Pros and Cons of the Cannabinoid System in Cancer: Focus on Hematological Malignancies
Abstract
:1. Introduction
2. Adverse Effects of Marijuana Intake: CBs and Solid Cancers
3. CBs, Tumors, and Treatment-Related Symptoms
4. Anticancer Mechanisms and Carcinogenic Actions of Cannabinoids
5. CBs and Hematological Malignancies
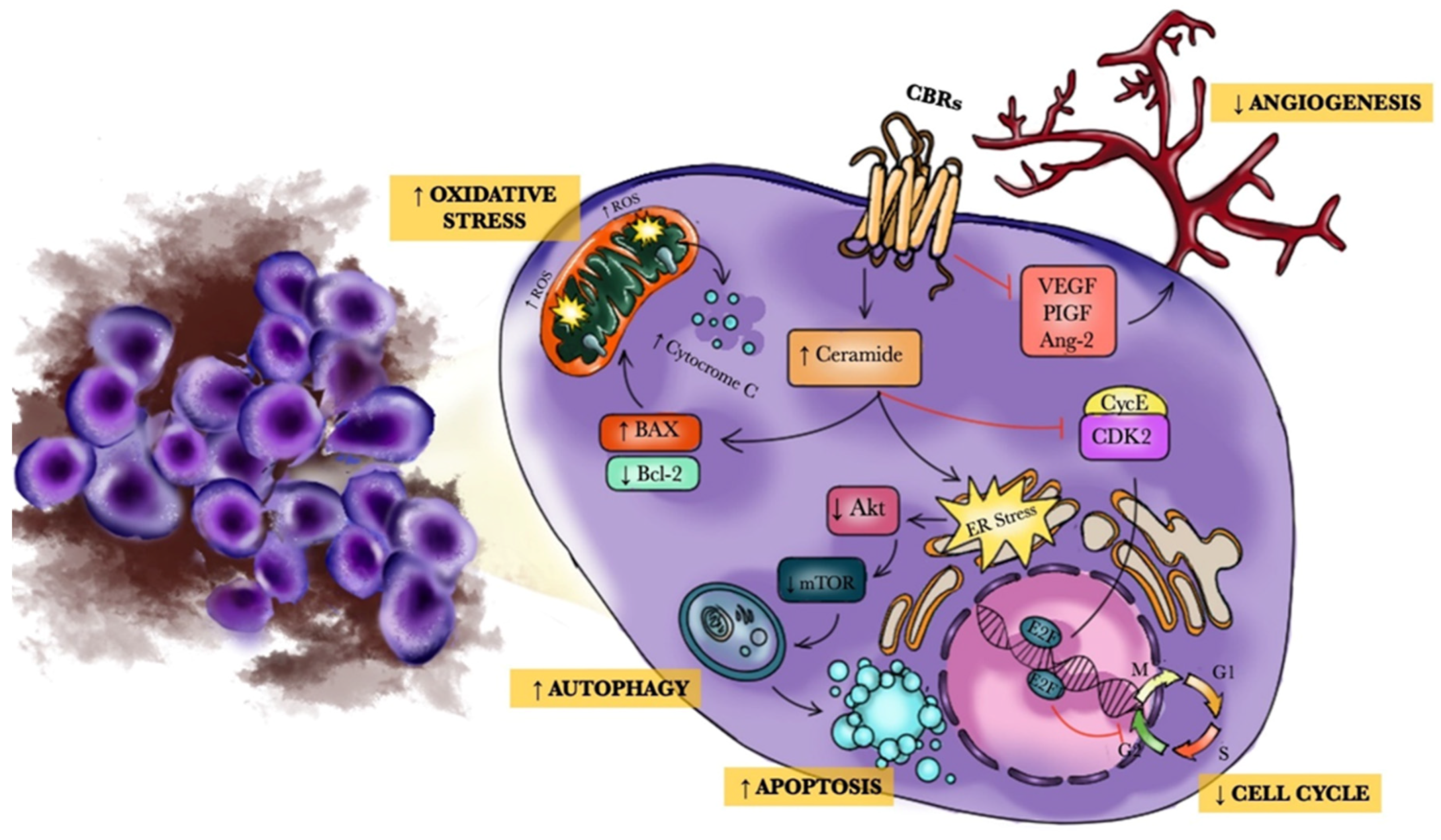
5.1. Preclinical Studies
5.2. Clinical Studies
6. CBs and Bone Marrow Transplantation
7. Future Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- National Institute on Drug Abuse. Marijuana. Revised February 2016. Available online: https://www.drugabuse.gov/drugs-abuse/marijuana (accessed on 16 June 2016).
- American Society of Addiction Medicine. Marijuana Use Fact Sheet. Available online: http://www.asam.org/docs/default-source/default-document-library/marijuana-use-fact-sheet.pdf?sfvrsn=0 (accessed on 16 June 2016).
- Pertwee, R.G.; Howlett, A.; Abood, M.E.; Alexander, S.P.H.; Di Marzo, V.; Elphick, M.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid Receptors and Their Ligands: Beyond CB1and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [Green Version]
- Thomas, B.F.; ElSohly, M.A. Biosynthesis and Pharmacology of Phytocannabinoids and Related Chemical Constituents. In The Analytical Chemistry of Cannabis; Elsevier: Amsterdam, The Netherlands, 2016; pp. 27–41. [Google Scholar]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Fraguas-Sánchez, A.I.; Martín-Sabroso, C.; Torres-Suárez, A.I. Insights into the effects of the endocannabinoid system in cancer: A review. Br. J. Pharmacol. 2018, 175, 2566–2580. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, K.T.; Bronstein, A.C.; Newquist, K.L. Marijuana Poisoning. Top. Companion Anim. Med. 2013, 28, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Arturo, I.F.; Fabiana, P. Endocannabinoidome. eLS 2018, 5, 1–10. [Google Scholar] [CrossRef]
- Ramer, R.; Schwarz, R.; Hinz, B. Modulation of the Endocannabinoid System as a Potential Anticancer Strategy. Front. Pharmacol. 2019, 10, 430. [Google Scholar] [CrossRef] [Green Version]
- Lauritsen, K.J.; Rosenberg, H. Comparison of outcome expectancies for synthetic cannabinoids and botanical marijuana. Am. J. Drug Alcohol Abus. 2016, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Weinstein, A. The Effects of Cannabinoids on Executive Functions: Evidence from Cannabis and Synthetic Canna-binoids—A Systematic Review. Brain Sci. 2018, 8, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almada, M.; Alves, P.; Fonseca, B.M.; Carvalho, F.; Queirós, C.R.; Gaspar, H.; Amaral, C.; Teixeira, N.; Correia-Da-Silva, G. Synthetic cannabinoids JWH-018, JWH-122, UR-144 and the phytocannabinoid THC activate apoptosis in placental cells. Toxicol. Lett. 2020, 319, 129–137. [Google Scholar] [CrossRef]
- Atwood, B.K.; Mackie, K. CB2: A cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 2010, 160, 467–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Hua, T.; Vemuri, K.; Ho, J.-H.; Wu, Y.; Wu, L.; Popov, P.; Benchama, O.; Zvonok, N.; Locke, K.; et al. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell 2019, 176, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nat. Cell Biol. 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002, 68-69, 619–631. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional ex-pression of the cloned cDNA. Nat. Cell Biol. 1990, 346, 561–564. [Google Scholar]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; A Stevenson, L.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Stella, N.; Schweitzer, P.J.; Piomelli, D. A second endogenous cannabinoid that modulates long-term potentiation. Nat. Cell Biol. 1997, 388, 773–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiura, T.; Kishimoto, S.; Oka, S.; Gokoh, M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog. Lipid Res. 2006, 45, 405–446. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.-O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- O’sullivan, S. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br. J. Pharmacol. 2007, 152, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Breijyeh, Z.; Jubeh, B.; Bufo, S.; Karaman, R.; Scrano, L. Cannabis: A Toxin-Producing Plant with Potential Therapeutic Uses. Toxins 2021, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Lange, P. Cannabis and the lung. Thorax 2007, 62, 1036–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrams, D. Integrating Cannabis into Clinical Cancer Care. Curr. Oncol. 2016, 23, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Howden, M.L.; Naughton, M.T. Pulmonary effects of marijuana inhalation. Expert Rev. Respir. Med. 2011, 5, 87–92. [Google Scholar] [CrossRef]
- Tan, W.C.; Lo, C.; Jong, A.; Xing, L.; Fitzgerald, M.J.; Vollmer, W.M.; Buist, S.A.; Sin, D.D.; for the Vancouver Burden of Obstructive Lung Disease (BOLD) Research Group. Marijuana and chronic obstructive lung disease: A population-based study. Can. Med. Assoc. J. 2009, 180, 814–820. [Google Scholar] [CrossRef] [Green Version]
- Feldman, A.L.; Sullivan, J.T.; Passero, M.A.; Lewis, D.C. Pneumothorax in polysubstance-abusing marijuana and tobacco smokers: Three cases. J. Subst. Abus. 1993, 5, 183–186. [Google Scholar] [CrossRef]
- Aldington, S.; Williams, M.; Nowitz, M.; Weatherall, M.; Pritchard, A.; McNaughton, A.; Robinson, G.; Beasley, R. Effects of cannabis on pulmonary structure, function and symptoms. Thorax 2007, 62, 1058–1063. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.K.; Smith, R.P.; Morrison, D.; Laszlo, G.; White, R.J. Large lung bullae in marijuana smokers. Thorax 2000, 55, 340–342. [Google Scholar] [CrossRef] [Green Version]
