska, J.; Nowicka, J.; Mielcarek, J.; Goslinski, T. Photodynamic inactivation of Enterococcus faecalis by conjugates of zinc (II) phthalocyanines with thymol and carvacrol loaded into lipid vesicles. Inorg. Chim. Acta. 2019, 489, 180–190. [Google Scholar] [CrossRef]
- Jeankumar, V.U.; Sridevi, J.P.; Matikonda, S.S.; Peddi, S.; Alvala, M.; Yogeeswari, P.; Sriram, D. Identification and Structure–Activity Relationship Study of Carvacrol Derivatives as Mycobacterium Tuberculosis Chorismate Mutase Inhibitors. J. Enzyme Inhib. Med. Chem. 2014, 29, 547–554. [Google Scholar]
- Talavera-Aleman, A.; Rodriguez-Garcia, G.; Lopez, Y.; Garcia-Gutierrez, H.A.; TorresValencia, J.M.; del Rio, R.E.; Cerda-Garcia-Rojas, C.M.; Joseph-Nathan, P.; Gomez-Hurtado, M.A. Systematic evaluation of thymol derivatives possessing stereogenic or prostereogenic centers. Phytochem. Rev. 2016, 15, 251–277. [Google Scholar] [CrossRef]
- Cherkasov, R.A.; Nizamov, I.S.; Gabdullina, G.T.; Almetkina, L.A.; Shamilov, R.R.; Sofronov, A.V. Dithiophosphoric and Dithiophosphonic Acids and Their Derivatives on the Basis of Thymol: Synthesis and Antimicrobial Activity. Phosphorus Sulfur. Silicon Relat. Elements 2013, 188, 33–35. [Google Scholar] [CrossRef]
- Chen, L.C.; Lee, T.H.; Sung, P.J.; Shu, C.W.; Lim, Y.P.; Cheng, M.J.; Kuo, W.L.; Chen, J.J. New thymol derivatives and cytotoxic constituents from the root of Eupatorium cannabinum ssp. asiaticum. Chem. Biodivers. 2014, 11, 1374–1380. [Google Scholar] [CrossRef]
- Kulabaş, N.; Tatar, E.; Bingöl Özakpınar, Ö.; Özsavci, D.; Pannecouque, C.; De Clercq, E.; Küçükgüzel, İ. Synthesis and antiproliferative evaluation of novel 2-(4H-1,2,4-triazole-3-ylthio)acetamide derivatives as inducers of apoptosis in cancer cells. Eur. J. Med. Chem. 2016, 121, 58–70. [Google Scholar] [CrossRef]
- Rajput, J.D.; Bagul, S.D.; Bendre, R.S. Synthesis, biological activities and molecular docking simulation of hydrazone scaffolds of carvacrol, thymol and eugenol. Res. Chem. Intermed. 2017, 43, 6601–6616. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Therapeutic potential of selenium and tellurium compounds: Opportunities yet unrealized. Dalton Trans. 2012, 41, 6390–6395. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef] [PubMed]
- Ruberte, A.C.; Sanmartin, C.; Aydillo, C.; Sharma, A.K.; Plano, D. Development and Therapeutic Potential of Selenazo Compounds. J. Med. Chem. 2020, 63, 1473–1489. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-C.; Kuhn, H.; Daniliuc, C.-G.; Ivanov, I.; Jones, P.G.; du Mont, W.-W. 5-Selenization of salicylic acid derivatives yielded isoform-specific 5-lipoxygenase inhibitors. Org. Biomol. Chem. 2010, 8, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Potapov, V.A. Organic diselenides, ditellurides, polyselenides and polytellurides. Synthesis and reactions. In Patai’s Chemistry of Functional Groups. Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; John Wiley and Sons: Chichester, UK, 2013; Volume 4, pp. 765–843. [Google Scholar]
- Braga, A.L.; Rafique, J. Synthesis of biologically relevant small molecules containing selenium. Part B. Anti-infective and anticancer compounds. In Patai’s Chemistry of Functional Groups. Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; John Wiley and Sons: Chichester, UK, 2013; Volume 4, pp. 1053–1117. [Google Scholar]
- Azad, G.K.; Tomar, R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014, 41, 4865–4879. [Google Scholar] [CrossRef]
- Woollins, J.D.; Laitinen, R.S. (Eds.) Selenium and Tellurium Chemistry. From Small Molecules to Biomolecules and Materials; Springer: Berlin/Heidelberg, Germany, 2011; p. 334. [Google Scholar]
- Santi, C. (Ed.) Organoselenium Chemistry: Between Synthesis and Biochemistry; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014; p. 563. [Google Scholar]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of Biologically Important Synthetic Organoselenium Compounds. Chem. Rev. 2001, 101, 2125–2180. [Google Scholar] [CrossRef] [PubMed]
