Cytoprotection against Oxidative Stress by Methylnissolin-3-O-?-d-glucopyranoside from Astragalus membranaceus Mainly via the Activation of the Nrf2/HO-1 Pathway
Abstract
:1. Introduction
2. Results
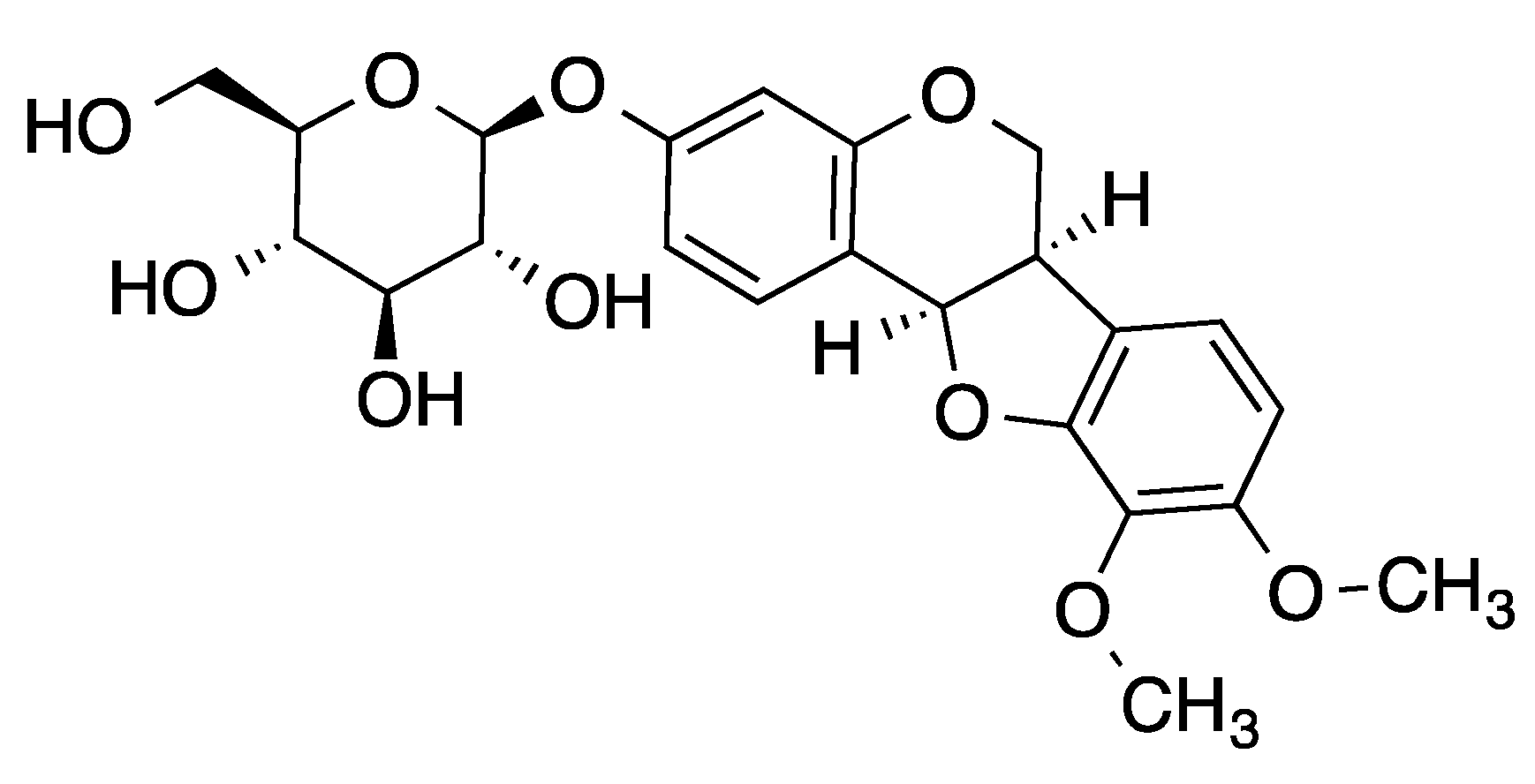
2.1. Identification of MNG as an Nrf2 Activator and Its Cytotoxicity on EA.hy926 Cells
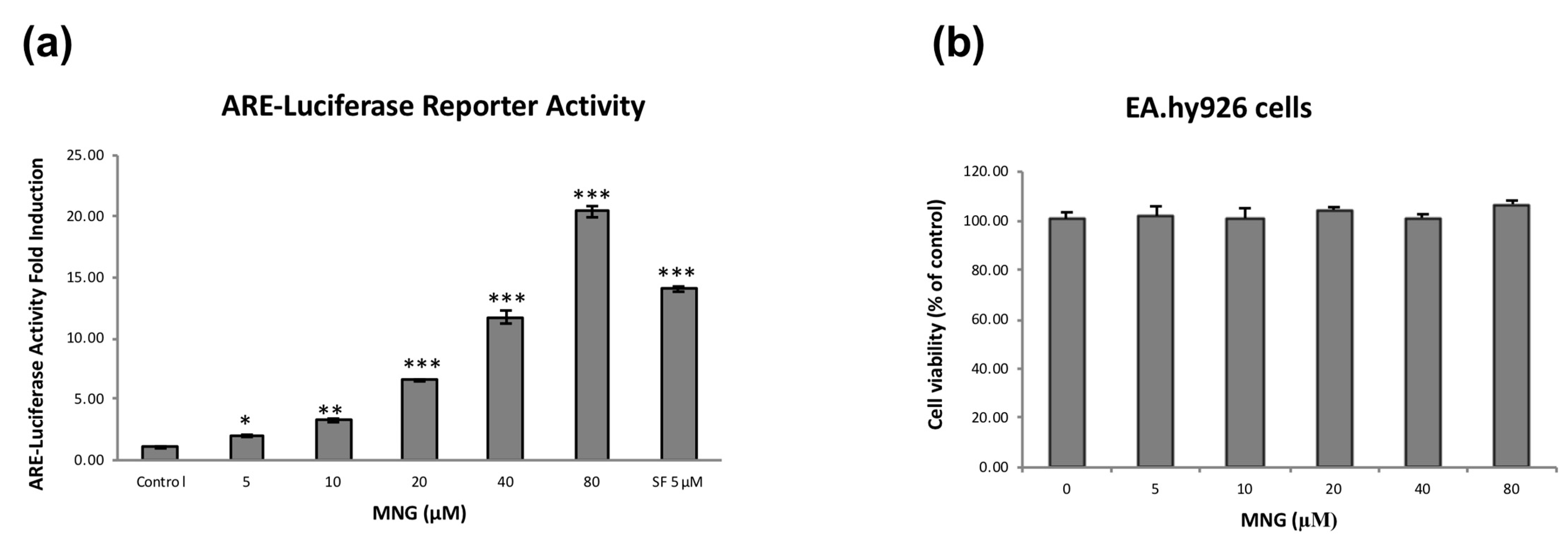
2.2. MNG Clearly Increased Expression of Nrf2 and Its Target Genes in EA.hy926 Cells
2.3. MNG Effectively Induced Nrf2 Nuclear Translocation in EA.hy926 Cells
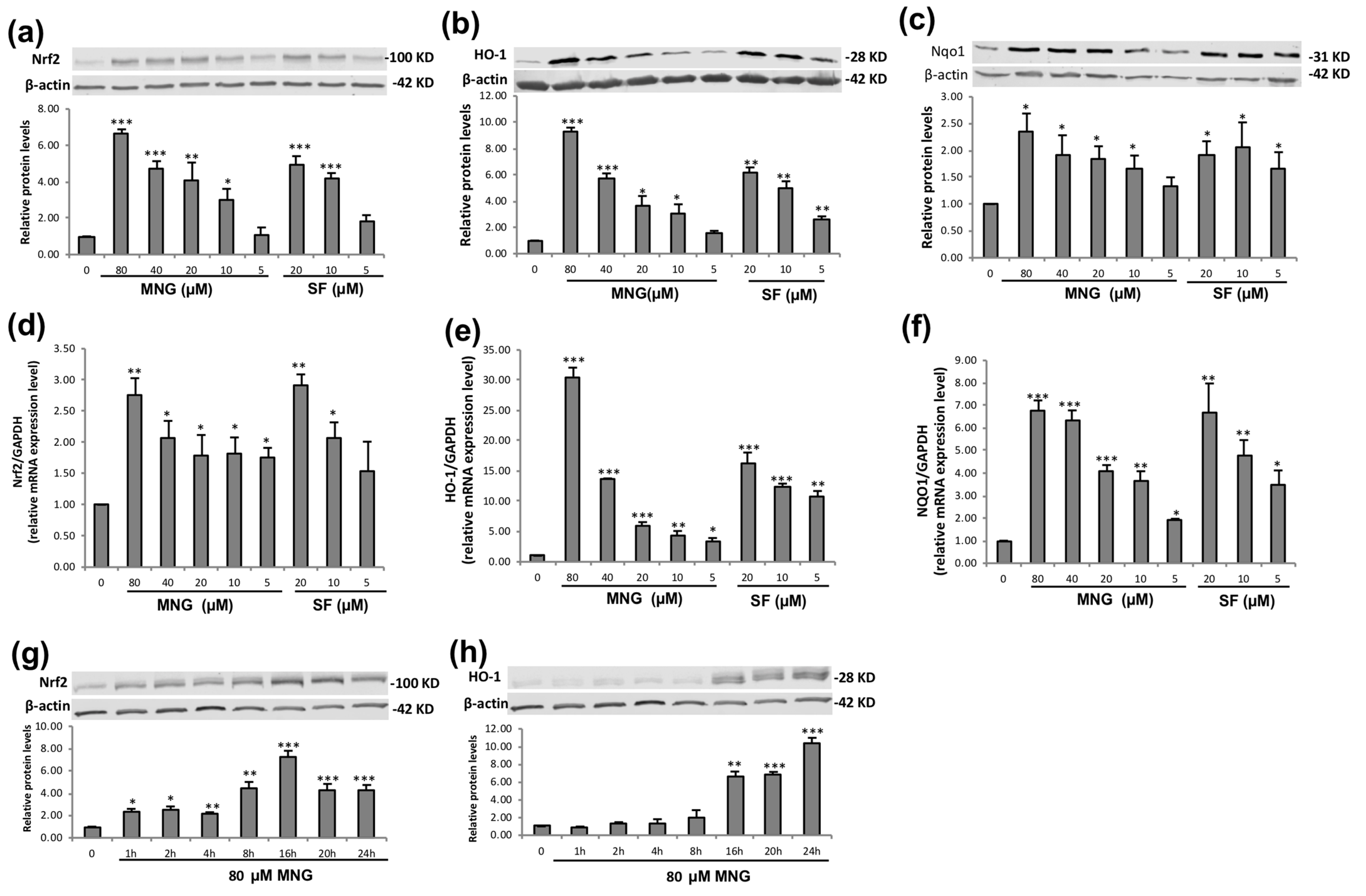
2.4. MNG Protected EA.hy926 Cells against H2O2-Induced Cell Death
2.5. Inhibition of ROS Production by MNG
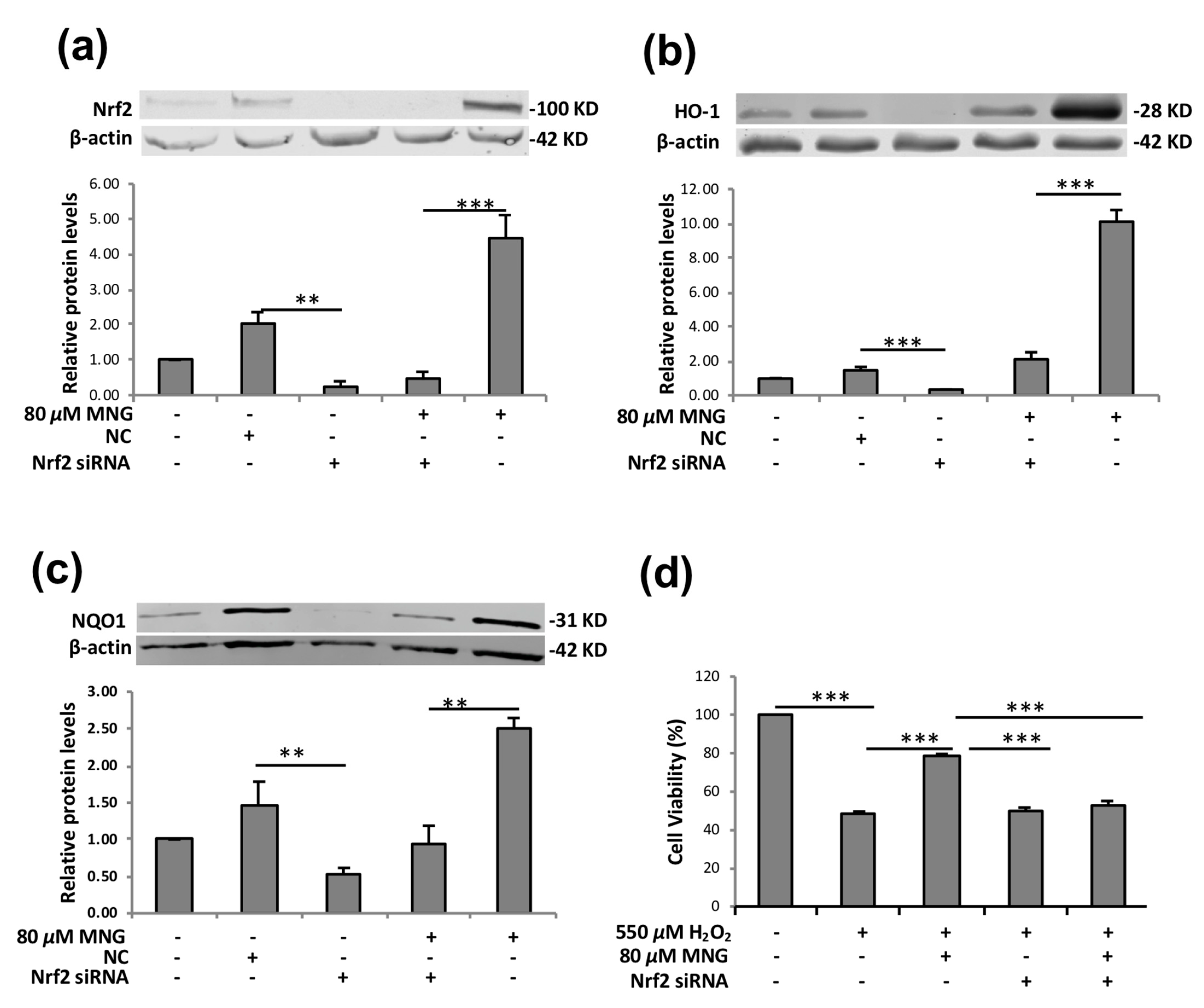
2.6. Nrf2 siRNA Attenuated the MNG-Mediated Cytoprotective Effect and Induction of HO-1 and NQO1
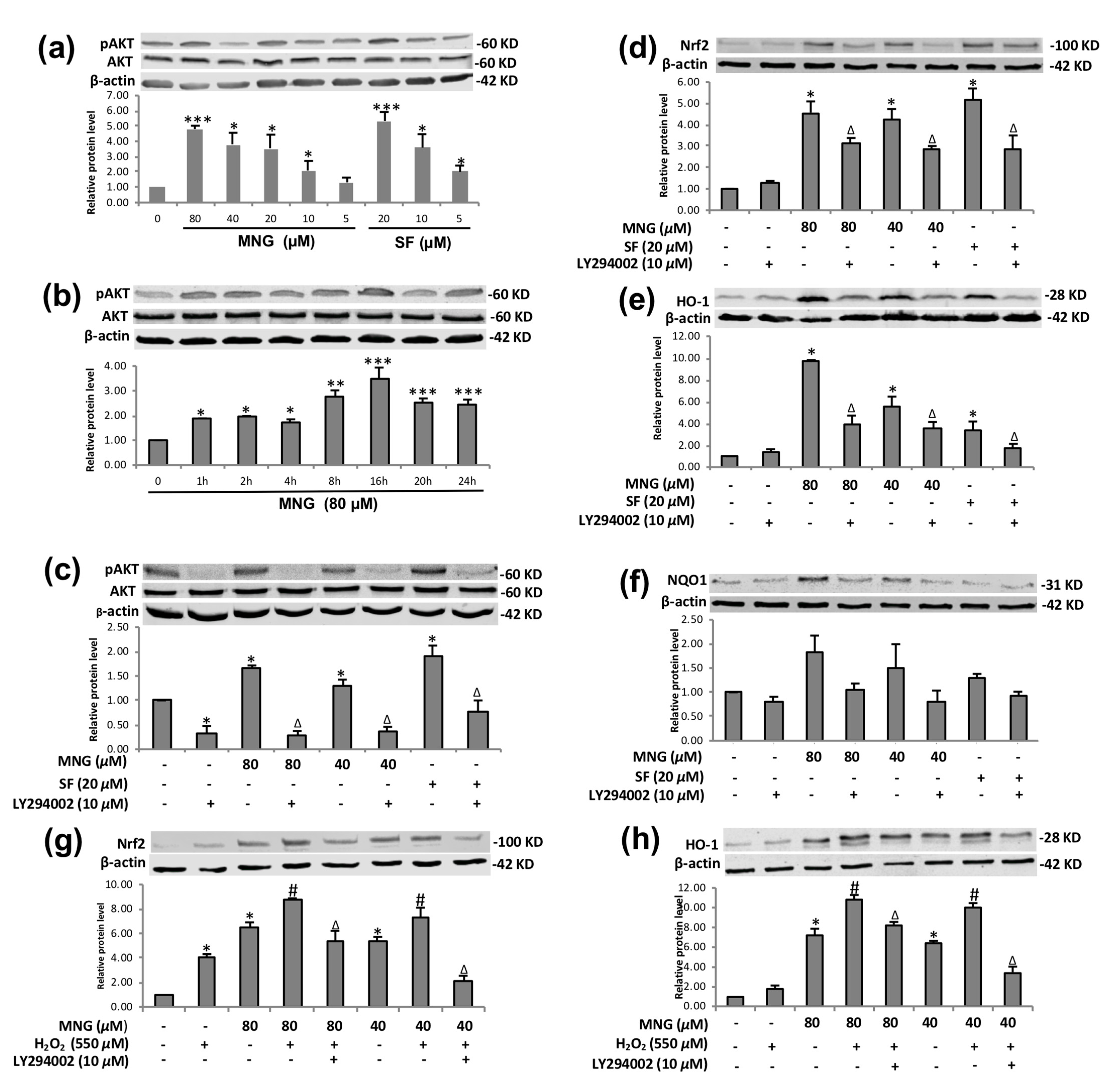
2.7. PI3K/AKT Regulated MNG-Induced Nrf2/HO-1 Expression
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Chemicals
4.3. Cell Culture
4.4. Luciferase Reporter Gene Assay
4.5. Cell Viability Assay
4.6. Hoechst 33342/PI Fluorescent Staining
4.7. ROS Detection
4.8. Isolation of Nuclear and Cytoplasmic Extract
4.9. Western Blot Analysis
4.10. Real-Time PCR Analysis
4.11. Immunofluorescence Analysis
4.12. RNA Interference of Nrf2
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations and Nomenclature
References
- Li, R.; Jia, Z.; Trush, M.A. Defining ROS in Biology and Medicine. React. Oxyg. Species 2016, 1, 9–21. [Google Scholar]
