Green Synthesized Silver Nanoparticles Immobilized on Activated Carbon Nanoparticles: Antibacterial Activity Enhancement Study and Its Application on Textiles Fabrics
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Equipment
2.3. Fabrication of Activated Carbon Black Nanoparticles (ACNPs)
2.4. Synthesis of Silver Nanoparticles (AgNPs) Using Aqueous-Aloe Vera Extract
2.5. Incorporating AgNPs on ACNPs Surface
2.6. Surface Morphology Confirmation and Particles Size Determination of ACNPs; AgNPs and AgNPs-Immobilized ACNPs
2.7. Antibacterial Activity Test
2.8. Application of the ACNPs; AgNPs, and AgNPs-Immobilized on Polyester and Cotton Fabrics
3. Result and Discussion
3.1. Synthesis of AgNPs
3.2. Surface Morphology
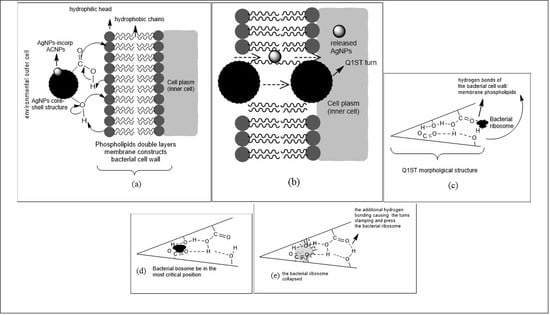
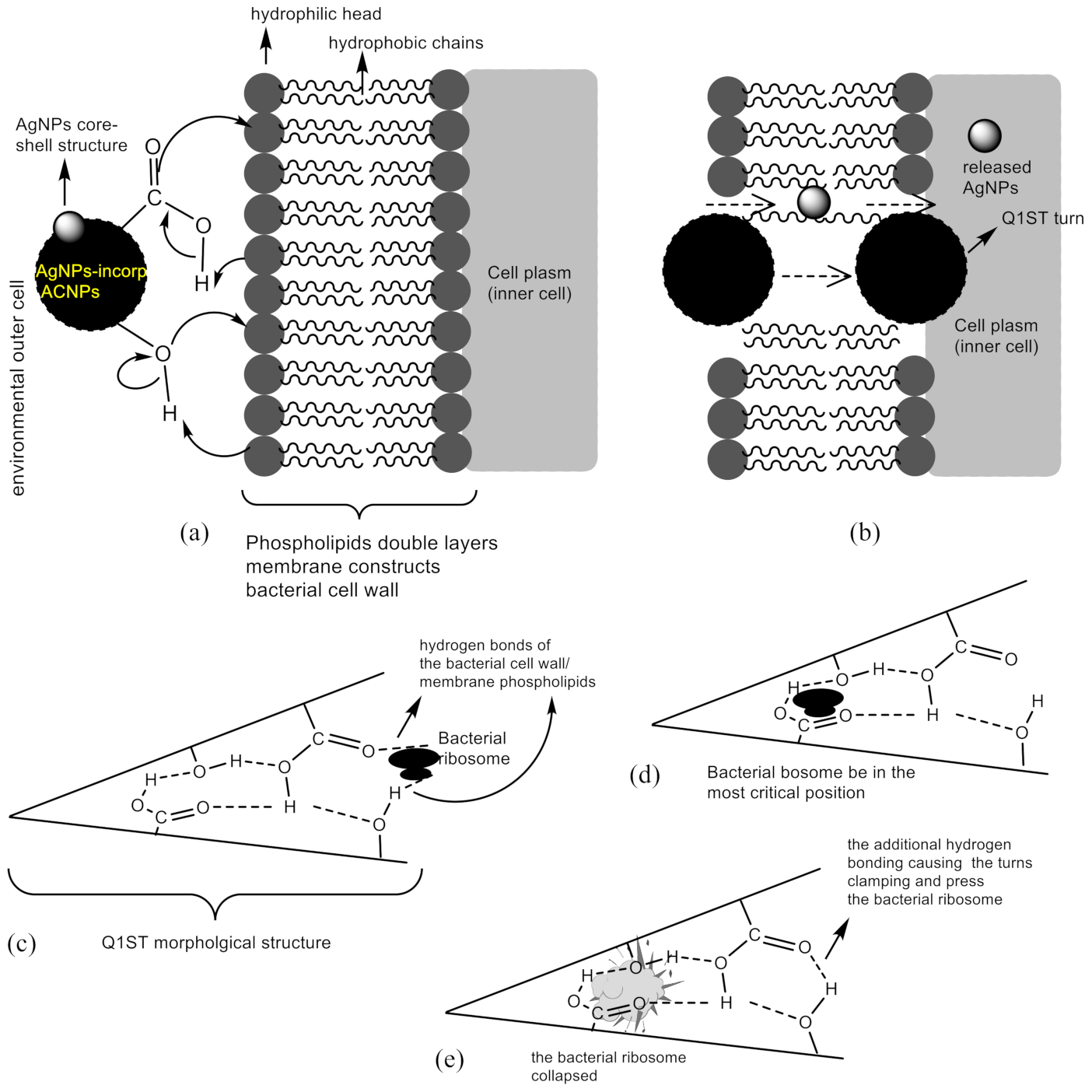
3.3. Antibacterial Activity Test
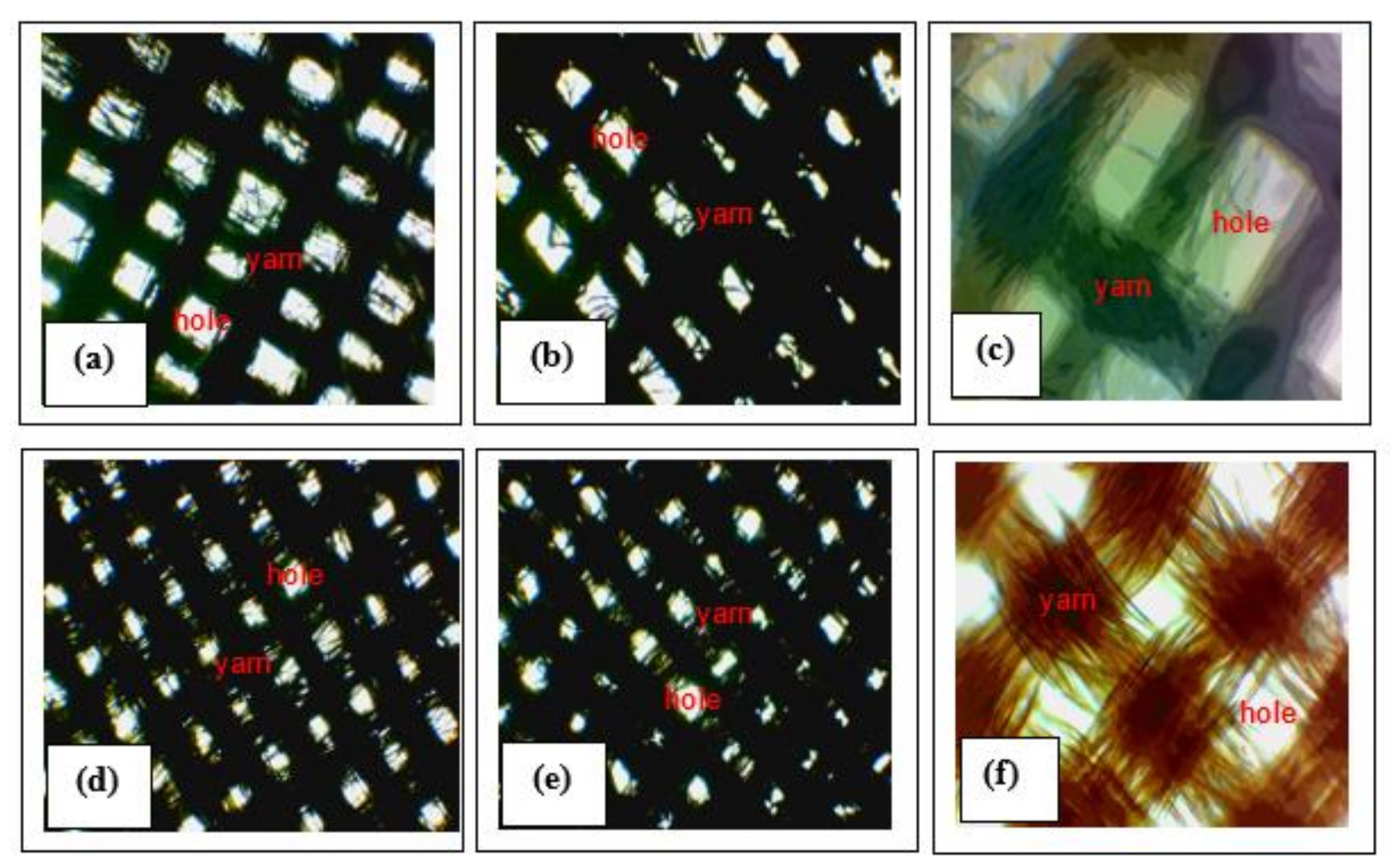
3.4. Application of the Synthesized AgNPs on Textile Fabrics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Burdusel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoanta, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Y.; Wu, C.; Wu, T.; Yuan, C.; Chen, S.; Ding, T.; Ye, X.; Hu, Y. Green synthesis of sodium alginate-silver nanoparticles and their antibacterial activity. Int. J. Biol. Macromol. 2018, 111, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Sondi, B.S. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kraeling, M.E.K.; Topping, V.D.; Keltner, Z.M.; Belgrave, K.R.; Bailey, K.D.; Gao, X.; Yourick, J.J. In vitro percutaneous penetration of silver nanoparticles in pig and human skin. Regul. Toxicol. Pharmacol. 2018, 95, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Gajda, K.D.; Nocun, M.; Roszak, J.; Janasik, B.; Quarles, C.D., Jr.; Wasowicz, W.; Grobelny, J.; Tomaszewska, E.; Celichowski, G.; Soliwoda, K.R.; et al. A study on the in vitro percutaneous absorption of silver nanoparticles in combination with aluminum chloride, methyl paraben or di-n-butyl phthalate. Toxicol. Lett. 2017, 272, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag. Shelf Life 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Fortunati, E.; Peltzer, M.; Armentano, I.; Jiménez, A.; Kenny, J.M. Combined effects of cellulose nanocrystals and silver nanoparticles on the barrier and migration properties of PLA nano-biocomposites. J. Food Eng. 2013, 118, 117–124. [Google Scholar] [CrossRef]
