Tunable Viscoelastic Properties of Sodium Polyacrylate Solution via CO2-Responsive Switchable Water
Abstract
:1. Introduction
2. Results and Discussion
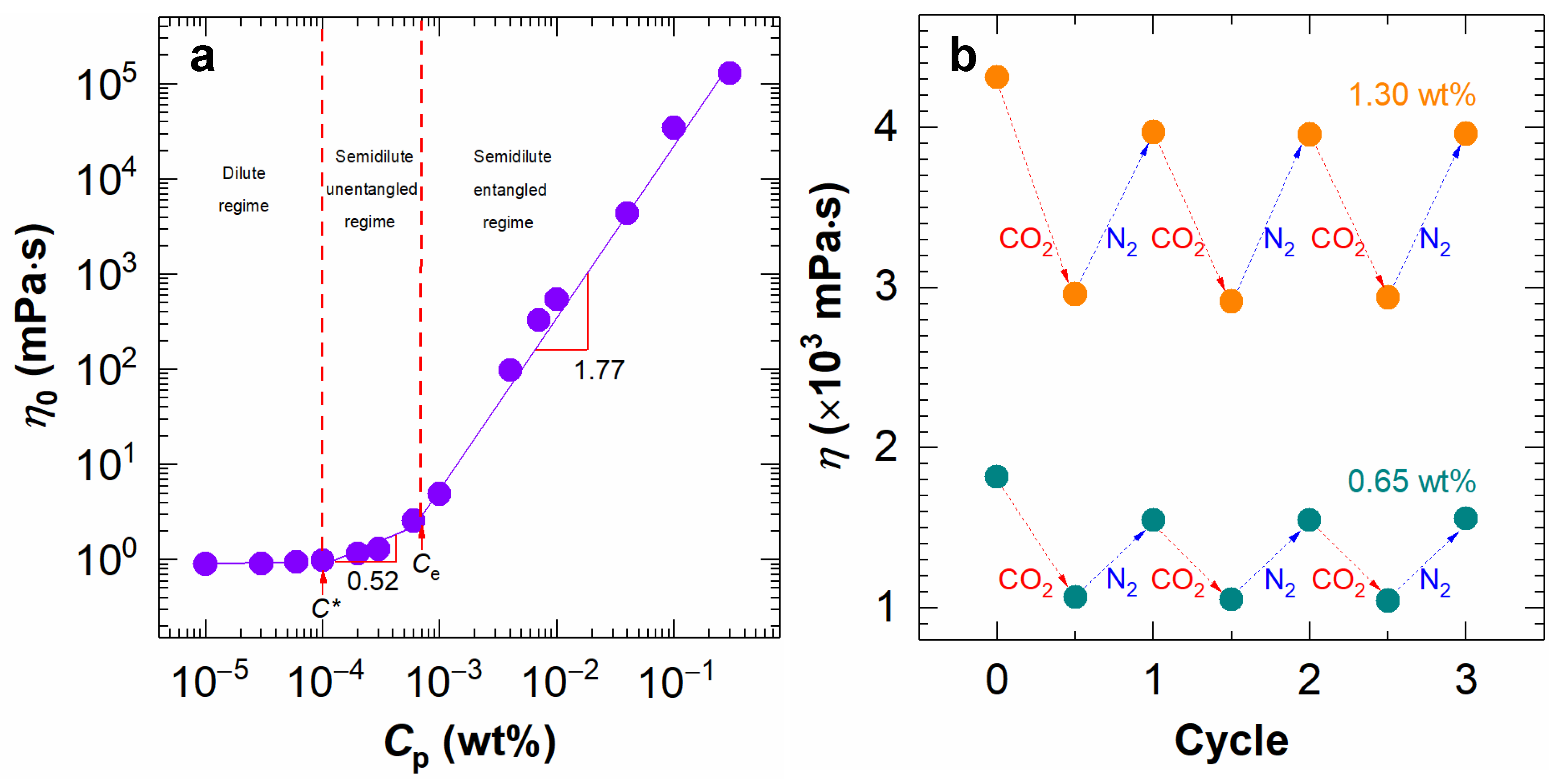
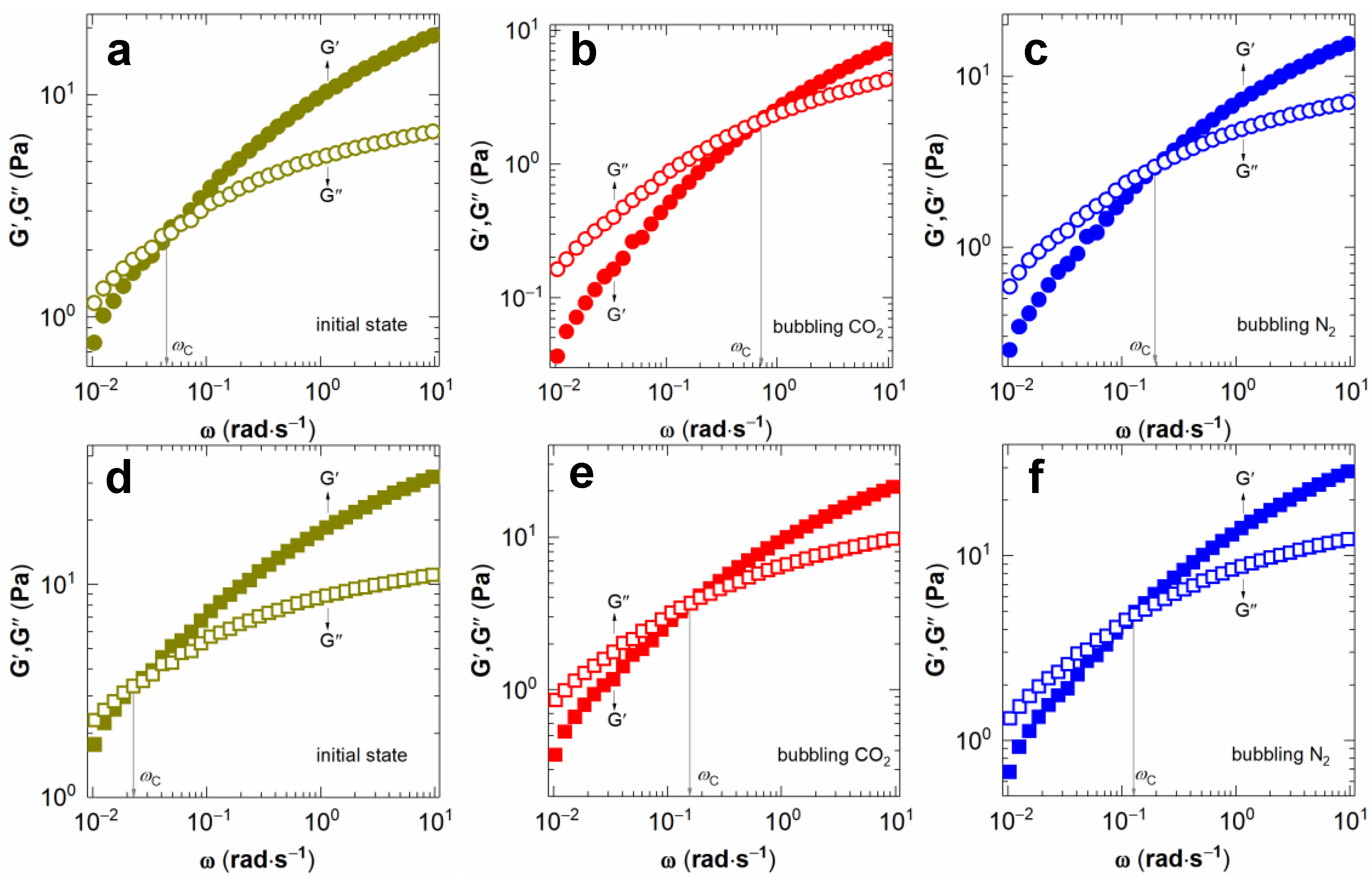
2.1. Rheological Properties of NaPAA Aqueous Solutions and Their CO2-Responsive Behavior
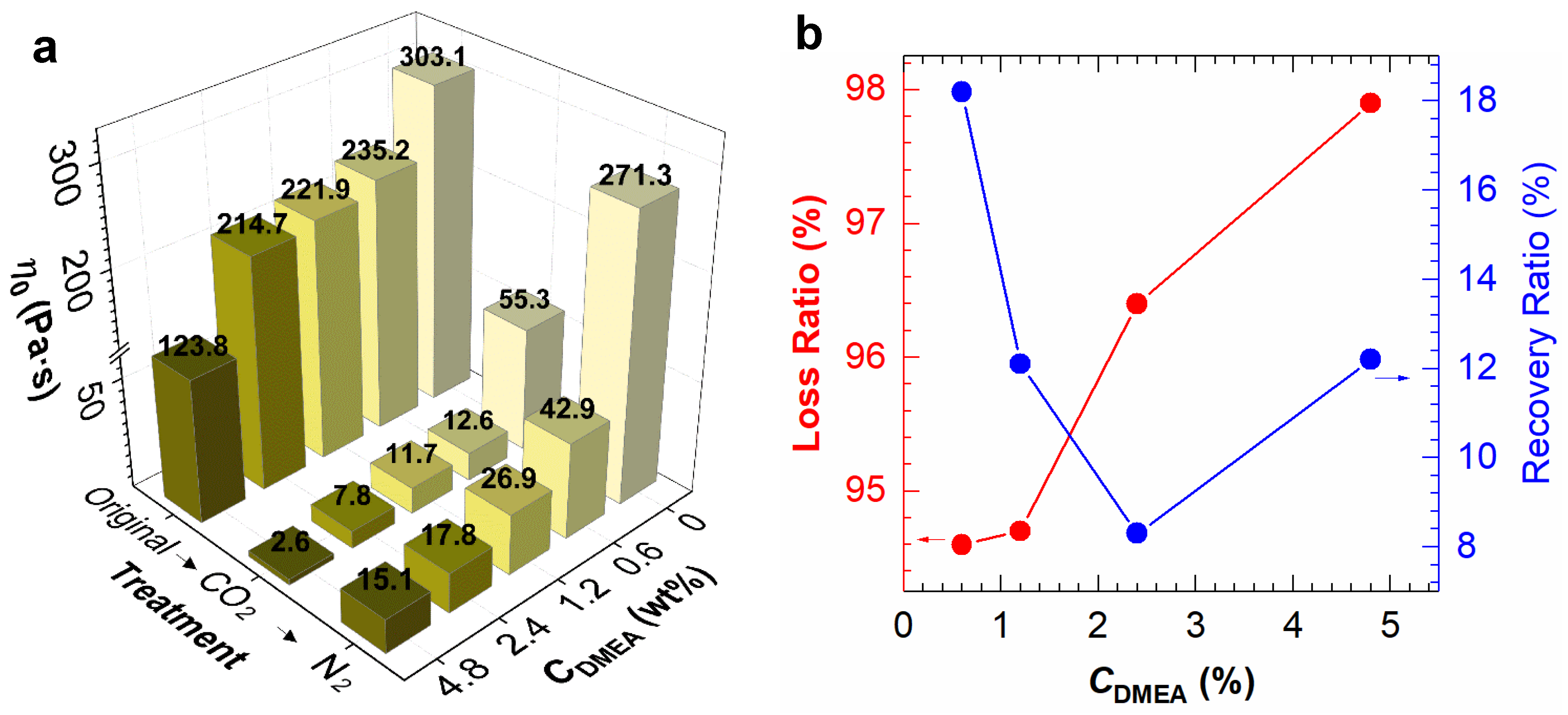
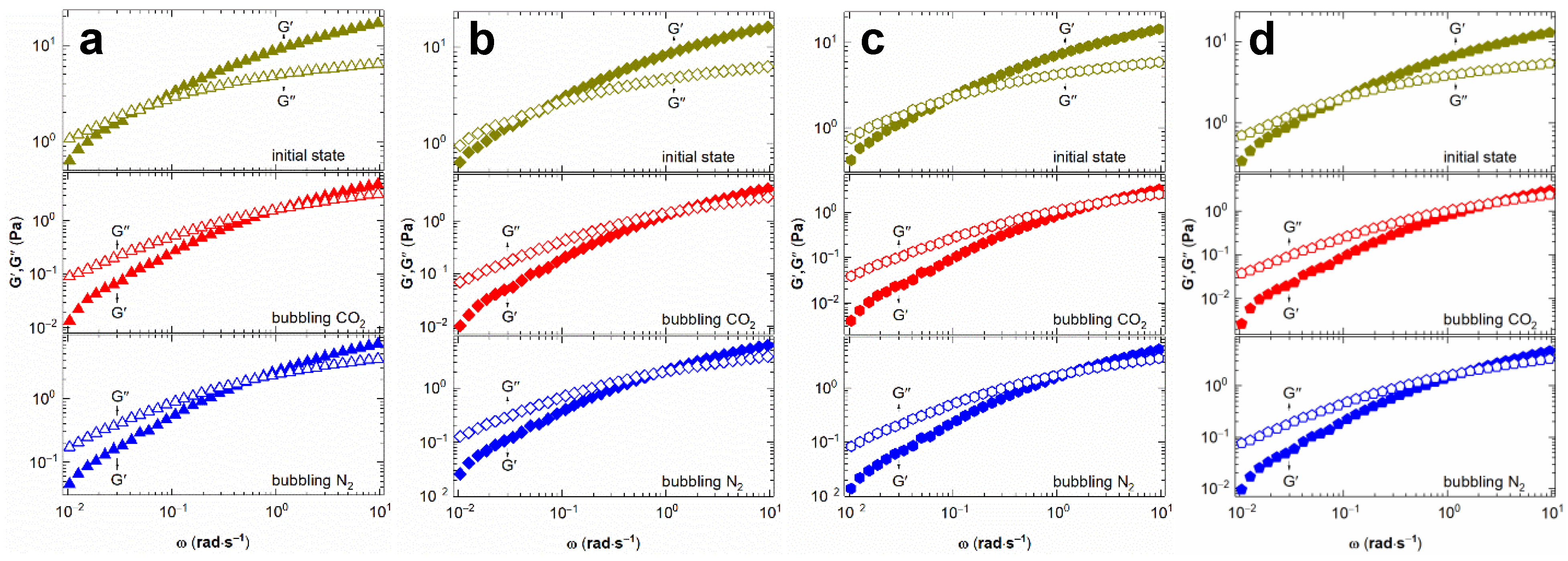
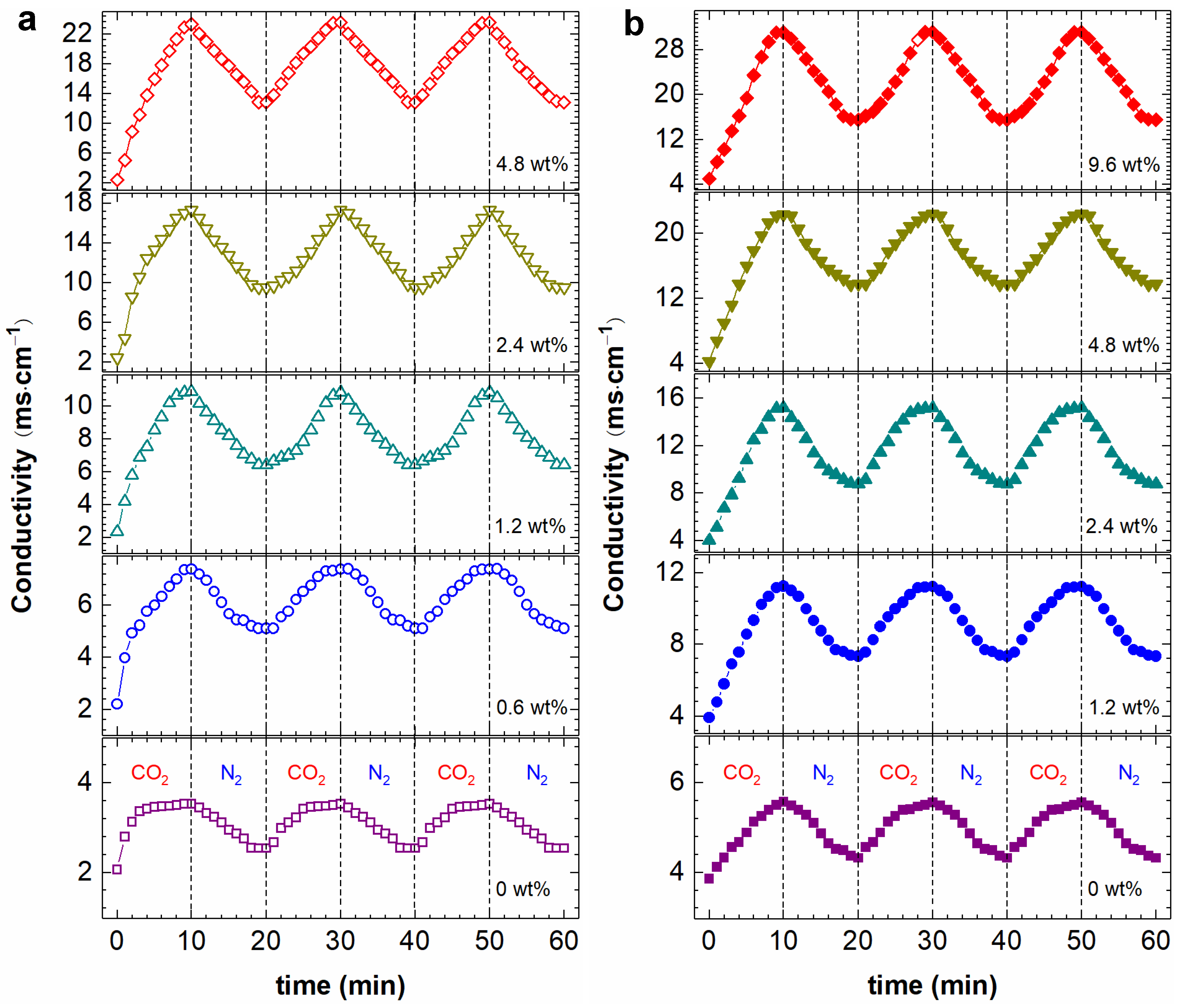
2.2. Effect of DMEA CO2-Switchable Water on Rheological Properties of NaPAA Solutions
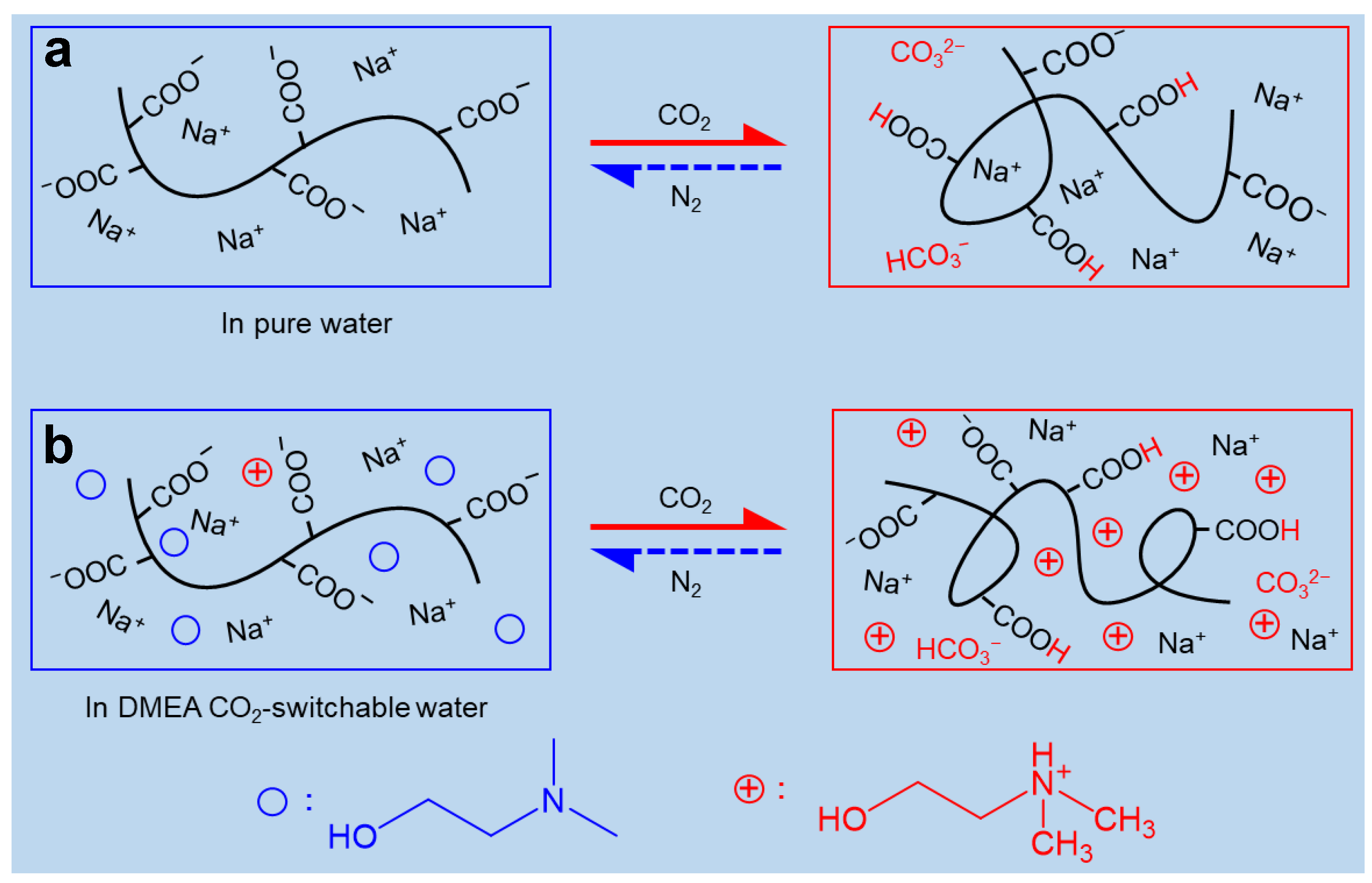
2.3. Mechanism of CO2-Switchable Viscoelasticity
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.2.1. CO2 Treatment
3.2.2. N2 Treatment
3.3. Rheological Tests
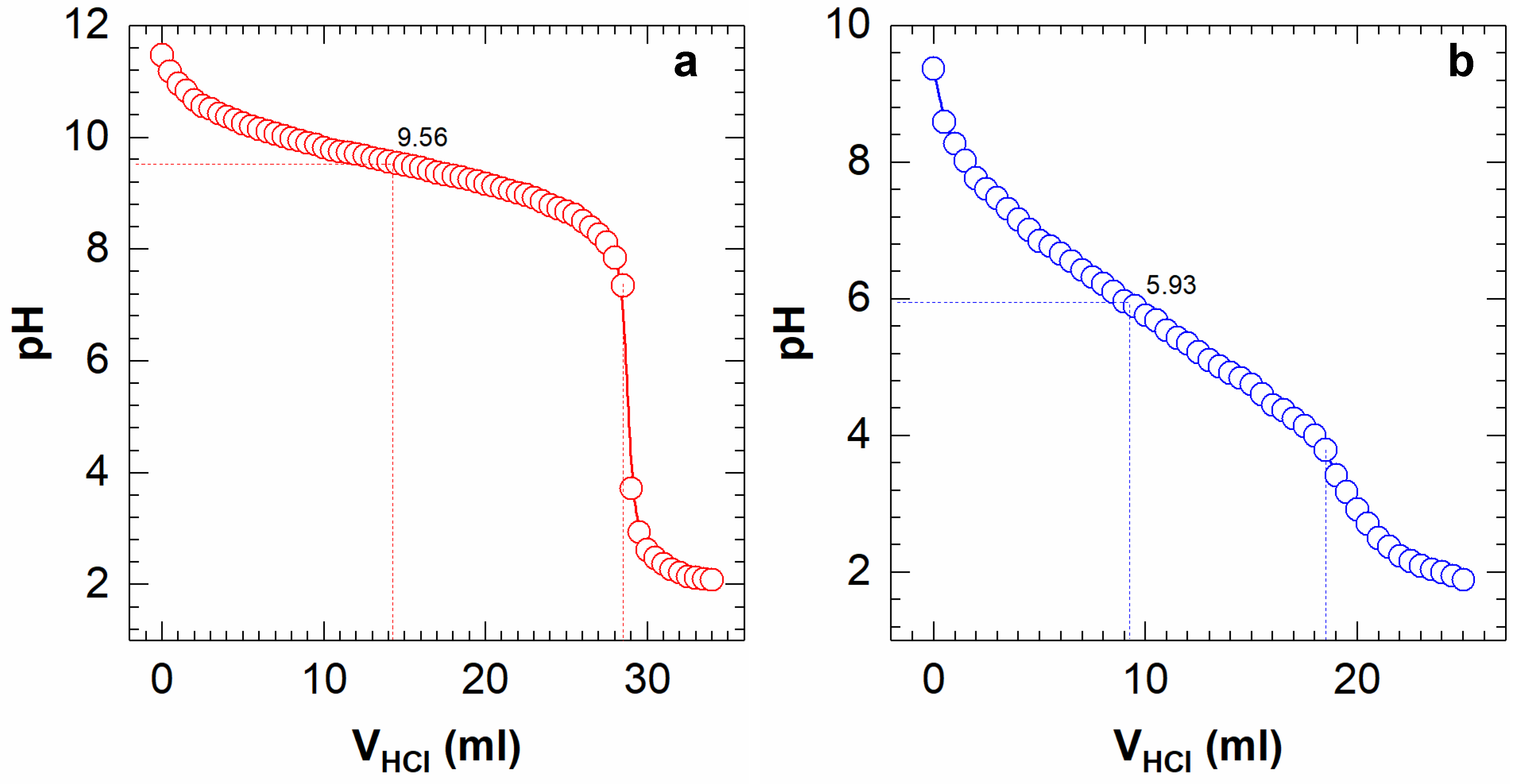
3.4. pKaH Determination
3.5. Conductivity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lin, F.; Liu, C.; Zhang, P. On hydrodynamics of viscoelastic fluids. Commun. Pur. Appl. Math. 2005, 58, 1437–1471. [Google Scholar] [CrossRef]
- Denn, M.M. Fifty years of non-Newtonian fluid dynamics. AIChE J. 2004, 50, 2335–2345. [Google Scholar] [CrossRef]
- Zhou, J.; Papautsky, I. Viscoelastic microfluidics: Progress and challanges. Microsyst. Nanoeng. 2020, 6, 113. [Google Scholar] [CrossRef]