- Zhai, K.-F.; Duan, H.; Chen, Y.; Khan, G.J.; Cao, W.-G.; Gao, G.-Z.; Shan, L.-L.; Wei, Z.-J. Apoptosis effects of imperatorin on synoviocytes in rheumatoid arthritis through mitochondrial/caspase-mediated pathways. Food Funct. 2018, 9, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef] [Green Version]
- Zhai, K.-F.; Duan, H.; Khan, G.J.; Xu, H.; Han, F.-K.; Cao, W.-G.; Gao, G.-Z.; Shan, L.-L.; Wei, Z.-J. Salicin from Alangium chinense Ameliorates Rheumatoid Arthritis by Modulating the Nrf2-HO-1-ROS Pathways. J. Agric. Food Chem. 2018, 66, 6073–6082. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Suzuki, T.; Motohashi, H.; Yamamoto, M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol. Sci. 2013, 34, 340–346. [Google Scholar] [CrossRef]
- Itoh, K.; Mimura, J.; Yamamoto, M. Discovery of the negative regulator of Nrf2, Keap1: A historical overview. Antioxid. Redox Signal. 2010, 13, 1665–1678. [Google Scholar] [CrossRef]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003, 278, 21592–21600. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yu, S.; Liu, T.; Kim, J.-H.; Blank, V.; Li, H.; Kong, A.-N.T. Heterodimerization with Small Maf Proteins Enhances Nuclear Retention of Nrf2 via Masking the NESzip Motif. Biochim. Biophys. Acta. 2008, 1783, 1847–1856. [Google Scholar] [CrossRef] [Green Version]
- Burgering, B.M.T.; Kops, G.J.P.L. Cell Cycle and Death Control: Long Live Forkheads. Trends Biochem. Sci. 2002, 27, 352–360. [Google Scholar] [CrossRef]