- Hii, S.W.; Tam, J.D.C.; Thompson, B.R.; Naughton, M.T. Bullous lung disease due to marijuana. Respirology 2008, 13, 122–127. [Google Scholar] [CrossRef]
- Fisher, B.A.C.; Ghuran, A.; Vadamalai, V.; Antonios, T. Cardiovascular complications induced by cannabis smoking: A case report and review of the literature. Emerg. Med. J. 2005, 22, 679–680. [Google Scholar] [CrossRef]
- Korantzopoulos, P.; Liu, T.; Papaioannides, D.; Li, G.; Goudevenos, J.A. Atrial fibrillation and marijuana smoking. Int. J. Clin. Pr. 2007, 62, 308–313. [Google Scholar] [CrossRef]
- Ishida, J.H.; Peters, M.G.; Jin, C.; Louie, K.; Tan, V.; Bacchetti, P.; Terrault, N.A. Influence of Cannabis Use on Severity of Hepatitis C Disease. Clin. Gastroenterol. Hepatol. 2008, 6, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Solowij, N.; Stephens, R.; A Roffman, R.; Babor, T. Does marijuana use cause long-term cognitive deficits? JAMA 2002, 287, 2653. [Google Scholar]
- McHale, S.; Hunt, N. Executive function deficits in short-term abstinent cannabis users. Hum. Psychopharmacol. Clin. Exp. 2008, 23, 409–415. [Google Scholar] [CrossRef]
- Scott, J.C.; Slomiak, S.T.; Jones, J.D.; Rosen, A.F.G.; Moore, T.M.; Gur, R.C. Association of cannabis with cognitive functioning in adolescents and young adults: A systematic review and meta-analysis. JAMA Psychiatry 2018, 75, 585–595. [Google Scholar] [CrossRef]
- Khwaja, S.; Yacoub, A.; Cheema, A.; Rihana, N.; Russo, R.; Velez, A.P.; Nanjappa, S.; Sandin, R.L.; Bohra, C.; Gajanan, G.; et al. Marijuana Smoking in Patients with Leukemia. Cancer Control. 2016, 23, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, D.; Brunnerman, D.K.; Gori, G.B.; Wynder, E.L. On the carcinogenicity of marijuana smoke. Recent Adv. Phytochem. 1975, 9, 63–81. [Google Scholar]
- Reid, P.T.; Macleod, J.; Robertson, J.R. Cannabis and the lung. J. R. Coll. Physicians Edinb. 2010, 40, 328–333. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.-C.; Tashkin, D.P.; Djahed, B.; Rose, J.E. Pulmonary Hazards of Smoking Marijuana as Compared with Tobacco. N. Engl. J. Med. 1988, 318, 347–351. [Google Scholar] [CrossRef]
- Fligiel, S.E.; Roth, M.D.; Kleerup, E.C.; Barsky, S.H.; Simmons, M.S.; Tashkin, D.P. Tracheobronchial Histopathology in Habitual Smokers of Cocaine, Marijuana, and/or Tobacco. Chest 1997, 112, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Barsky, S.H.; Roth, M.D.; Kleerup, E.C.; Simmons, M.; Tashkin, D.P. Histopathologic and Molecular Alterations in Bronchial Epithelium in Habitual Smokers of Marijuana, Cocaine, and/or Tobacco. J. Natl. Cancer Inst. 1998, 90, 1198–1205. [Google Scholar] [CrossRef] [Green Version]
- Callaghan, R.C.; Allebeck, P.; Sidorchuk, A. Marijuana use and risk of lung cancer: A 40-year cohort study. Cancer Causes Control. 2013, 24, 1811–1820. [Google Scholar] [CrossRef]
- Donald, P.J. Marijuana Smoking—Possible Cause of Head and Neck Carcinoma in Young Patients. Otolaryngol. Neck Surg. 1986, 94, 517–521. [Google Scholar] [CrossRef]
- Chacko, J.A.; Heiner, J.G.; Siu, W.; Macy, M.; Terris, M.K. Association between marijuana use and transitional cell carcinoma. Urology 2006, 67, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Tennstedt, D.; Saint-Remy, A. Cannabis and skin diseases. Eur. J. Dermatol. EJD 2011, 21, 5–11. [Google Scholar] [CrossRef]
- Tetrault, J.M.; Crothers, K.; Moore, B.A.; Mehra, R.; Concato, J.; Fiellin, D.A. Effects of marijuana smoking on pulmonary function and respiratory complications: A systematic review. Arch. Intern. Med. 2007, 167, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Kondrad, E.; Reid, A. Colorado Family Physicians’ Attitudes Toward Medical Marijuana. J. Am. Board Fam. Med. 2013, 26, 52–60. [Google Scholar] [CrossRef]
- Gargani, Y.; Bishop, P.; Denning, D.W. Too Many Mouldy Joints—Marijuana and Chronic Pulmonary Aspergillosis. Mediterr. J. Hematol. Infect. Dis. 2011, 3, e2011005. [Google Scholar] [CrossRef]
- E Verweij, P.; Kerremans, J.J.; Voss, A.; Meis, J.F. Fungal contamination of tobacco and marijuana. JAMA 2000, 284, 2875. [Google Scholar] [CrossRef]
- Hall, W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addict. 2015, 110, 19–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, W. The costs and benefits of cannabis control policies. Dialog Clin. Neurosci. 2020, 22, 281–287. [Google Scholar] [CrossRef]
- Munson, A.E.; Harris, L.S.; Friedman, M.A.; Dewey, W.L.; Carchman, R.A. Antineoplastic Activity of Cannabinoids2. J. Natl. Cancer Inst. 1975, 55, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.; Ramer, R.; Hinz, B. Targeting the endocannabinoid system as a potential anticancer approach. Drug Metab. Rev. 2018, 50, 26–53. [Google Scholar] [CrossRef]
- Efird, J.T.; Friedman, G.D.; Sidney, S.; Klatsky, A.; Habel, L.A.; Udaltsova, N.V.; Van Den Eeden, S.; Nelson, L.M. The risk for malignant primary adult-onset glioma in a large, multiethnic, managed-care cohort: Cigarette smoking and other lifestyle be-haviors. J. Neurooncol. 2004, 68, 57–69. [Google Scholar] [CrossRef]
- Randall, M.D. Endocannabinoids and the haematological system. Br. J. Pharmacol. 2007, 152, 671–675. [Google Scholar] [CrossRef] [Green Version]
