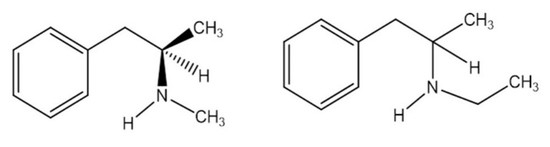
The Vaporization Enthalpy and Vapor Pressure of (±) N-Ethyl Amphetamine by Correlation Gas Chromatography
Abstract
:1. Introduction
2. Experimental Methods
2.1. Analytical Methods
2.2. Thermochemical Methods: Vaporization Enthalpies
2.3. Thermochemical Methods: Vapor Pressures
2.4. Uncertainties
2.5. Estimation of Vaporization Enthalpy
2.6. Vaporization Enthalpy: Temperature Adjustments
2.7. Vapor Pressures
3. Results
3.1. Vaporization Enthalpies
3.2. Vapor Pressures
3.3. Estimation of Boiling Temperatures and Their Uncertainties
4. Discussion
4.1. Vaporization Enthalpy
4.2. Vapor pressure
5. Summary
Supplementary Materials
Funding
Conflicts of Interest
References
- National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Etilamfetamine (accessed on 17 March 2021).
- Cayman Chemical. Available online: https://www.caymanchem.com/product/11557/n-ethylamphetamine-(hydrochloride) (accessed on 17 March 2021).
- Fitzgerald, R.L.; Ramos, J.M., Jr.; Bogema, S.C.; Poklis, A. Resolution of Methamphetamine Stereoisomers in Urine Drug Testing: Urinary Excretion of R(−)-Methamphetamine Following Use of Nasal Inhalers. J. Anal. Toxicol. 1988, 12, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Beckett, A.H.; Brookes, L.G.; Shenoy, E.V.B. Urinary excretion of the drug and its main metabolite in man, after the administration of (±)-, (+)- and (−)-ethylamphetamine. J. Pharm. Pharm. 2011, 21, 151S–156S. [Google Scholar] [CrossRef]
- Orf, M.; Kurian, M.; Espinosa, L.; Nelson, C.; Simmons, D.; Chickos, J. Thermochemical properties of sesquiterpenes in natural products by correlation gas chromatography: Application to bergamotene oil. J. Chem. Thermodyn. 2018, 126, 128–136. [Google Scholar] [CrossRef]
- Siripoltangman, N.; Chickos, J. Vapor Pressure and vaporization enthalpy studies of the major components of ginger, α-zingiberene, β-sesquiphellandrene and (−) ar curcumene by correlation gas chromatography. J. Chem. 2019, 138, 107–115. [Google Scholar] [CrossRef]
- Zafar, A.; Chickos, J. The vapor pressure and vaporization enthalpy of squalene and squalene by correlation gas chromatography. J. Chem. Thermodyn. 2019, 135, 192–197. [Google Scholar] [CrossRef]
- Thornton, M.; Gobble, C.; Chickos, J. The vaporization enthalpy and vapor pressure of S (+)-methamphetamine at T = 298.15 K by correlation gas chromatography. J. Chem. Thermodyn. 2014, 73, 51–56. [Google Scholar] [CrossRef]
- Peacock, A.; Fuchs, R. Enthalpy of vaporization measurements by gas chromatography. J. Am. Chem. Soc. 1977, 99, 5524–5525. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hanshaw, W. Vapor pressures and vaporization enthalpies of the n-alkanes from C21-C30 at T = 298.15 by correlation-gas chromatography. J. Chem. Eng. Data 2004, 49, 77–85. [Google Scholar] [CrossRef]
- Nichols, G.; Kweskin, S.; Frericks, M.; Reiter, S.; Wang, G.; Orf, J.; Carvallo, B.; Hillesheim-Cox, D.; Chickos, J.S. An Evaluation of the The Vaporization, Fusion and Sublimation Enthalpies of the 1-Alkanols. The Vaporization Enthalpy of 1-, 6-, 7-, and 9-Heptadecanol, 1-Octadecanol, 1-Eicosanol, 1-Docosanol, 1-Hexacosanol and Cholesterol at T = 298.15 K by Correlation-Gas Chromatography. J. Chem. Eng. Data 2006, 51, 475–482. [Google Scholar] [CrossRef]
- Guide to the Expression of Uncertainty in Measurement BIPM. Available online: http://www.bipm.org/en/publications/guides/gum.html (accessed on 17 March 2021).