- Tripathi, D.; Pandey, S.K.; Bég, O.A. Mathematical modelling of heat transfer effects on swallowing dynamics of viscoelastic food bolus through the human oesophagus. Int. J. Therm. Sci. 2013, 70, 41–53. [Google Scholar] [CrossRef]
- Luo, X.L. Numerical simulation of Weissenberg phenomena—the rod-climbing of viscoelastic fluids. Comput. Methods Appl. Mech. Eng. 1999, 180, 393–412. [Google Scholar] [CrossRef]
- Plohr, B.J. Instabilities in shear flow of viscoelastic fluids with fading memory. Lect. Notes Phys. 1989, 344, 113–127. [Google Scholar]
- Ashrafi, N.; Eskafi, M.R. Application of Viscoelastic Fluids in Industrial Dampers. In Proceedings of the IMECE2011, Denver, CO, USA, 11–17 November 2011. [Google Scholar]
- Leung, L.H.; Naguib, H.E. Viscoelastic properties of poly(ε-caprolactone)—Hydroxyapatite micro- and nano-composites. Polym. Adv. Technol. 2013, 24, 144–150. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.; Zhang, S.; Feng, Y. Smart thermoviscosifying polymer for improving drag reduction in slick-water hydrofracking. Fuel 2020, 278, 118408. [Google Scholar] [CrossRef]
- Li, S.; Braun, O.; Lauber, L.; Leblanc, T.; Su, X.; Feng, Y. Enhancing oil recovery from high–temperature and high-salinity reservoirs with smart thermoviscosifying polymers: A laboratory study. Fuel 2021, 288, 119777. [Google Scholar] [CrossRef]