- Li, H.; Tang, Z.; Chu, P.; Song, Y.; Yang, Y.; Sun, B.; Niu, M.; Qaed, E.; Shopit, A.; Han, G.; et al. Neuroprotective Effect of Phosphocreatine on Oxidative Stress and Mitochondrial Dysfunction Induced Apoptosis in Vitro and in Vivo: Involvement of Dual PI3K/Akt and Nrf2/HO-1 Pathways. Free Radical Biol. Med. 2018, 120, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xu, J.; Chen, M.; Wang, D.; Yang, Y.; Manavalan, A.; Wu, X.; Liu, Y.; Cao, S. Compound Analysis of Jing Liqueur and Nrf2 Activation by Jing Liqueur–One of the Most Popular Beverages in China. Beverages 2020, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.-B.; Du, X.-M.; Jian, L.-Y. Studies on purification process of total saponins in Radix Astragali with resin and structural identification of compounds. Asian J. Chem. 2014, 26, 4610–4614. [Google Scholar] [CrossRef]
- Auyeung, K.K.; Han, Q.-B.; Ko, J.K. Astragalus membranaceus: A Review of its Protection Against Inflammation and Gastrointestinal Cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef]
- Yu, D.; Duan, Y.; Bao, Y.; Wei, C.; An, L. Isoflavonoids from Astragalus mongholicus protect PC12 cells from toxicity induced by L-glutamate. J. Ethnopharmacol. 2005, 98, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, F.; Zhang, X.; Li, P.; Zhang, X.; Wu, Z.; Li, D. In vitro synergistic antioxidant activity and identification of antioxidant components from Astragalus membranaceus and Paeonia lactiflora. PLoS ONE 2014, 9, e96780. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1- NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The Role of Hydrogen Peroxide in Redox-Dependent Signaling: Homeostatic and Pathological Responses in Mammalian Cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, Y.; Wang, Y.; Fu, A.; Gong, L.; Li, W.; Li, Y. Bacillus amyloliquefaciens SC06 Alleviates the Oxidative Stress of IPEC-1 via Modulating Nrf2/Keap1 Signaling Pathway and Decreasing ROS Production. Appl. Microbiol. Biotechnol. 