- Tavaf, Z.; Tabatabaei, M.; Nezhad, A.K.; Panahi, F. Evaluation of antibacterial, antibiofilm, and antioxidant activities of synthesized silver nanoparticles (AgNPs) and casein peptide fragments against streptococcus mutans. Eur. J. Integr. Med. 2017, 12, 163–171. [Google Scholar] [CrossRef]
- Chowdhury, N.R.; MacGregor-Ramiasa, M.; Zilm, P.; Majewski, P.; Vasilev, K. Chocolate’ silver nanoparticles: Synthesis, antibacterial activity, and cytotoxicity. J. Colloid Interface Sci. 2016, 482, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, R.C. Facile and eco-friendly fabrication of AgNPs coated silk for antibacterial and antioxidant textiles using honeysuckle extract. J. Photochem. Photobiol. B 2018, 178, 463–471. [Google Scholar] [CrossRef]
- Pannerselvam, B.; Jothinathan, M.K.D.; Rajendran, M.; Perumal, P.; Thangavelu, K.P.; Kim, H.J.; Singh, V.; Rangarajulu, S.K. An in vitro study on the burn wound healing activity of cotton fabrics immobilized with photosynthesized silver nanoparticles in male Wistar albino rats. Eur. J. Pharm. Sci. 2017, 100, 187–196. [Google Scholar] [CrossRef]
- Wang, Y.S.; Liu, L.; Fu, Q.; Sun, J.; An, Z.Y.; Ding, R.; Li, Y.; Zhao, X.D. Effect of Bacillus subtilis on corrosion behavior of 10MnNiCrCu steel in marine environment. Sci. Rep. 2020, 10, 5744. [Google Scholar] [CrossRef] [Green Version]
- Ishi, S.; Sadowsky, M.J. Escherichia coli in the Environment: Implications for Water Quality and Human Health. Microbes Environ. 2008, 23, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Parham, S.; Chandren, S.; Wicaksono, D.H.B.; Bagherbaigi, S.; Lee, S.L.; Yuan, L.S.; Nur, H. Textile/Al2O3–TiO2 nanocomposite as an antimicrobial and radical scavenger wound dressing. R. Soc. Chem. Adv. 2016, 6, 8188–8197. [Google Scholar] [CrossRef]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics 2018, 10, 557. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Liu, Y.; Yin, K.; Wang, D.; Li, B.; Yu, H.; Xing, M. ROS-Induced Hepatotoxicity under Cypermethrin: Involvement of the Crosstalk between Nrf2/Keap1 and NF-κB/iκB-α Pathways Regulated by Proteasome. Environ. Sci. Technol. 2021, 55, 6171–6183. [Google Scholar] [CrossRef]