- Lenardao, E.J.; Santi, C.; Sancineto, L. New Frontiers in Organoselenium Compounds; Springer International Publishing AG: Cham, Switzerland, 2018; 189p. [Google Scholar]
- Petragnani, N.; Stefani, H.A.; Valduga, C.J. Recent advances in selenocyclofunctionalization reactions. Tetrahedron 2001, 57, 1411–1448. [Google Scholar] [CrossRef]
- Kostić, M.D.; Divac, V.M.; Bugarčić, Z.M. Electrophilic Selenocyclofunctionalization in the Synthesis of Biologically Relevant Molecules. Curr. Org. Chem. 2016, 20, 2606–2619. [Google Scholar] [CrossRef]
- Kostić, M.D.; Divac, V.M.; Bugarčić, Z.M. An introduction to the kinetics of the triethylamine-mediated selenocyclofunctionalization of 4-pentenoic acid. J. Mol. Struct. 2019, 1175, 24–27. [Google Scholar] [CrossRef]
- Nieto, J.; Andres, C.; Perez-Encabo, A. 7-Endo Selenocyclization reactions on chiral 3-prenyl- and 3-cinnamyl-2-hydroxymethylperhydro-1,3-benzoxazine derivatives. A way to enantiopure 1,4-oxazepanes. Org. Biomolec. Chem. 2015, 13, 9118–9126. [Google Scholar] [CrossRef] [Green Version]
- Pedrosa, R.; Andres, C.; Mendiguchia, P.; Nieto, J. Diastereoselective Synthesis of Enantiopure Morpholines by Electrophilic Selenium-Induced 6-exo Cyclizations on Chiral 3-Allyl-2-hydroxymethylperhydro-1,3-benzoxazine Derivatives. J. Org. Chem. 2006, 71, 8854–8863. [Google Scholar] [CrossRef]
- Stefani, H.A.; Costa, I.M.; Silva, D.D.O.; Menezes, P.H.; Rodrigues, A. Selenocyclofunctionalization of β-ketoamides: Synthesis of substituted dihydrofurans. Phosphorus Sulfur. Silicon Relat. Elements 2001, 171–172, 395–406. [Google Scholar] [CrossRef]
- Saikia, I.; Borah, A.J.; Phukan, P. Use of Bromine and Bromo-Organic Compounds in Organic Synthesis. Chem. Rev. 2016, 116, 6837–7042. [Google Scholar] [CrossRef]
- Kloeckner, U.; Finkbeiner, P.; Nachtsheim, B.J. Iodide-Catalyzed Halocyclization/Cycloaddition/Elimination Cascade Reaction. J. Org. Chem. 2013, 78, 2751–2756. [Google Scholar] [CrossRef]
- Parker, P.D.; Lemercier, B.C.; Pierce, J.G. Synthesis of Quaternary-Substituted Thiazolines via Halocyclization of S-Allyl Thioimidate Salts. J. Org. Chem. 2018, 83, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Accurso, A.A.; Cho, S.-H.; Amin, A.; Potapov, V.A.; Amosova, S.V.; Finn, M.G. Thia-, Aza-, and Selena[3.3.1]bicyclononane Dichlorides: Rates vs Internal Nucleophile in Anchimeric Assistance. J. Org. Chem. 2011, 76, 4392–4395. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Belozerova, O.V.; Albanov, A.I.; Yarosh, O.G.; Voronkov, M.G. Synthesis of 3,6-dihalo-4,4-dimethyl-1,4-selenasilafulvenes. Chem. Heterocycl. Compd. 2003, 39, 549–550. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V. New Methods for Preparation of Organoselenium and Organotellurium Compounds from Elemental Chalcogens. Russ. J. Org. Chem. 2003, 39, 1373–1380. [Google Scholar] [CrossRef]
- Abakumov, G.A.; Piskunov, A.V.; Cherkasov, V.K.; Fedushkin, I.L.; Ananikov, V.P.; Eremin, D.B.; Gordeev, E.G.; Beletskaya, I.P.; Averin, A.D.; Bochkarev, M.N.; et al. Organoelement chemistry: Promising growth areas and challenges. Russ. Chem. Rev. 2018, 87, 393–507. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Musalova, M.V.; Amosova, S.V. Regioselective synthesis of bis[(2,3-dihydro-1-benzofuran-2-yl)methyl]selenide. Russ. J. Org. Chem. 2014, 50, 1702–1703. [Google Scholar] [CrossRef]
- Musalov, M.V.; Ishigeev, R.S.; Udalova, S.I.; Musalova, M.V.; Kurkutov, E.O.; Khabibulina, A.G.; Albanov, A.I.; Potapov, V.A.; Amosova, S.V. Synthesis of Fused Compounds on the Basis of Chalcogen Chlorides and 2-Allylphenols. Russ. J. Org. Chem. 2018, 54, 1035–1040. [Google Scholar] [CrossRef]
- Kurkutov, E.O.; Potapov, V.A.; Musalov, M.V.; Amosova, S.V. Bis(1,3-dioxolan-2-ylmethyl)selenide. Russ. J. Gen. Chem. 2017, 87, 357–358. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A. Selenium dihalides: New possibilities for the synthesis of selenium-containing heterocycles. Chem. Heterocycl. Comp. 2017, 53, 150–152. [Google Scholar] [CrossRef]