2017, 101, 3015–3026. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.-F.; Duan, H.; Cui, C.-Y.; Cao, Y.-Y.; Si, J.-L.; Yang, H.-J.; Wang, Y.-C.; Cao, W.-G.; Gao, G.-Z.; Wei, Z.-J. Liquiritin from Glycyrrhiza uralensis Attenuating Rheumatoid Arthritis via Reducing Inflammation, Suppressing Angiogenesis, and Inhibiting MAPK Signaling Pathway. J. Agric. Food Chem. 2019, 67, 2856–2864. [Google Scholar] [CrossRef] [PubMed]
- Tabei, Y.; Murotomi, K.; Umeno, A.; Horie, M.; Tsujino, Y.; Masutani, B.; Yoshida, Y.; Nakajima, Y. Antioxidant Properties of 5- Hydroxy-4-Phenyl-Butenolide via Activation of Nrf2/ARE Signaling Pathway. Food Chem. Toxicol. 2017, 107, 129–137. [Google Scholar] [CrossRef]
- Hou, D.-X.; Korenori, Y.; Tanigawa, S.; Yamada-Kato, T.; Nagai, M.; He, X.; He, J. Dynamics of Nrf2 and Keap1 in ARE-Mediated NQO1 Expression by Wasabi 6-(Methylsulfinyl)hexyl Isothiocyanate. J. Agric. Food Chem. 2011, 59, 11975–11982. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Y.; Sternberg, P.; Cai, J. Essential Roles of the PI3 Kinase/Akt Pathway in Regulating Nrf2-Dependent Antioxidant Functions in the RPE. Invest. Ophthalmol. Vis. Sci. 2008, 49, 1671–1678. [Google Scholar] [CrossRef] [Green Version]
- Zou, W.; Chen, C.; Zhong, Y.; An, J.; Zhang, X.; Yu, Y.; Yu, Z.; Fu, J. PI3K/Akt Pathway Mediates Nrf2/ARE Activation in Human L02 Hepatocytes Exposed to Low-Concentration HBCDs. Environ. Sci. Technol. 2013, 47, 12434–12440. [Google Scholar] [CrossRef]