- Callaghan, R.C.; Allebeck, P.; Akre, O.; McGlynn, K.A.; Sidorchuk, A. Cannabis Use and Incidence of Testicular Cancer: A 42-Year Follow-up of Swedish Men between 1970 and 2011. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1644–1652. [Google Scholar] [CrossRef] [Green Version]
- McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. D-9-Tetrahydrocannabinol Enhances Breast Cancer Growth and Metastasis by Suppression of the Antitumor Immune Response. J. Immunol. 2005, 174, 3281–3289. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.X.; Sharma, S.; Stolina, M.; Gardner, B.; Roth, M.D.; Tashkin, D.P.; Dubinett, S.M. D-9-Tetrahydrocannabinol Inhibits Antitumor Immunity by a CB2 Receptor-Mediated, Cytokine-Dependent Pathway. J. Immunol. 2000, 165, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.; Fischer, O.M.; Ullrich, A. Cannabinoids Induce Cancer Cell Proliferation via Tumor Necrosis Factor α-Converting Enzyme (TACE/ADAM17)-Mediated Transactivation of the Epidermal Growth Factor Receptor. Cancer Res. 2004, 64, 1943–1950. [Google Scholar] [CrossRef] [Green Version]
- Sarfaraz, S.; Adhami, V.M.; Syed, D.N.; Afaq, F.; Mukhtar, H. Cannabinoids for Cancer Treatment: Progress and Promise. Cancer Res. 2008, 68, 339–342. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, M.; Duarte, M.; Blazquez, C.; Ravina, J.; Rosa, M.; Galveroperh, I.; Sanchez, C.R.M.; Velasco, G.; González-Feria, L. A pilot clinical study of D9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br. J. Cancer 2006, 95, 197–203. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Ning, W.; Backlund, M.G.; Dey, S.K.; Dubois, R.N. Loss of Cannabinoid Receptor 1 Accelerates Intestinal Tumor Growth. Cancer Res. 2008, 68, 6468–6476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, B.; Schuebel, K.; Mukhopadhyay, P.; Cinar, R.; Godlewski, G.; Xiong, K.; Mackie, K.; Lizak, M.; Yuan, Q.; Goldman, D.; et al. Cannabinoid receptor 1 promotes hepatocellular carcinoma initiation and progression through multiple mechanisms. Hepatology 2015, 61, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Messalli, E.M.; Grauso, F.; Luise, R.; Angelini, A.; Rossiello, R. Cannabinoid receptor type 1 immunoreactivity and disease severity in human epithelial ovarian tumors. Am. J. Obstet. Gynecol. 2014, 211, 234.e1–234.e6. [Google Scholar] [CrossRef]
- Benz, A.H.; Renné, C.; Maronde, E.; Koch, M.; Grabiec, U.; Kallendrusch, S.; Rengstl, B.; Newrzela, S.; Hartmann, S.; Hansmann, M.-L.; et al. Expression and Functional Relevance of Cannabinoid Receptor 1 in Hodgkin Lymphoma. PLoS ONE 2013, 8, e81675. [Google Scholar] [CrossRef]
- Perez-Gomez, E.; Andradas, C.; Benito, S.B.; Caffarel, M.M.; García-Taboada, E.; Villa-Morales, M.C.; Moreno, E.; Hamann, S.; Martin-Villar, E.; Flores, J.M.; et al. Role of Cannabinoid Receptor CB2 in HER2 Pro-oncogenic Signaling in Breast Cancer. J. Natl. Cancer Inst. 2015, 107, djv077. [Google Scholar] [CrossRef] [Green Version]
- Dumitru, C.A.; Sandalcioglu, I.E.; Karsak, M. Cannabinoids in Glioblastoma Therapy: New Applications for Old Drugs. Front. Mol. Neurosci. 2018, 11, 159. [Google Scholar] [CrossRef]
- Jung, C.K.; Kang, W.K.; Park, J.M.; Ahn, H.J.; Kim, S.W.; Oh, S.T.; Choi, K.Y. Expression of the cannabinoid type I receptor and prognosis following surgery in colorectal cancer. Oncol. Lett. 2012, 5, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, E.; Gómez, I.; Martín, P.; Sánchez, A.; Román, L.; Tejerina, E.; Bonilla, F.; Merino, A.G.; De Herreros, A.G.; Provencio, M.; et al. Cannabinoids receptor type 2, CB2, expression correlates with human colon cancer progression and pre-dicts patient survival. Oncoscience 2015, 2, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Sidney, S.; Quesenberry, C.P., Jr.; Friedman, G.D.; Tekawa, I.S. Marijuana use and cancer incidence (California, United States). Cancer Causes Control. 1997, 8, 722–728. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Morgenstern, H.; Spitz, M.R.; Tashkin, D.P.; Yu, G.P.; Marshall, J.R.; Hsu, T.C.; Schantz, S.P. Marijuana use and increased risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol. Biomark. Prev. 1999, 8, 1071–1078. [Google Scholar]
- Rosenblatt, K.A.; Daling, J.R.; Chen, C.; Sherman, K.J.; Schwartz, S.M. Marijuana use and risk of oral squamous cell carcinoma. Cancer Res. 2004, 64, 4049–4054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barchi, M.; Innocenzi, E.; Giannattasio, T.; Dolci, S.; Rossi, P.; Grimaldi, P. Cannabinoid Receptors Signaling in the Development, Epigenetics, and Tumours of Male Germ Cells. Int. J. Mol. Sci. 2019, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Malfitano, A.M.; Ciaglia, E.; Gangemi, G.; Gazzerro, P.; Laezza, C.; Bifulco, M. Update on the endocannabinoid system as an anticancer target. Expert Opin. Ther. Targets 2011, 15, 297–308. [Google Scholar] [CrossRef]
- Sailler, S.; Schmitz, K.; Jäger, E.; Ferreiros, N.; Wicker, S.; Zschiebsch, K.; Pickert, G.; Geisslinger, G.; Walter, C.; Tegeder, I.; et al. Regulation of circulating endocannabinoids associated with cancer and metastases in mice and humans. Oncoscience 2014, 1, 272–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Carchman, R.; Harris, L.S.; E Munson, A. The inhibition of DNA synthesis by cannabinoids. Cancer Res. 1976, 36, 95–100. [Google Scholar]
- Reddy, P.M.; Maurya, N.; Velmurugan, B.K. Medicinal Use of Synthetic Cannabinoids—A Mini Review. Curr. Pharmacol. Rep. 2019, 5, 1–13. [Google Scholar] [CrossRef]
- Bifulco, M.; Laezza, C.; Pisanti, S.; Gazzerro, P. Cannabinoids and cancer: Pros and cons of an antitumour strategy. Br. J. Pharmacol. 2006, 148, 123–135. [Google Scholar] [CrossRef]