- Roux, M.V.; Temprado, M.; Chickos, J.S. Vaporization, fusion and sublimation enthalpies of the dicarboxylic acids from C4 to C14 and C16. J. Chem. Thermodyn. 2005, 37, 941–953. [Google Scholar] [CrossRef]
- Chickos, J.S. Computational Thermochemistry: Prediction and Estimation of Molecular Thermodynamics; Irikura, K.K., Frurip, D.J., Eds.; ACS Symposium Series 677; American Chemical Society: Washington, DC, USA, 1996; Chapter 4; pp. 77–79. [Google Scholar]
- Acree, W., Jr.; Chickos, J.S. Phase Transition Enthalpy Measurements of Organic and Organometallic Compounds. Sublimation, Vaporization and Fusion Enthalpies From 1880 to Part C1–C10. J. Phys. Chem. Ref. Data 2016, 45, 1–565. [Google Scholar] [CrossRef]
- Petros, L.; Majer, V.; Koubek, J.; Svoboda, V.; Pick, J. Temperature Dependence of Heats of Vaporization, Saturated Vapour Pressures and Cohesive Energies of Secondary Amines. Coll. Czechoslov. Chem. Commun. 1979, 44, 3533–3549. [Google Scholar] [CrossRef]
- Wadso, I. Enthalpies of Vaporization of Organic Compounds. III. Amines. Acta Chem. Scand. 1969, 23, 2061. [Google Scholar] [CrossRef]
- Stephenson, R.; Malanowski, S. Handbook of the Thermodynamics of Organic Compounds; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1987. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Cabral, J.I.T.A. Thermochemical Properties of Three Piperidine Derivatives1-Benzyl-4-piperidinol, 4-benzylpiperidine and 4-piperidine-piperidine. J. Therm. Anal. Calorim. 2007, 90, 865–871. [Google Scholar] [CrossRef]
- Zabransky, M.; Ruzicka, V., Jr.; Majer, V.; Domalski, E.S. Heat Capacity of Liquids: Critical Review and Recommended Values. Vol. I and II. J. Phys. Chem. Ref. Data 2001, 30, 1199. [Google Scholar] [CrossRef] [Green Version]
- EPI Suite Version 4.11 (Estimation Programs Interface). Available online: http://www.epa.gov/oppt/exposure/pubs/episuitedl.htm (accessed on 8 April 2021).
- Aldrich Handbook of Fine Chemicals (2012–2014); Sigma Aldrich: St. Louis, MO, USA, 2012.
- Ledo, J.M.; Flores, H.; Freitas, V.L.S.; Solano-Altamirano, J.M.; Hernández-Pérez, J.M.; Camarillo, E.A.; Ramos, F.; Ribeiro da Silva, M.D.M.C. Benzocaine: A comprehensive thermochemical study. J. Chem. Thermodyn. 2020, 147, 1–8. [Google Scholar] [CrossRef]
- Almeida, A.R.R.P.; Monte, M.J.S. Thermodynamic study of phase transitions in methyl esters of ortho- meta- and para-aminobenzoic acids. J. Chem. Thermodyn. 2012, 53, 100–107. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hanshaw, W. Vapor Pressures and Vaporization Enthalpies of the n-Alkanes from C31 to C38 at T = 298.15 K by Correlation Gas Chromatography. J. Chem. Eng. Data 2004, 49, 620–630. [Google Scholar] [CrossRef]
- Temmler, T.H.; Keil, F.; Dobke, W. β-Aralkylamines. DE 767263; SciFinder Scholar; Chemical Abstracts Service: Columbus, OH, USA, 1952. [Google Scholar]
- Shiho, D.; Kanayama, K. A new process of alkylation of amines. II. Nippon Kagaku Kaishi 1944, 65, 237–239. [Google Scholar] [CrossRef] [Green Version]