- Han, D.; Chen, W.; Gu, X.; Shan, R.; Zou, J.; Liu, G.; Shahid, M.; Gao, J.; Han, B. Cytoprotective Effect of Chlorogenic Acid against Hydrogen Peroxide-Induced Oxidative Stress in MC3T3-E1 Cells through PI3K/Akt-Mediated Nrf2/HO-1 Signaling Pathway. Oncotarget 2017, 8, 14680–14692. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Sun, Y.N.; Yan, X.T.; Yang, S.Y.; Kim, S.; Lee, Y.M.; Koh, Y.-S.; Kim, Y.H. Flavonoids from Astragalus membranaceus and their inhibitory effects on LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Arch. Pharm. Res. 2014, 37, 186–192. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W. Anti-Aging Pharmaceutical a Composition. CN Patent 104,523,733, 22 April 2015. [Google Scholar]
- Zhu, M.; Li, J.; Wang, K.; Hao, X.; Ge, R.; Li, Q. Isoquercitrin Inhibits Hydrogen Peroxide-Induced Apoptosis of EA.hy926 Cells via the PI3K/Akt/GSK3β Signaling Pathway. Molecules 2016, 21, 356. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, L.J.M.; Kuijper, I.A.; Schimming, J.P.; Wolters, L.; Ter Braak, B.; Langenberg, J.P.; Noort, D.; Beltman, J.B.; van de Water, B. A systematic analysis of Nrf2 pathway activation dynamics during repeated xenobiotic exposure. Arch. Toxicol. 2019, 93, 435–451. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, T.C.; Lu, X.; Wang, Z.; Wu, J.M. Induction of quinone reductase NQO1 by resveratrol in human K562 cells involves the antioxidant response element ARE and is accompanied by nuclear translocation of transcription factor Nrf2. Med. Chem. 2006, 2, 275–285. [Google Scholar] [CrossRef]