- Wang, M.; Sun, G.; Han, P.; Su, X.; Feng, Y. Thermoviscosifying polymers based on polyether prepared from inverse emulsion polymerization. J. Appl. Polym. Sci. 2018, 135, 46696. [Google Scholar] [CrossRef]
- Su, X.; Feng, Y. Thermoviscosifying Smart Polymers for Oil and Gas Production: State of the Art. Chemphyschem 2018, 19, 1941–1955. [Google Scholar] [CrossRef]
- Tamer, Y.; Yıldırım, H. Biodegradable and stimuli sensitive amphiphilic graft copolymers and their sol-gel phase transition behavior. Polym. Adv. Technol. 2015, 26, 399–407. [Google Scholar] [CrossRef]
- Daniel, C.; Alejandro, S.; Ram, M.; Revital, Z.; Shai, G.; Levy, A. Chain extension as a strategy for the development of improved reverse thermo-responsive polymers. Polym. Adv. Technol. 2007, 18, 731–736. [Google Scholar]
- Takeuchi, Y.; Tsujimoto, T.; Uyama, H. Thermogelation of amphiphilic poly(asparagine) derivatives. Polym. Adv. Technol. 2011, 22, 620–626. [Google Scholar] [CrossRef]
- Pilipenko, I.M.; Korzhikov-Vlakh, V.A.; Zakharova, N.V.; Urtti, A.; Tennikova, T.B. Thermo- and pH-sensitive glycosaminoglycans derivatives obtained by controlled grafting of poly(N-isopropylacrylamide). Carbohydr. Polym. 2020, 248, 116764. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Liu, X. pH-responsive viscoelastic fluid formed by cleavable sodium hexadecyl phthalate monoester alone. J. Mol. Liq. 2020, 313, 113514. [Google Scholar] [CrossRef]