- Bifulco, M.; Malfitano, A.M.; Pisanti, S.; Laezza, C. Endocannabinoids in endocrine and related tumours. Endocr. Relat. Cancer 2008, 15, 391–408. [Google Scholar] [CrossRef]
- Vanderah, T.; Hanlon, K.; Lozano-Ondoua, A.; Umaretiya, P.; Symons-Ligouri, A.; Chandramouli, A.; Moy, J.; Kwass, W.; Mantyh, P.; Nelson, M. Modulation of breast cancer cell viability by a cannabinoid receptor 2 agonist, JWH-015, is calcium dependent. Breast Cancer 2016, 8, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Ravi, J.; Elbaz, M.; Wani, N.A.; Nasser, M.W.; Ganju, R.K. Cannabinoid receptor-2 agonist inhibits macrophage induced EMT in non-small cell lung cancer by downregulation of EGFR pathway. Mol. Carcinog. 2016, 55, 2063–2076. [Google Scholar] [CrossRef]
- Qamri, Z.; Preet, A.; Nasser, M.W.; Bass, C.; Leone, G.; Barsky, S.H.; Ganju, R.K. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol. Cancer Ther. 2009, 8, 3117–3129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Mirelman, D.; Waissengrin, B.; Goldway, N.; Sharon, H.; Brill, S.; Wolf, I. Use of medical cannabis: Perceptions of Israeli oncolo-gists. Lancet Oncol. 2019, 20, 475–477. [Google Scholar] [CrossRef]
- Coyne, Z.; Cowzer, D.; Hennessy, M.; Linehan, A.; Hennessy, B.T.; Grogan, W.; Breathnach, O.S.; Morris, P.G. Cannabis and cancer: Examining the use and perceived benefits in an Irish cancer cohort. J. Clin. Oncol. 2020, 38, e24178. [Google Scholar] [CrossRef]
- Abrahamov, A.; Abrahamov, A.; Mechoulam, R. An efficient new cannabinoid antiemetic in pediatric oncology. Life Sci. 1995, 56, 2097–2102. [Google Scholar] [CrossRef]
- Sallan, S.E.; Zinberg, N.E.; Frei, E., 3rd. Antiemetic effect of delta-9-tetrahydrocannabinol in patients receiving cancer chemo-therapy. N. Engl. J. Med. 1975, 293, 795–797. [Google Scholar] [CrossRef]
- Chang, A.E.; Shiling, D.J.; Stillman, R.C.; Goldberg, N.H.; Seipp, C.A.; Barofsky, I.; Simon, R.M.; Rosenberg, S.A. Delata-9-tetrahydrocannabinol as an antiemetic in cancer patients receiving high-dose methotrexate. A prospective, randomized evaluation. Ann. Intern. Med. 1979, 91, 819–824. [Google Scholar] [CrossRef]
- Sallan, S.E.; Cronin, C.; Zelen, M.; Zinberg, N.E. Antiemetics in patients receiving chemotherapy for cancer: A randomized comparison of delta-9-tetrahydrocannabinol and prochlorperazine. N. Engl. J. Med. 1980, 302, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Shiling, D.J.; Stillman, R.C.; Chang, A.E.; Goldberg, N.H.; Rn, C.A.S.; Barofsky, I.; Rosenberg, S.A. A prospective evaluation of delta-9-tetrahydrocannabinol as an antiemetic in patients receiving adriamycin and cytoxan chemotherapy. Cancer 1981, 47, 1746–1751. [Google Scholar] [CrossRef]
- A Darmani, N. Delta-9-tetrahydrocannabinol differentially suppresses cisplatin-induced emesis and indices of motor function via cannabinoid CB1 receptors in the least shrew. Pharmacol. Biochem. Behav. 2001, 69, 239–249. [Google Scholar] [CrossRef]
- Ferrari, F.; Ottani, A.; Giuliani, D. Cannabimimetic activity in rats and pigeons of HU 210, a potent antiemetic drug. Pharmacol. Biochem. Behav. 1999, 62, 75–80. [Google Scholar] [CrossRef]
- London, S.W.; McCarthy, L.E.; Borison, H.L. Suppression of Cancer Chemotherapy-Induced Vomiting in the Cat by Nabilone, a Synthetic Cannabinoid. Exp. Biol. Med. 1979, 160, 437–440. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, L.E.; Borison, H.L. Antiemetic Activity of N-Methyllevonantradol and Nabilone in Cisplatin-Treated Cats. J. Clin. Pharmacol. 1981, 21, 30–37. [Google Scholar] [CrossRef]
- Tramer, M.R.; Carroll, D.; Campbell, F.A.; Reynolds, D.J.; Moore, R.A.; McQuay, H.J. Cannabinoids for control of chemotherapy induced nausea and vomiting: Quantitative systematic review. BMJ 2001, 323, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, R.H.; Voth, E.A.; Sheridan, M.J. Marijuana to Prevent Nausea and Vomiting in Cancer Patients: A Survey of Clinical Oncologists. South Med. J. 1997, 90, 167–172. [Google Scholar] [CrossRef] [PubMed]
- E Badowski, M.; Yanful, P.K. Dronabinol oral solution in the management of anorexia and weight loss in AIDS and cancer. Ther. Clin. Risk Manag. 2018, 14, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Fride, E.; Bregman, T.; Kirkham, T.C. Endocannabinoids and food intake: Newborn suckling and appetite regulation in adult-hood. Exp. Biol. Med. 2005, 230, 225–234. [Google Scholar] [CrossRef]
- Kirkham, T.C.; Williams, C.M.; Fezza, F.; Di Marzo, V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: Stimulation of eating by 2-arachidonoyl glycerol. Br. J. Pharmacol. 2002, 136, 550–557. [Google Scholar] [CrossRef]
- Buxbaum, D. Analgesic activity of delta-9-tetrahydrocannabinol in the rat and mouse. Psychopharmacologia 1972, 25, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Sofia, R.; Nalepa, S.; Harakal, J.; Vassar, H. Anti-edema and analgesic properties of delta-9-tetrahydrocannabinol (THC). J. Pharmacol. Exp. Ther. 1973, 186, 646–655. [Google Scholar]
- Meng, I.D.; Manning, B.H.; Martin, W.; Fields, H.L. An analgesia circuit activated by cannabinoids. Nat. Cell Biol. 1998, 395, 381–383. [Google Scholar] [CrossRef]
- E Lynch, M. Preclinical Science Regarding Cannabinoids as Analgesics: An Overview. Pain Res. Manag. 2005, 10, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahn, E.J.; Makriyannis, A.; Hohmann, A.G. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br. J. Pharmacol. 2007, 152, 765–777. [Google Scholar] [CrossRef] [Green Version]