- Li, B.; Tian, S.; Liu, X.; He, C.; Ding, Z.; Shan, Y. Sulforaphane protected the injury of human vascular endothelial cell induced by LPC through up-regulating endogenous antioxidants and phase II enzymes. Food Funct. 2015, 6, 1984–1991. [Google Scholar] [CrossRef]
- Yang, G.; Chang, C.C.; Yang, Y.; Yuan, L.; Xu, L.; Ho, C.T.; Li, S. Resveratrol Alleviates Rheumatoid Arthritis via Reducing ROS and Inflammation, Inhibiting MAPK Signaling Pathways, and Suppressing Angiogenesis. J. Agric. Food Chem. 2018, 66, 12953–12960. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.-R.; Chen, J.; Chen, Y.-J.; Wang, Z.-X.; Geng, M.; Xu, D.-C.; Wang, Z.-Y.; Li, J.-H.; Xu, Z.-D.; et al. Pterostilbene Attenuates Experimental Atherosclerosis through Restoring Catalase-Mediated Redox Balance in Vascular Smooth Muscle Cells. J. Agric. Food Chem. 2019, 67, 12752–12760. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, H.; Duan, Y.; Chen, G. Protective Effects of Radish (Raphanus sativus L.) Leaves Extract against Hydrogen Peroxide-Induced Oxidative Damage in Human Fetal Lung Fibroblast (MRC-5). Cells. Biomed. Pharmacother. 2018, 103, 406–414. [Google Scholar] [CrossRef]
- Mateés, J.M. Effects of Antioxidant Enzymes in the Molecular Control of Reactive Oxygen Species Toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Kenessey, A.; Kaie Ojamaa, K. Thyroid hormone stimulates protein synthesis in the cardiomyocyte by activating the Akt-mTOR and p70S6K pathways. J. Biol. Chem. 2006, 281, 20666–20672. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Ye, P.; Li, H.; Wang, Y.; Xu, Y.; Tian, Q.; Lei, G.; Zhao, C.; Gao, Z.; Zhao, W.; et al. Lunasin attenuates oxidant-induced endothelial injury and inhibits atherosclerotic plaque progression in ApoE −/− mice by up-regulating heme oxygenase-1 via PI3K/Akt/Nrf2/ARE pathway. FASEB J. 2019, 33, 4836–4850. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, B.B.; Han, L.; Gao, C.F.; Wang, M. D-Fagomine attenuates high glucose-induced endothelial cell oxidative damage by upregulating the expression of PGC-1α. J. Agric. Food Chem. 2018, 66, 2758–2764. [Google Scholar] [CrossRef]
- Hou, Y.; Li, X.; Peng, S.; Yao, J.; Bai, F.; Fang, J. Lipoamide Ameliorates Oxidative Stress via Induction of Nrf2/ARE Signaling Pathway in PC12 Cells. J. Agric. Food Chem. 2019, 67, 8227–8234. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; Li, D.; Jaminet, S.C.; Cao, S. Activation of the Nrf2 Cell Defense Pathway by Ancient Foods: Disease Prevention by Important Molecules and Microbes Lost from the Modern Western Diet. PLoS ONE 2016, 11, e0148042. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, X.; Miao, J.; Yuan, H.; Liu, E.; Liang, T.; Li, Q. Farrerol Directly Targets GSK-3β to Activate Nrf2-ARE Pathway and Protect EA.hy926 Cells against Oxidative Stress-Induced Injuries. Oxid. Med. Cell. Longev. 2020, 2020, 5967434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.F.; Shen, X.Y.; Lio, C.K.; Dai, Y.; Cheng, C.S.; Liu, J.X.; Yao, Y.D.; Yu, Y.; Xie, Y.; Luo, P.; et al. Activation of Nrf2/HO-1 Pathway by Nardochinoid C Inhibits Inflammation and Oxidative Stress in Lipopolysaccharide-Stimulated Macrophages. Front. Pharmacol. 