- Wibawa, P.J.; Nur, M.; Asy’ari, M.; Nur, H. SEM, XRD and FTIR analyses of both ultrasonic and heat generated activated carbon black microstructures. Heliyon 2020, 6, e03546. [Google Scholar] [CrossRef]
- Elkady, M.F.; Hussein, M.M.; Atiaa, H.M. Preparation of nano-activated carbon from carbon-based material for copper decontamination from wastewater. Am. J. Appl. Chem. 2015, 3, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Haham, H.; Grinblat, J.; Sougrati, M.T.; Stievano, L.; Margel, S. Engineering of Iron-Based Magnetic Activated Carbon Fabrics for Environmental Remediation. Materials 2015, 8, 4593–4607. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, L.C.A.; Rios, R.V.R.A.; Fabris, J.D.; Garg, V.; Sapag, K.; Lago, R.M. Activated carbon/ iron oxide magnetic composites for the adsorption of contaminants in water. Carbon 2002, 40, 2177–2183. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Huang, P.-C.; Lin, Y.-Q. Reusing Cow Manure for the Production of Activated Carbon Using Potassium Hydroxide (KOH) Activation Process and Its Liquid-Phase Adsorption Performance. Processes 2019, 7, 737. [Google Scholar] [CrossRef] [Green Version]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Carmo, M.; Linardi, M.; Poco, J.G.R. H2O2 treated carbon black as electrocatalyst support for polymer electrolyte membrane fuel cell applications. Int. J. Hydrogen Energy 2008, 33, 6289–6297. [Google Scholar] [CrossRef]
- Knight, E.W.; Gillespie, A.K.; Prosniewski, M.J.; Stalla, D.; Dohnke, E.; Rash, T.A.; Pfeifer, P.; Wexler, C. Determination of the enthalpy of adsorption of hydrogen in activated carbon at room temperature. Int. J. Hydrogen Energy 2020, 45, 15541–15552. [Google Scholar] [CrossRef]
- Utrilla, J.R.; Díaz, J.M.; Polo, M.S.; García, M.A.F.; Toledo, I.B. Removal of the surfactant sodium dodecylbenzene sulphonate from water by the simultaneous use of ozone and powdered activated carbon: Comparison with systems based on O3 and O3/H2O2. Water Res. 2006, 40, 1717–1725. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Nagornaya, M.N.; Razdyakonova, G.I.; Khodakova, S.Y. The effect of functional groups of carbon black on rubber properties: International Conference on Oil and Gas Engineering. Procedia Eng. 2016, 152, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.J.F.; Liu, Z.; Suenaga, K. Imaging the atomic structure of activated carbon. J. Phys. Condens. Matter 2008, 20, 36220. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Van der Waals Forces between Particles and Surfaces. In Intermolecular and Surface Forces, 3rd ed.; Academic Press-Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 253–289. ISBN 978-0-12-375182-9. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012, 87, 564–573. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods: A Guide to Modern Technique of Plant Analysis, 2nd ed.; Chapman and Hall: New York, NY, USA, 1984; pp. 1–221. ISBN 13 978-94-010-8956-2/978-94-009-5570-7. [Google Scholar] [CrossRef]
- Omrani, A.A.; Taghavinia, N. Photo-induced growth of silver nanoparticles using UV sensitivity of cellulose fibers. Appl. Surf. Sci. 2012, 258, 2373–2377. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Jiang, Y.; Fang, Y. Spectroscopy property of Ag nanoparticles. Spectrochim. Acta Part A 2006, 65, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.J.; Barbic, M.; Smith, D.R.; Schultz, D.A.; Schultz, S. Shape effects in plasmon resonance of individual colloidal silver Nanoparticles. J. Chem. Phys. 2002, 116, 6755. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hussein, M.H.; Abo-elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 13071. [Google Scholar] [CrossRef]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia Spinosa grown in vitro and their antibacterial activity assessment. J. Nanostruct. Chem. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Jyoti, K.; Singh, A. Green synthesis of nanostructured silver particles and their catalytic application in dye degradation. J. Genet. Eng. Biotechnol. 2016, 14, 311–317. [Google Scholar] [CrossRef] [Green Version]
- IUPAC: Commission on Molecular Structure and Spectroscopy: Tables of Wavenumbers for the Calibration of Infrared Spectrometers. International Union of Pure and Applied Chemistry (IUPAC). pp. 537–699. Available online: http://publications.iupac.org/pac/pdf/1960/pdf/0104x0537.pdf (accessed on 13 August 2019).