- Kelly, E.A.; Willis-Fox, N.; Houston, J.E.; Blayo, C.; Divitini, G.; Cowieson, N.; Daly, R.; Evans, R.C. A single-component photorheological fluid with light-responsive viscosity. Nanoscale 2020, 12, 6300–6306. [Google Scholar]
- Hao, X.; Leng, Z.; Wang, H.; Peng, F.; Yan, Q. CO2-switchable non-Newtonian fluids. Green Chem. 2020, 22, 3784–3790. [Google Scholar] [CrossRef]
- Yin, H.; Feng, Y.; Liu, H.; Mu, M.; Fei, C. Insights into the relationship between CO2 switchability and basicity: Examples of melamine and its derivatives. Langmuir 2014, 30, 9911–9919. [Google Scholar] [CrossRef]
- Darabi, A.; Jessop, P.G.; Cunningham, M.F. CO2-responsive polymeric materials: Synthesis, self-assembly, and functional applications. Chem Soc. Rev. 2016, 45, 4391–4436. [Google Scholar] [CrossRef]
- Su, X.; Robert, T.; Mercer, S.M.; Humphries, C.; Cunningham, M.F.; Jessop, P.G. A Conventional Surfactant Becomes CO2-Responsive in the Presence of Switchable Water Additives. Chem. Eur. J. 2013, 19, 5595–5601. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Wang, Y.; Li, X. CO2-switchable viscoelastic fluids based on a pseudogemini surfactant. Langmuir 2013, 29, 4187–4192. [Google Scholar] [CrossRef]