- Russo, E.B.; Hohmann, A.G. Role of Cannabinoids in Pain Management. In Comprehensive Treatment of Chronic Pain by Medical, Interventional, and Integrative Approaches; Springer Science and Business Media LLC: New York, NY, USA, 2012; pp. 181–197. [Google Scholar]
- Bloomfield, M.A.; Ashok, A.H.; Volkow, N.D.; Howes, O.D. The effects of Delta-9-tetrahydrocannabinol on the dopamine system. Nature 2016, 539, 369–377. [Google Scholar] [CrossRef]
- Hoffman, A.F.; Oz, M.; Yang, R.; Lichtman, A.H.; Lupica, C.R. Opposing actions of chronic Delta9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation. Learn Mem. 2007, 14, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, K.H.; Lim, S.; Ryu, J.; Lee, C.-W.; Kim, Y.; Kang, J.-H.; Kang, S.-S.; Ahn, Y.K.; Park, C.-S.; Kim, J.J. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc. Res. 2009, 84, 378–386. [Google Scholar] [CrossRef]
- Jean-Gilles, L.; Braitch, M.; Latif, M.L.; Aram, J.; Fahey, A.J.; Edwards, L.J.; Robins, R.A.; Tanasescu, R.; Tighe, P.J.; Gran, B.; et al. Effects of pro-inflammatory cytokines on cannabinoid CB1and CB2receptors in immune cells. Acta Physiol. 2015, 214, 63–74. [Google Scholar] [CrossRef]
- Campbell, G.; Hall, W.; Peacock, A.; Lintzeris, N.; Bruno, R.; Larance, B.; Nielsen, S.; Cohen, M.; Chan, G.; Mattick, R.P.; et al. Effect of cannabis use in people with chronic non-cancer pain prescribed opioids: Findings from a 4-year prospective cohort study. Lancet Public Health 2018, 3, e341–e350. [Google Scholar] [CrossRef]
- Velasco, G.; Sanchez, C.; Guzmán, M. Anticancer Mechanisms of Cannabinoids. Curr. Oncol. 2016, 23, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mould, R.R.; Botchway, S.W.; Parkinson, J.R.C.; Thomas, E.L.; Guy, G.W.; Bell, J.D.; Nunn, A.V.W. Cannabidiol Modulates Mitochondrial Redox and Dynamics in MCF7 Cancer Cells: A Study Using Fluorescence Lifetime Imaging Microscopy of NAD(P)H. Front. Mol. Biosci. 2021, 8, 630107. [Google Scholar] [CrossRef]
- Flygare, J.; Sander, B. The endocannabinoid system in cancer—Potential therapeutic target? Semin. Cancer Biol. 2008, 18, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, E.S.; Watters, A.K.; MacKenzie, J.D.; Granat, L.M.; Zhang, D. Cannabidiol (CBD) as a Promising Anti-Cancer Drug. Cancers 2020, 12, 3203. [Google Scholar] [CrossRef]
- Braile, M.; Marcella, S.; Marone, G.; Galdiero, M.; Varricchi, G.; Loffredo, S. The Interplay between the Immune and the Endocannabinoid Systems in Cancer. Cells 2021, 10, 1282. [Google Scholar] [CrossRef]
- Kogan, N.M.; Blazquez, C.; Alvarez, L.; Gallily, R.; Schlesinger, M.; Guzman, M.; Mechoulam, R. A cannabinoid quinone in-hibits angiogenesis by targeting vascular endothelial cells. Mol. Pharmacol. 2006, 70, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Kogan, N.M.; Schlesinger, M.; Priel, E.; Rabinowitz, R.; Berenshtein, E.; Chevion, M.; Mechoulam, R. HU-331, a novel canna-binoid-based anticancer topoisomerase II inhibitor. Mol. Cancer Ther. 2007, 6, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Morales, P.; Vara, D.; Goméz-Cañas, M.; Zúñiga, M.C.; Olea-Azar, C.; Goya, P.; Fernández-Ruiz, J.; Diaz-Laviada, I.; Jagerovic, N. Synthetic cannabinoid quinones: Preparation, in vitro antiproliferative effects and in vivo prostate antitumor activity. Eur. J. Med. Chem. 2013, 70, 111–119. [Google Scholar] [CrossRef]
- Badolato, M.; Carullo, G.; Caroleo, M.C.; Cione, E.; Aiello, F.; Manetti, F. Discovery of 1,4-Naphthoquinones as a New Class of Antiproliferative Agents Targeting GPR55. ACS Med. Chem. Lett. 2019, 10, 402–406. [Google Scholar] [CrossRef]
- Casanova, M.L.; Blázquez, C.; Martínez-Palacio, J.; Villanueva, C.; Fernández-Aceñerp, M.J.; Huffman, J.W.; Jorcano, J.L.; Guzmán, M. Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J. Clin. Investig. 2003, 111, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Portella, G.; Laezza, C.; Laccetti, P.; De Petrocellis, L.; Di Marzo, V.; Bifulco, M. Inhibitory effects of cannabinoid CB 1 receptor stimulation on tumor growth and metastatic spreading: Actions on signals involved in angiogenesis and metastasis. FASEB J. 2003, 17, 1771–1773. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Borselli, C.; Oliviero, O.; Laezza, C.; Gazzerro, P.; Bifulco, M. Antiangiogenic activity of the endocannabinoid anandamide: Correlation to its tumor-suppressor efficacy. J. Cell. Physiol. 2007, 211, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, C.; González-Feria, L.; Álvarez, L.; Haro, A.; Casanova, M.L.; Guzmán, M. Cannabinoids Inhibit the Vascular En-dothelial Growth Factor Pathway in Gliomas. Cancer Res. 2004, 64, 5617–5623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarnelli, G.; Gigli, S.; Capoccia, E.; Iuvone, T.; Cirillo, C.; Seguella, L.; Nobile, N.; D’Alessandro, A.; Pesce, M.; Steardo, L.; et al. Palmitoylethanolamide Exerts Antiproliferative Effect and Downregulates VEGF Signaling in Caco-2 Human Colon Carcinoma Cell Line Through a Selective PPAR-α-Dependent Inhibition of Akt/mTOR Pathway. Phytother. Res. 2016, 30, 963–970. [Google Scholar] [CrossRef]
- Quartarone, E.; Alonci, A.; Allegra, A.; Bellomo, G.; Calabro, L.; D’Angelo, A.; Del Fabro, V.; Grasso, A.; Cincotta, M.; Musolino, C. Differential levels of soluble angiopoietin-2 and Tie-2 in patients with haematological malignancies. Eur. J. Haematol. 2006, 77, 480–485. [Google Scholar] [CrossRef]