- Baranović, G.; Šegota, S. Infrared spectroscopy of flavones and flavonols. Reexamination of the hydroxyl and carbonyl vibrations in relation to the interactions of flavonoids with membrane lipids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 192, 473–486. [Google Scholar] [CrossRef]
- El Aziz, M.M.A.; Ashour, A.S.; Gomha Melad, A.S.G. A review on saponins from medicinal plants: Chemistry, isolation, and determination. J. Nanomed. Res. 2019, 7, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Moghimipour, E.; Handali, S. Saponin: Properties, Methods of Evaluation and Applications. Annu. Res. Rev. Biol. 2015, 5, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Okuda, T.; Ito, H. Tannins of Constant Structure in Medicinal and Food Plants-Hydrolyzable Tannins and Polyphenols Related tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Rauwel, P.; Küünal, S.; Ferdov, S.; Erwan Rauwel, E. A Review on the Green Synthesis of Silver Nanoparticles and Their Morphologies Studied via TEM. Adv. Mater. Sci. Eng. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Atapour, A.; Amani, A.M.; Dashtaki, A.S.; Babapoor, A.; Arjmand, O. Green synthesis of silver nanoparticles toward bio and medical applications: Review study. Artif. Cells Nanomed. Biotechnol. 2018, 46, S855–S872. [Google Scholar] [CrossRef] [Green Version]
- House, J.E. Inorganic Chemistry: Covalent Bonding in Diatomic Molecules; Academic Press: Cambridge, MA, USA, 2008; pp. 76–78. [Google Scholar]
- Dowling, A. Nanoscience and Nanotechnology: Opportunities and Uncertainties; Royal Society/Royal Academy of Engineering: London, UK, 2005. [Google Scholar]
- Lead, J.R.; Wilkinson, K.J. Environmental Colloids and Particles: Current Knowledge and Future Development, Book Chapter I; Wiley: Chichester, UK, 2007; pp. 1–15. [Google Scholar]
- Garbin, R.P.B.; Otaguiri, E.S.; Morey, A.T.; da Silva, M.F.; Morguette, A.E.B.; Lancheros, C.A.C.; Kian, D.; Perugini, M.R.E.; Nakazato, G.; Durán, N.; et al. Effect of eugenol against Streptococcus agalactiae and synergistic interaction with biologically produced silver nanoparticles. Evid. Based Complement. Altern. Med. 2015, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cardozo, V.F.; Oliveira, A.G.; Nishio, E.K.; Perugini, M.R.E.; Andrade, C.G.T.; Silveira, W.D.; Durán, N.; Andrade, G.; Kobayashi, R.K.T.; Nakazato, G. Antibacterial activity of extracellular compounds produced by a Pseudomonas strain against methicillin-resistant Staphylococcus aureus (MRSA) strains. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 12. [Google Scholar] [CrossRef] [Green Version]
- Bogdanov, M.; Pyrshev, K.; Yesylevskyy, S.; Ryabichko, S.; Boiko, V.; Ivanchenko, P.; Kiyamova, R.; Guan, Z.; Ramseyer, C.; Dowhan, W. Phospholipid distribution in the cytoplasmic membrane of Gram-negative bacteria is highly asymmetric, dynamic, and cell shape-dependent. Sci. Adv. 2020, 6, eaaz6333. [Google Scholar] [CrossRef]
- Sohlenkamp, C.; Geiger, O. Review articles: Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2016, 40, 133–159. [Google Scholar] [CrossRef] [Green Version]