- Lu, H.; Jiang, J.; Huang, Z.; Dai, S. A water-soluble CO2-triggered viscosity-responsive copolymer of N,N-dimethylaminoethyl methacrylate and acrylamide. J. Appl. Polym. Sci. 2014, 131, 40872. [Google Scholar] [CrossRef]
- Su, X.; Cunningham, M.F.; Jessop, P.G. Use of a switchable hydrophobic associative polymer to create an aqueous solution of CO2-switchable viscosity. Polym. Chem. 2014, 5, 940–944. [Google Scholar] [CrossRef]
- Fujita, H.; Mitsuhashi, K.; Homma, T. Viscosities of sodium polyacrylate in aqueous sodium chloride. J. Colloid Sci. 1954, 9, 466–478. [Google Scholar] [CrossRef]
- Huber, K. Calcium-induced shrinking of polyacrylate chains in aqueous solution. J. Phys. Chem. 1993, 97, 9825–9830. [Google Scholar] [CrossRef]
- Jessop, P.G.; Mercer, S.M.; Heldebrant, D.J. CO2-triggered switchable solvents, surfactants, and other materials. Energy Environ. Sci. 2012, 5, 7240–7253. [Google Scholar] [CrossRef]
- Robert, T.; Mercer, S.M.; Clark, T.J.; Mariampillai, B.E.; Champagne, P.; Cunningham, M.F.; Jessop, P.G. Nitrogen-containing polymers as potent ionogens for aqueous solutions of switchable ionic strength: Application to separation of organic liquids and clay particles from water. Green Chem. 2012, 14, 3053. [Google Scholar] [CrossRef]