- Javid, F.A.; Phillips, R.M.; Afshinjavid, S.; Verde, R.; Ligresti, A. Cannabinoid pharmacology in cancer research: A new hope for cancer patients? Eur. J. Pharmacol. 2016, 775, 1–14. [Google Scholar] [CrossRef]
- Hinz, B.; Ramer, R. Anti-tumour actions of cannabinoids. Br. J. Pharmacol. 2019, 176, 1384–1394. [Google Scholar] [CrossRef]
- Joosten, M. Leukemic predisposition of pSca-1/Cb2 transgenic mice. Exp. Hematol. 2002, 30, 142–149. [Google Scholar] [CrossRef]
- Salazar, M.; Carracedo, A.; Salanueva Íñigo, J.; Hernández-Tiedra, S.; Lorente, M.; Egia, A.; Vázquez, P.; Blázquez, C.; Torres, S.; García, S.; et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Investig. 2009, 119, 1359–1372. [Google Scholar] [CrossRef] [Green Version]
- Salazar, M.; Lorente, M.; García-Taboada, E.; Hernández-Tiedra, S.; Davila, D.; Francis, S.; Guzmán, M.; Kiss-Toth, E.; Velasco, G. The pseudokinase tribbles homologue-3 plays a crucial role in cannabinoid anticancer action. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2013, 1831, 1573–1578. [Google Scholar] [CrossRef]
- Armstrong, J.L.; Hill, D.S.; McKee, C.S.; Hernandez-Tiedra, S.; Lorente, M.; Lopez-Valero, I.; Anagnostou, M.E.; Babatunde, F.; Corazzari, M.; Redfern, C.; et al. Exploiting Cannabinoid-Induced Cytotoxic Autophagy to Drive Melanoma Cell Death. J. Investig. Dermatol. 2015, 135, 1629–1637. [Google Scholar] [CrossRef] [Green Version]
- Vara, D.; Salazar, M.; Olea-Herrero, N.; Guzmán, M.; Velasco, G.; Díaz-Laviada, I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: Role of AMPK-dependent activation of autophagy. Cell Death Differ. 2011, 18, 1099–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouaboula, M.; Rinaldi, M.; Carayon, P.; Carillon, C.; Delpech, B.; Shire, D.; Fur, G.; Casellas, P. Cannabinoid-receptor expression in human leukocytes. JBIC J. Biol. Inorg. Chem. 1993, 214, 173–180. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Zhang, M.; Cao, Y. Exposure to morphine affects the expression of endocannabinoid receptors and immune functions. J. Neuroimmunol. 2012, 247, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, C.; Dai, X.; Ao, Y.; Li, Y. Inhibitory effect of trans-caryophyllene (TC) on leukocyte-endothelial attachment. Toxicol. Appl. Pharmacol. 2017, 329, 326–333. [Google Scholar] [CrossRef]
- Tanasescu, R.; Constantinescu, C.S. Cannabinoids and the immune system: An overview. Immunobiology 2010, 215, 588–597. [Google Scholar] [CrossRef]
- Croxford, J.L.; Yamamura, T. Cannabinoids and the immune system: Potential for the treatment of inflammatory diseases? J. Neuroimmunol. 2005, 166, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Singh, S.; Niyogi, R.G.; Lamont, G.J.; Wang, H.; Lamont, R.J.; Scott, D.A. Marijuana-Derived Cannabinoids Trigger a CB2/PI3K Axis of Suppression of the Innate Response to Oral Pathogens. Front. Immunol. 2019, 10, 2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servettaz, A.; Kavian, N.; Nicco, C.; Deveaux, V.; Chéreau, C.; Wang, A.; Zimmer, A.; Lotersztajn, S.; Weill, B.; Batteux, F. Targeting the Cannabinoid Pathway Limits the Development of Fibrosis and Autoimmunity in a Mouse Model of Systemic Sclerosis. Am. J. Pathol. 2010, 177, 187–196. [Google Scholar] [CrossRef]
- Börner, C.; Bedini, A.; Höllt, V.; Kraus, J. Analysis of Promoter Regions Regulating Basal and Interleukin-4-Inducible Expression of the Human CB1 Receptor Gene in T Lymphocytes. Mol. Pharmacol. 2007, 73, 1013–1019. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; He, S.; Hu, Y.; Zheng, H. Antagonism of Cannabinoid Receptor 1 Attenuates the Anti-Inflammatory Effects of Electroacupuncture in a Rodent Model of Migraine. Acupunct. Med. 2016, 34, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Musolino, C.; Allegra, A.; Innao, V.; Allegra, A.G.; Pioggia, G.; Gangemi, S. Inflammatory and Anti-Inflammatory Equilibrium, Proliferative and Antiproliferative Balance: The Role of Cytokines in Multiple Myeloma. Mediat. Inflamm. 2017, 2017, 1852517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, L.; Franklin, A.; Witting, A.; Wade, C.; Xie, Y.; Kunos, G.; Mackie, K.; Stella, N. Nonpsychotropic Cannabinoid Receptors Regulate Microglial Cell Migration. J. Neurosci. 2003, 23, 1398–1405. [Google Scholar] [CrossRef] [Green Version]
- Kampa-Schittenhelm, K.M.; Salitzky, O.; Akmut, F.; Illing, B.; Kanz, L.; Salih, H.R.; Schittenhelm, M.M. Dronabinol has pref-erential antileukemic activity in acute lymphoblastic and myeloid leukemia with lymphoid differentiation patterns. BMC cancer 2016, 16, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto-Mercado, V.; Mendivil-Perez, M.; Jimenez-Del-Rio, M.; E Fox, J.; Velez-Pardo, C. Cannabinoid CP55940 selectively induces apoptosis in Jurkat cells and in ex vivo T-cell acute lymphoblastic leukemia through H2O2 signaling mechanism. Leuk. Res. 2020, 95, 106389. [Google Scholar] [CrossRef]
- McKallip, R.J.; Jia, W.; Schlomer, J.; Warren, J.W.; Nagarkatti, P.S.; Nagarkatti, M. Cannabidiol-Induced Apoptosis in Human Leukemia Cells: A Novel Role of Cannabidiol in the Regulation of p22phox and Nox4 Expression. Mol. Pharmacol. 2006, 70, 897–908. [Google Scholar] [CrossRef] [Green Version]
- Powles, T.; Poele, R.T.; Shamash, J.; Chaplin, T.; Propper, D.; Joel, S.; Oliver, T.; Liu, W.M. Cannabis-induced cytotoxicity in leukemic cell lines: The role of the cannabinoid receptors and the MAPK pathway. Blood 2005, 105, 1214–1221. [Google Scholar] [CrossRef] [Green Version]