- Clifton, L.A.; Skoda, M.W.A.; Daulton, E.L.; Hughes, A.V.; Le Brun, A.P.; Lakey, J.H.; Holt, S.A. Asymmetric phospholipid: Lipopolysaccharide bilayers; a Gram-negative bacterial outer membrane mimic. J. R. Soc. Interface 2013, 10, 20130810. [Google Scholar] [CrossRef]
- Kedziora, A.; Speruda, M.; Krzyzewska, E.; Rybka, J.; Łukowiak, A.; Płoskonska, G.B. Similarities and Differences between Silver Ions and Silver in Nanoforms as Antibacterial Agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [Green Version]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [Green Version]


| Materials, 10% in Concentration | Clear Zone Radius/mm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | S. aureus | |||||||||
| I | II | III | Average | % Antibacterial Activity * | I | II | III | Average | % Antibacterial Activity * | |
| AgNPs | 2.50 | 4.50 | 2.50 | 3.17 ± 1.15 | 57.58 | 3.50 | 3.50 | 2.50 | 3.17 ± 0.58 | 61.25 |
| AgNPs-immobilized ACNPs | 3.50 | 3.50 | 3.50 | 3.50 ± 0.00 | 63.64 | 5.50 | 4.50 | 4.50 | 4.83 ± 0.50 | 93.49 |
| Amoxicillin (positive control) | 5.50 | 5.50 | 5.50 | 5.50 ± 0.00 | 100.00 | 4.50 | 5.50 | 5.50 | 5.17 ± 0.58 | 99.94 |
| Distilled water (negative control) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Textile Fabrics Wetted with Materials of 5% w/v in Concentration * | Clear Zone | ||||
|---|---|---|---|---|---|
| E. coli | S. aureus | ||||
| Radius/mm | % Antibacterial Activity ** | Radius/mm | % Antibacterial Activity ** | ||
| Cotton | ACNPs | 0 | 0 | 0 | 0 |
| AgNPs-immobilized ACNPs | 2.00 | 19.23 | 1.40 | 13.36 | |
| Amoxicillin (positive control) | 10.40 | 100 | 8.50 | 81.73 | |
| Distilled water (negative control | 0 | 0 | 0 | 0 | |
| Polyester | ACNPs | 0 | 0 | 0 | 0 |
| AgNPs-immobilized ACNPs | 3.30 | 31.73 | 2.20 | 21.15 | |
| Amoxicillin (positive control) | 8.30 | 79.81 | 7.90 | 75.96 | |
| Distilled water (negative control | 0 | 0 | 0 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wibawa, P.J.; Nur, M.; Asy’ari, M.; Wijanarka, W.; Susanto, H.; Sutanto, H.; Nur, H. Green Synthesized Silver Nanoparticles Immobilized on Activated Carbon Nanoparticles: Antibacterial Activity Enhancement Study and Its Application on Textiles Fabrics. Molecules 2021, 26, 3790. https://doi.org/10.3390/molecules26133790
Wibawa PJ, Nur M, Asy’ari M, Wijanarka W, Susanto H, Sutanto H, Nur H. Green Synthesized Silver Nanoparticles Immobilized on Activated Carbon Nanoparticles: Antibacterial Activity Enhancement Study and Its Application on Textiles Fabrics. Molecules. 2021; 26(13):3790. https://doi.org/10.3390/molecules26133790
Chicago/Turabian StyleWibawa, Pratama Jujur, Muhammad Nur, Mukhammad Asy’ari, Wijanarka Wijanarka, Heru Susanto, Heri Sutanto, and Hadi Nur. 2021. "Green Synthesized Silver Nanoparticles Immobilized on Activated Carbon Nanoparticles: Antibacterial Activity Enhancement Study and Its Application on Textiles Fabrics" Molecules 26, no. 13: 3790. https://doi.org/10.3390/molecules26133790
APA StyleWibawa, P. J., Nur, M., Asy’ari, M., Wijanarka, W., Susanto, H., Sutanto, H., & Nur, H. (2021). Green Synthesized Silver Nanoparticles Immobilized on Activated Carbon Nanoparticles: Antibacterial Activity Enhancement Study and Its Application on Textiles Fabrics. Molecules, 26(13), 3790. https://doi.org/10.3390/molecules26133790