- Leonard, N.J.; Adamcik, J.A.; Djerassi, C.; Halpern, O. Cyclic amino acyloins and amino ketones. X. Trans-annular nitrogen-carbonyl interaction in cyclic aminoketones and optical rotatory dispersión. J. Am. Chem. Soc. 1958, 80, 4858–4862. [Google Scholar] [CrossRef]
- Chiavarelli, S.; Marini-Bettolo, G.B. Synthetic sympatholytic substances in the ergotamine series. II. Derivatives of benzylamine, phenethylamine, and α-methylphenethylamines with amine and amide functions. Gazz. Chim. Ital. 1951, 81, 89–97. [Google Scholar]


| Compound | CAS Registry No. | Supplier | Mass Fraction Supplier | GC |
|---|---|---|---|---|
| Methanol | 67-56-1 | Fisher Scientific | 0.998 | |
| N,N-Dibutylamine | 111-92-2 | Aldrich | 0.99 | |
| (dl)-N-Ethylamphetamine | 457-87-4 | Millipore Sigma | reference standard | >0.99 1 |
| N,N-Dipentylamine | 2050-92-2 | TCI | >0.97 | |
| 4-Benzylpiperidine | 31252-42-3 | Aldrich | 0.99 | |
| N,N-Dihexylamine | 143-16-8 | TCI | >0.99 | |
| N,N-Diheptylamine | 2470-68-0 | TCI | >0.98 |
| Tm/K | Cp(l, 298 K) 1 J·mol−1·K−1 | ΔCpΔT 2 kJ·mol−1 | Ref. | |||
|---|---|---|---|---|---|---|
| N,N-Dibutylamine | 50.8 ± 4.1 | 298.15 | 308.2 | 50.8 ± 4.1 | [8] | |
| 46.0 | 343.2 | 308.2 | 4.1 ± 1.0 | 50.1 ± 1.0 | [16] | |
| 44.75 | 358.2 | 308.2 | 5.4 ± 1.0 | 50.2 ± 1.0 | [16] | |
| 49.4 ± 0.1 | 298.15 | 308.2 | 49.4 ± 0.1 | [17] | ||
| Average | 50.1 ± 0.6 3 | |||||
| N,N-Dipentylamine | 61.2 ± 2.6 | 298.15 | 372 | 61.2 ± 2.6 | [8] | |
| 51.2 | 394 | 372 | 10.3 ± 1.6 | 61.4 ± 1.6 | [18] | |
| Average | 61.3 ± 2.1 3 | |||||
| N,N-Dihexylamine | 70.8 ± 4.7 | 298.15 | 435.8 | 70.8 ± 4.7 | [8] | |
| 55.1 | 423 | 435.8 | 15.5 ± 2.1 | 70.5 ± 2.1 | [17,18] | |
| Average | 70.7 ± 3.4 3 | |||||
| 4-Benzylpiperidine | 74.2 ± 1.0 | 298 | 74.2 ± 1.0 | [19] | ||
| N,N-Diheptylamine | 60.0 | 450 | 499.6 | 21.3 ± 2.6 | 81.3 ± 2.6 | [18] |
| 81.2 ± 7.1 | 298.15 | 499.6 | 81.2 ± 7.1 | [8] | ||
| Average | 81.3 ± 4.9 3 | |||||
| Antoine Equation (4) | AA | BA | −CA | Trange/K | p(l, 298 K)/Pa | Ref. |
|---|---|---|---|---|---|---|
| N,N-Dibutylamine | 14.6511 | 3687.84 | 65.37 | 286–371 | 304 | [16] |
| N,N-Dipentylamine | 14.7935 | 4105.74 | 72.15 | 379–527 | 34 | [18] |
| N,N-Dihexylamine | 15.1013 | 4635.56 | 69.15 | 408–569 | 5.8 | [18] |
| N,N-Diheptylamine | 14.8948 | 4716.85 | 86.15 | 435–605 | 0.64 | [18] |
| 3rd Order Polynomial Equation (5) | AT | BT | CT × 10−4 | DT × 10−6 | p(l, 298 K)/Pa | |
| 4-Benzylpiperidine | 6.74 | −1642.3 | −130.851 | 62.508 | 1.5 | [8] |
| (S) (+)-Methamphetamine | 7.592 | –2119.6 | –84.929 | 31.824 | 39 | [8] |
| Run 1 | −Slope T/K | Intercept | kJ·mol−1 | ||
|---|---|---|---|---|---|
| (lit) 2 | (calc) | ||||
| N,N-Di-n-butylamine | 4197.2 ± 20 | 10.984 ± 0.05 | 34.89 ± 0.16 | 50.1 ± 0.6 | 50.4 ± 3.4 |
| N,N-Di-n-pentylamine | 5121.3 ± 23 | 12.181 ± 0.06 | 42.58 ± 0.19 | 61.3 ± 2.1 | 61.0 ± 3.7 |
| N-Ethylamphetamine | 5238.1 ± 24 | 11.973 ± 0.06 | 43.55 ± 0.20 | 62.4 ± 3.8 | |
| N,N-Di-n-hexylamine | 6037.1 ± 24 | 13.374 ± 0.06 | 50.19 ± 0.20 | 70.7 ± 3.4 | 71.5 ± 4.0 |