- Olivas-Aguirre, M.; Torres-López, L.; Valle-Reyes, J.S.; Hernández-Cruz, A.; Pottosin, I.; Dobrovinskaya, O. Cannabidiol di-rectly targets mitochondria and disturbs calcium homeostasis in acute lymphoblastic leukemia. Cell Death Dis. 2019, 10, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalenderoglou, N.; MacPherson, T.; Wright, K.L. Cannabidiol Reduces Leukemic Cell Size—But Is It Important? Front. Pharmacol. 2017, 8, 144. [Google Scholar] [CrossRef] [Green Version]
- Mazuz, M.; Tiroler, A.; Moyal, L.; Hodak, E.; Nadarajan, S.; Vinayaka, A.C.; Gorovitz-Haris, B.; Lubin, I.; Drori, A.; Drori, G.; et al. Synergistic cytotoxic activity of cannabinoids from cannabis sativa against cutaneous T-cell lymphoma (CTCL) in-vitro and ex-vivo. Oncotarget 2020, 11, 1141–1156. [Google Scholar] [CrossRef] [Green Version]
- Flygare, J.; Gustafsson, K.; Kimby, E.; Christensson, B.; Sander, B. Cannabinoid receptor ligands mediate growth inhibition and cell death in mantle cell lymphoma. FEBS Lett. 2005, 579, 6885–6889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, C.; Aikins, J.; Warshal, D.; Ostrovsky, O. Can Cannabidiol Affect the Efficacy of Chemotherapy and Epigenetic Treatments in Cancer? Biomolecules 2021, 11, 766. [Google Scholar] [CrossRef]
- Scott, K.A.; Dalgleish, A.G.; Liu, W.M. Anticancer effects of phytocannabinoids used with chemotherapy in leukaemia cells can be improved by altering the sequence of their administration. Int. J. Oncol. 2017, 51, 369–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, K.A.; Shah, S.; Dalgleish, A.G.; Liu, W.M. Enhancing the activity of cannabidiol and other cannabinoids in vitro through modifications to drug combinations and treatment schedules. Anticancer. Res. 2013, 33, 4373–4380. [Google Scholar] [PubMed]
- Gallily, R.; Even-Chena, T.; Katzavian, G.; Lehmann, D.; Dagan, A.; Mechoulam, R. γ-Irradiation Enhances Apoptosis Induced by Cannabidiol, a Non-psychotropic Cannabinoid, in Cultured HL-60 Myeloblastic Leukemia Cells. Leuk. Lymphoma 2003, 44, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Togano, T.; Kim, N.; Kim, N.; Park, G.S.; Park, A.K.; Bennet, M.; Park, J. The evaluation of Cannabidiol’s effect on the immu-notherapy of Burkitt lymphoma. Biochem. Biophys. Res. Commun. 2019, 520, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Lombard, C.; Nagarkatti, M.; Nagarkatti, P.S. Targeting cannabinoid receptors to treat leukemia: Role of cross-talk between extrinsic and intrinsic pathways in Delta9-tetrahydrocannabinol (THC)-induced apoptosis of Jurkat cells. Leuk. Res. 2005, 29, 915–922. [Google Scholar] [CrossRef]
- Herrera, B.; Carracedo, A.; Zaera, M.D.; Del Pulgar, T.G.; Guzmán, M.; Velasco, G. The CB2 cannabinoid receptor signals apoptosis via ceramide-dependent activation of the mitochondrial intrinsic pathway. Exp. Cell Res. 2006, 312, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Musolino, C.; Allegra, A.; Saija, A.; Alonci, A.; Russo, S.; Spatari, G.; Penna, G.; Gerace, D.; Cristani, M.; David, A.; et al. Changes in advanced oxidation protein products, advanced glycation end products, and s-nitrosylated proteins, in patients affected by polycythemia vera and essential thrombocythemia. Clin. Biochem. 2012, 45, 1439–1443. [Google Scholar] [CrossRef]
- Gangemi, S.; Allegra, A.; Alonci, A.; Cristani, M.; Russo, S.; Speciale, A.; Penna, G.; Spatari, G.; Cannavò, A.; Bellomo, G.; et al. Increase of novel biomarkers for oxidative stress in patients with plasma cell disorders and in multiple myeloma patients with bone lesions. Inflamm. Res. 2012, 61, 1063–1067. [Google Scholar] [CrossRef]
- Gangemi, S.; Allegra, A.; Aguennouz, M.; Alonci, A.; Speciale, A.; Cannavò, A.; Cristani, M.; Russo, S.; Spatari, G.; Alibrandi, A.; et al. Relationship Between Advanced Oxidation Protein Products, Advanced Glycation End Products, andS-Nitrosylated Proteins With Biological Risk and MDR-1 Polymorphisms in Patients Affected by B-Chronic Lymphocytic Leukemia. Cancer Investig. 2012, 30, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Imbesi, S.; Musolino, C.; Allegra, A.; Saija, A.; Morabito, F.; Calapai, G.; Gangemi, S. Oxidative stress in oncohematologic diseases: An update. Expert Rev. Hematol. 2013, 6, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.; Wey, S.-P.; Liao, M.-H.; Hsu, W.-L.; Wu, H.-Y.; Jan, T.-R. A comparative study on cannabidiol-induced apoptosis in murine thymocytes and EL-4 thymoma cells. Int. Immunopharmacol. 2008, 8, 732–740. [Google Scholar] [CrossRef]
- Prince, H.M.; Querfeld, C. Integrating novel systemic therapies for the treatment of mycosis fungoides and Sézary syndrome. Best Pr. Res. Clin. Haematol. 2018, 31, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, K.; Wang, X.; Severa, D.; Eriksson, M.; Kimby, E.; Merup, M.; Christensson, B.; Flygare, J.; Sander, B. Expression of cannabinoid receptors type 1 and type 2 in non-Hodgkin lymphoma: Growth inhibition by receptor activation. Int. J. Cancer 2008, 123, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.C.; Asplund, A.C.; Lindvall, J.M.; Nygren, L.; Liden, J.; Kimby, E.; Christensson, B.; Smith, C.; Sander, B.; Islam, T.C.; et al. High level of cannabinoid receptor 1, absence of regulator of G protein signalling 13 and differential expression of Cyclin D1 in mantle cell lymphoma. Leukaemia 2003, 17, 1880–1890. [Google Scholar] [CrossRef] [Green Version]
- A Nelson, R.; Levine, A.M.; Marks, G.B.; E Bernstein, L. Alcohol, tobacco and recreational drug use and the risk of non-Hodgkin’s lymphoma. Br. J. Cancer 1997, 76, 1532–1537. [Google Scholar] [CrossRef] [Green Version]