- Mercer, S.M.; Robert, T.; Dixon, D.V.; Chen, C.; Ghoshouni, Z.; Harjani, J.R.; Jahangiri, S.; Peslherbe, G.H.; Jessop, P.G. Design, synthesis, and solution behaviour of small polyamines as switchable water additives. Green Chem. 2012, 14, 832–839. [Google Scholar] [CrossRef]
- Zhu, R.; Feng, Y.; Luo, P. Net Contribution of Hydrophobic Association to the Thickening Power of Hydrophobically Modified Polyelectrolytes Prepared by Micellar Polymerization. Macromolecules 2020, 53, 1326–1337. [Google Scholar] [CrossRef]
- Jimenez, L.N.; Dinic, J.; Parsi, N.; Sharma, V. Extensional Relaxation Time, Pinch-Off Dynamics, and Printability of Semidilute Polyelectrolyte Solutions. Macromolecules 2018, 51, 5191–5208. [Google Scholar] [CrossRef]
- Dobrynin, A.; Rubinstein, M. Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci. 2005, 30, 1049–1118. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Colby, R.H.; Rubinstein, M. Scaling Theory of Polyelectrolyte Solutions. Macromolecules 1995, 28, 1859–1871. [Google Scholar] [CrossRef]
- Volpert, E.; Selb, J.; Candau, F. Associating behaviour of polyacrylamides hydrophobically modified with dihexylacrylamide. Polymer 1998, 39, 1025–1033. [Google Scholar] [CrossRef]