- Potapov, V.A.; Kurkutov, E.O.; Musalov, M.V.; Amosova, S.V. Effective synthesis of bis(1,4-dioxan-2-ylmethyl)selenide and selenoxide. Russ. J. Org. Chem. 2016, 52, 1715–1716. [Google Scholar] [CrossRef]
- Musalov, M.V.; Ishigeev, R.S.; Potapov, V.A.; Amosova, S.V. Reaction of selenium dihalides with 2-(allylsulfanyl)ethanol. Russ. J. Org. Chem. 2016, 52, 1533–1534. [Google Scholar] [CrossRef]
- Potapov, V.A.; Kurkutov, E.O.; Musalov, M.V.; Amosova, S.V. Efficient method for the synthesis of bis(tetrahydro-2H-pyran-2-ylmethyl) selenide. Russ. Chem. Bull. 2015, 64, 2973–2974. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Kurkutov, E.O.; Musalova, M.V.; Albanov, A.I.; Amosova, S.V. Synthesis of new functionalized organoselenium compounds by heterocyclization of selenium dihalides with pent-4-en-1-ol. Russ. J. Org. Chem. 2016, 52, 339–342. [Google Scholar] [CrossRef]
- Musalov, M.V.; Yakimov, V.A.; Potapov, V.A.; Amosova, S.V.; Borodina, T.N.; Zinchenko, S.V. A novel methodology for the synthesis of condensed selenium heterocycles based on the annulation and annulation–methoxylation reactions of selenium dihalides. New J. Chem. 2019, 43, 18476–18483. [Google Scholar] [CrossRef]
- Musalov, M.V.; Yakimov, V.A.; Potapov, V.A. Synthesis of Functional Dihydro-1,4-benzoxaselenines from Carvacrol Allyl Ether and Selenium Dihalides. Russ. J. Org. Chem. 2020, 56, 2258–2262. [Google Scholar] [CrossRef]
- Coleman, M.T.; LeBlanc, G. Use of Diethoxymethane as a Solvent for Phase Transfer-Catalyzed O-Alkylation of Phenols. Org. Proc. Res. Develop. 2010, 14, 732–736. [Google Scholar] [CrossRef]
- Smit, V.A.; Zefirov, N.S.; Bodrikov, I.V.; Krimer, M.Z. Episulfonium ions: Myth and reality. Acc. Chem. Res. 1979, 12, 282–288. [Google Scholar] [CrossRef]
- Abu-yousef, I.A.; Harpp, D.N. New Sulfenyl Chloride Chemistry: Synthesis, Reactions and Mechanisms toward Carbon-Carbon Double Bonds. Sulfur. Rep. 2003, 24, 255–282. [Google Scholar] [CrossRef]
- Rasteikiene, L.; Greiciute, D.; Lin’kova, M.G.; Knunyants, I.L. The Addition of Sulphenyl Chlorides to Unsaturated Compounds. Russ. Chem. Rev. 1977, 46, 548–564. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Kurkutov, E.O.; Musalova, M.V.; Khabibulina, A.G.; Amosova, S.V. Regioselective syntheses of bis(2-haloalkyl) selenides and dihalo[bis(2-haloalkyl)]-λ4-selanes from selenium dihalides and 1-alkenes, and the methoxyselenenylation reaction. Arkivoc 2017, iii, 365–376. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Amosova, S.V. Reactions of selenium dichloride and dibromide with unsaturated ethers. Annulation of 2,3-dihydro-1,4-oxaselenine to the benzene ring. Tetrahedron Lett. 2011, 52, 4606–4610. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musalov, M.V.; Potapov, V.A.; Yakimov, V.A.; Musalova, M.V.; Maylyan, A.A.; Zinchenko, S.V.; Amosova, S.V. A Regioselective Synthesis of Novel Functionalized Organochalcogen Compounds by Chalcogenocyclofunctionalization Reactions Based on Chalcogen Halides and Natural Products. Molecules 2021, 26, 3729. https://doi.org/10.3390/molecules26123729
Musalov MV, Potapov VA, Yakimov VA, Musalova MV, Maylyan AA, Zinchenko SV, Amosova SV. A Regioselective Synthesis of Novel Functionalized Organochalcogen Compounds by Chalcogenocyclofunctionalization Reactions Based on Chalcogen Halides and Natural Products. Molecules. 2021; 26(12):3729. https://doi.org/10.3390/molecules26123729
Chicago/Turabian StyleMusalov, Maxim V., Vladimir A. Potapov, Vladimir A. Yakimov, Maria V. Musalova, Arkady A. Maylyan, Sergey V. Zinchenko, and Svetlana V. Amosova. 2021. "A Regioselective Synthesis of Novel Functionalized Organochalcogen Compounds by Chalcogenocyclofunctionalization Reactions Based on Chalcogen Halides and Natural Products" Molecules 26, no. 12: 3729. https://doi.org/10.3390/molecules26123729