2018, 9, 911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, H.; Li, X.; Jia, B.; Yang, Y.; Zhou, P.; Li, P.; Chen, J. Miltirone protects human EA.hy926 endothelial cells from oxidized low-density lipoprotein-derived oxidative stress via a heme oxygenase-1 and MAPK/Nrf2 dependent pathway. Phytomedicine 2016, 23, 1806–1813. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Xu, J.; Cai, Y.; Yang, Y.; Liu, Y.; Cao, S. Cytoprotection against Oxidative Stress by Methylnissolin-3-O-?-d-glucopyranoside from Astragalus membranaceus Mainly via the Activation of the Nrf2/HO-1 Pathway. Molecules 2021, 26, 3852. https://doi.org/10.3390/molecules26133852
Wu X, Xu J, Cai Y, Yang Y, Liu Y, Cao S. Cytoprotection against Oxidative Stress by Methylnissolin-3-O-?-d-glucopyranoside from Astragalus membranaceus Mainly via the Activation of the Nrf2/HO-1 Pathway. Molecules. 2021; 26(13):3852. https://doi.org/10.3390/molecules26133852
Chicago/Turabian StyleWu, Xiaohua, Jian Xu, Yousheng Cai, Yuejun Yang, Yuancai Liu, and Shugeng Cao. 2021. "Cytoprotection against Oxidative Stress by Methylnissolin-3-O-?-d-glucopyranoside from Astragalus membranaceus Mainly via the Activation of the Nrf2/HO-1 Pathway" Molecules 26, no. 13: 3852. https://doi.org/10.3390/molecules26133852
APA StyleWu, X., Xu, J., Cai, Y., Yang, Y., Liu, Y., & Cao, S. (2021). Cytoprotection against Oxidative Stress by Methylnissolin-3-O-?-d-glucopyranoside from Astragalus membranaceus Mainly via the Activation of the Nrf2/HO-1 Pathway. Molecules, 26(13), 3852. https://doi.org/10.3390/molecules26133852