- Valeria, C.; Hamley, I.W.; Xue, W.; Sommer, C.; Pedersen, J.S.; Olmsted, P.D. Rheological and Structural Characterization of Hydrophobically Modified Polyacrylamide Solutions in the Semidilute Regime. Macromolecules 2004, 37, 1492–1501. [Google Scholar]
- Wang, J.; Liu, D.; Huang, Z.; Zheng, C. CO2 responsive wormlike micelles based on sodium oleate, potassium chloride and N,N-dimethylethanolamine. J. Disper. Sci. Technol. 2018, 39, 1606–1612. [Google Scholar] [CrossRef]
- Yan, B.; Han, D.; Boissière, O.; Ayotte, P.; Zhao, Y. Manipulation of block copolymer vesicles using CO2: Dissociation or “breathing”. Soft. Matter. 2013, 9, 2011–2016. [Google Scholar] [CrossRef]
- Ko, Y.G.; Shin, S.S.; Choi, U.S. Primary, secondary, and tertiary amines for CO2 capture: Designing for mesoporous CO2 adsorbents. J. Colloid Interface Sci. 2011, 361, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, T.L.; Nguyen, Y.N. Carbon dioxide reaction kinetics and transport in aqueous amine membranes. Ind. Eng. Chem. Fundam. 1980, 19, 260–266. [Google Scholar] [CrossRef]
- Cao, F.; Gao, H.; Li, H.; Liang, Z. Experimental and Theoretical Studies on Mass Transfer Performance for CO2 Absorption into Aqueous N,N-Dimethylethanolamine Solution in the Polytetrafluoroethylene Hollow-Fiber Membrane Contactor. Ind. Eng. Chem. Res. 2018, 57, 16862–16874. [Google Scholar] [CrossRef]