| 4-Benzylpiperidine | 6149.2 ± 27 | 12.810 ± 0.07 | 51.12 ± 0.22 | 74.2 ± 1.0 | 72.8 ± 4.1 |
| N,N-Di-n-heptylamine | 6937.5 ± 36 | 14.546 ± 0.09 | 57.68 ± 0.30 | 81.3 ± 4.9 | 81.8 ± 4.3 |
| /kJ·mol−1 = (1.38 ± 0.06) (408 K) + (2.3 ± 2.8) | r2 = 0.9947 (6) | ||||
| Run 1 | Run 2 | Average | Lit. 2 | Estimate | |
|---|---|---|---|---|---|
| N,N-Di-n-butylamine | 50.4 ± 3.4 | 50.2 ± 4.5 | 50.3 ± 4.0 | 50.1 ± 0.6 3 | 49.4 ± 2.4 |
| N,N-Di-n-pentylamine | 61.0 ± 3.7 | 60.9 ± 4.9 | 61.0 ± 4.3 | 61.3 ± 2.1 4 | 58.8 ± 2.9 |
| N-Ethylamphetamine | 62.4 ± 3.8 | 62.3 ± 4.9 | 62.4 ± 4.4 | 63.5 ± 3.2 | |
| N,N-Di-n-hexylamine | 71.5 ± 4.0 | 71.8 ± 5.3 | 71.7 ± 4.7 | 70.7 ± 3.4 4 | 68.2 ± 3.4 |
| 4-Benzylpiperidine | 72.8 ± 4.1 | 73.3 ± 5.4 | 73.1 ± 4.8 | 74.2 ± 1.0 5 | 68.2 ± 3.4 |
| N,N-Di-n-heptylamine | 81.8 ± 4.3 | 82.5 ± 5.8 | 82.2 ± 5.1 | 81.3 ± 4.9 4 | 77.6 ± 3.9 |
| ln(to/tr) | ln(p/po) | ln(p/po) | p/Pa | p/Pa/Lit | Lit. | |
|---|---|---|---|---|---|---|
| N,N-Di-n-butylamine | −7.739 | −5.810 | −5.83 ± 0.49 | 300 ± 120 | 304 | [16] |
| N,N-Di-n-pentylamine | −9.634 | −7.992 | −7.90 ± 0.53 | 37 ± 20 | 34 | [18] |
| N-Ethylamphetamine | −10.235 | −8.56 ± 0.54 | 19 ± 11 | 8.2 1 | [21] | |
| N,N-Di-n-hexylamine | −11.517 | −9.760 | −9.97 ± 0.58 | 4.7 ± 2 | 5.8 | [18] |
| 4-Benzylpiperidine | −12.427 | −11.130 | −10.97 ± 0.60 | 1.8 ± 1 | 1.5 | [8] |
| N,N-Di-n-heptylamine | −13.369 | −11.973 | −12.00 ± 0.62 | 0.62 ± 0.4 | 0.64 | [18] |
| r2 = 0.9968 (7) | ||||||
| AS | Bs | Cs | TB/K | TBLit/K | |
|---|---|---|---|---|---|
| N,N-Di-n-butylamine | 9.483 ± 0.02 | −3128.7 ± 12 | −427980 ± 2190 | 434 ± 5 | 432.8 1 |
| N,N-Di-n-pentylamine | 9.395 ± 0.03 | −3271.1 ± 19 | −562,260 ± 3409 | 474 ± 3 | 475.2 1 |
| N-Ethylamphetamine | 8.347 ± 0.01 | −2872.4 ± 3.7 | −646650 ± 665 | 499 ± 3 | 508 2 |
| N,N-Di-n-hexylamine | 9.483 ± 0.04 | −3128.7 ± 27 | −427,980 ± 4919 | 512 ± 2 | 506–516 1 |
| 4-Benzylpiperidine | 7.429 ± 0.01 | −2679.1 ± 10 | −836,480 ± 1734 | 561 ± 1 | 552 3 |
| N,N-Di-n-heptylamine | 9.309 ± 0.05 | −3582.1 ± 36 | −825,650 ± 6424 | 547 ± 1 | 544.2 1 |
| p/Pa | AS | Bs | Cs | TB/K | |
|---|---|---|---|---|---|
| 62.4 ± 4.4 | 19 ± 11 | 8.347 ± 0.01 | –2,872.4 ± 3.7 | –646650 ± 665 | 499 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chickos, J.S. The Vaporization Enthalpy and Vapor Pressure of (±) N-Ethyl Amphetamine by Correlation Gas Chromatography. Molecules 2021, 26, 3809. https://doi.org/10.3390/molecules26133809
Chickos JS. The Vaporization Enthalpy and Vapor Pressure of (±) N-Ethyl Amphetamine by Correlation Gas Chromatography. Molecules. 2021; 26(13):3809. https://doi.org/10.3390/molecules26133809
Chicago/Turabian StyleChickos, James S. 2021. "The Vaporization Enthalpy and Vapor Pressure of (±) N-Ethyl Amphetamine by Correlation Gas Chromatography" Molecules 26, no. 13: 3809. https://doi.org/10.3390/molecules26133809
APA StyleChickos, J. S. (2021). The Vaporization Enthalpy and Vapor Pressure of (±) N-Ethyl Amphetamine by Correlation Gas Chromatography. Molecules, 26(13), 3809. https://doi.org/10.3390/molecules26133809