- Cao, F.; Gao, H.; Xiong, Q.; Liang, Z. Experimental studies on mass transfer performance for CO2 absorption into aqueous N,N-dimethylethanolamine (DMEA) based solutions in a PTFE hollow fiber membrane contactor. Int. J. Greenh. Gas. Con. 2019, 82, 210–217. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Shi, Y.; Lv, K.; Wei, B.; Zhu, Y.; Yin, H.; Feng, Y. Tunable Viscoelastic Properties of Sodium Polyacrylate Solution via CO2-Responsive Switchable Water. Molecules 2021, 26, 3840. https://doi.org/10.3390/molecules26133840
Wu D, Shi Y, Lv K, Wei B, Zhu Y, Yin H, Feng Y. Tunable Viscoelastic Properties of Sodium Polyacrylate Solution via CO2-Responsive Switchable Water. Molecules. 2021; 26(13):3840. https://doi.org/10.3390/molecules26133840
Chicago/Turabian StyleWu, Dianguo, Yiwen Shi, Kun Lv, Bing Wei, Youyi Zhu, Hongyao Yin, and Yujun Feng. 2021. "Tunable Viscoelastic Properties of Sodium Polyacrylate Solution via CO2-Responsive Switchable Water" Molecules 26, no. 13: 3840. https://doi.org/10.3390/molecules26133840
APA StyleWu, D., Shi, Y., Lv, K., Wei, B., Zhu, Y., Yin, H., & Feng, Y. (2021). Tunable Viscoelastic Properties of Sodium Polyacrylate Solution via CO2-Responsive Switchable Water. Molecules, 26(13), 3840. https://doi.org/10.3390/molecules26133840