- Holly, E.A.; Lele, C.; Bracci, P.M.; McGrath, M.S. Case control study of non-Hodgkin’s lymphoma among women and heter-osexual men in the San Francisco Bay Area, California. Am. J. Epidemiol. 1999, 150, 375–389. [Google Scholar] [CrossRef]
- Wen, W.-Q.; Shu, X.-O.; Steinbuch, M.; Severson, R.K.; Reaman, G.H.; Buckley, J.D.; Robison, L.L. Paternal military service and risk for childhood leukemia in offspring. Am. J. Epidemiol. 2000, 151, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Chang, G.; Meadows, M.-E.; Jones, J.A.; Antin, J.H.; Orav, E.J. Substance Use and Survival after Treatment for Chronic Myelogenous Leukemia (CML) or Myelodysplastic Syndrome (MDS). Am. J. Drug Alcohol Abus. 2010, 36, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasmin-Karim, S.; Moreau, M.; Mueller, R.; Sinha, N.; Dabney, R.; Herman, A.; Ngwa, W. Enhancing the Therapeutic Efficacy of Cancer Treatment With Cannabinoids. Front. Oncol. 2018, 8, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, Y.; Bali, C. Cannabis Extract Treatment for Terminal Acute Lymphoblastic Leukemia with a Philadelphia Chromosome Mutation. Case Rep. Oncol. 2013, 6, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Jagasia, M.; Arora, M.; Flowers, M.E.; Chao, N.J.; McCarthy, P.L.; Cutler, C.S.; Urbano-Ispizua, A.; Pavletic, S.Z.; Haagenson, M.D.; Zhang, M.J.; et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood 2012, 119, 296–307. [Google Scholar] [CrossRef] [Green Version]
- Khuja, I.; Yekhtin, Z.; Or, R.; Almogi-Hazan, O. Cannabinoids Reduce Inflammation but Inhibit Lymphocyte Recovery in Murine Models of Bone Marrow Transplantation. Int. J. Mol. Sci. 2019, 20, 668. [Google Scholar] [CrossRef] [Green Version]
- Berg, B.B.; Soares, J.S.; Paiva, I.R.; Rezende, B.M.; Rachid, M.A.; Cau, S.B.D.A.; Romero, T.R.L.; Pinho, V.; Teixeira, M.M.; e Castor, M.G.M. Cannabidiol Enhances Intestinal Cannabinoid Receptor Type 2 Receptor Expression and Activation Increasing Regulatory T Cells and Reduces Murine Acute Graft-versus-Host Disease without Interfering with the Graft-versus-Leukemia Response. J. Pharmacol. Exp. Ther. 2021, 377, 273–283. [Google Scholar] [CrossRef]
- Berdyshev, E.; Boichot, E.; Corbel, M.; Germain, N.; Lagente, V. Effects of cannabinoid receptor ligands on LPS-induced pul-monary inflammation in mice. Life Sci. 1998, 63, 125–129. [Google Scholar] [CrossRef]
- Pacifici, R.; Zuccaro, P.; Pichini, S.; Roset, P.N.; Poudevida, S.; Farré, M.; Segura, J.; De La Torre, R. Modulation of the Immune System in Cannabis Users. JAMA 2003, 289, 1929–1931. [Google Scholar] [CrossRef]
- Pandey, R.; Hegde, V.L.; Nagarkatti, M.; Nagarkatti, P.S. Targeting Cannabinoid Receptors as a Novel Approach in the Treatment of Graft-versus-Host Disease: Evidence from an Experimental Murine Model. J. Pharmacol. Exp. Ther. 2011, 338, 819–828. [Google Scholar] [CrossRef] [Green Version]
- Schicho, R.; Storr, M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology 2012, 89, 149–155. [Google Scholar] [CrossRef] [Green Version]
- De Filippis, D.; Esposito, G.; Cirillo, C.; Cipriano, M.; DE Winter, B.; Scuderi, C.; Sarnelli, G.; Cuomo, R.; Steardo, L.; De Man, J.G.; et al. Cannabidiol Reduces Intestinal Inflammation through the Control of Neuroimmune Axis. PLoS ONE 2011, 6, e28159. [Google Scholar] [CrossRef]
- Mechoulam, R.; Peters, M.; Murillo-Rodriguez, E.; Hanus, L.O. Cannabidiol recent advances. Chem. Biodivers. 2007, 4, 1678–1692. [Google Scholar] [CrossRef]
- Basu, S.; Dittel, B.N. Unraveling the complexities of cannabinoid receptor 2 (CB2) immune regulation in health and disease. Immunol. Res. 2011, 51, 26–38. [Google Scholar] [CrossRef]
- Adhikary, S.; Kocieda, V.P.; Yen, J.-H.; Tuma, R.F.; Ganea, D. Signaling through cannabinoid receptor 2 suppresses murine dendritic cell migration by inhibiting matrix metalloproteinase 9 expression. Blood 2012, 120, 3741–3749. [Google Scholar] [CrossRef] [Green Version]
- Yeshurun, M.; Shpilberg, O.; Herscovici, C.; Shargian, L.; Dreyer, J.; Peck, A.; Israeli, M.; Levy-Assaraf, M.; Gruenewald, T.; Mechoulam, R.; et al. Cannabidiol for the Prevention of Graft-versus-Host-Disease after Allogeneic Hematopoietic Cell Transplantation: Results of a Phase II Study. Biol. Blood Marrow Transplant. 2015, 21, 1770–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reshef, R.; Luger, S.M.; Hexner, E.O.; Loren, A.W.; Frey, N.V.; Nasta, S.D.; Goldstein, S.C.; Stadtmauer, E.A.; Smith, J.; Bailey, S.; et al. Blockade of Lymphocyte Chemotaxis in Visceral Graft-versus-Host Disease. N. Engl. J. Med. 2012, 367, 135–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koreth, J.; Stevenson, K.E.; Kim, H.T.; McDonough, S.M.; Bindra, B.; Armand, P.; Ho, V.T.; Cutler, C.; Blazar, B.R.; Antin, J.H.; et al. Bortezomib-Based Graft-Versus-Host Disease Prophylaxis in HLA-Mismatched Unrelated Donor Transplantation. J. Clin. Oncol. 2012, 30, 3202–3208. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.W.; Braun, T.; Chang, L.; Ferrara, J.L.; Pawarode, A.; Magenau, J.M.; Hou, G.; Beumer, J.H.; Levine, J.E.; Goldstein, S. Vorinostat plus tacrolimus and mycophenolate to prevent graft-versus-host disease after related donor reduced-intensity conditioning allogeneic haematopoietic stem-cell transplantation: A phase 1/2 trial. Lancet Oncol. 2014, 15, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Meyer, H.C.; Lee, F.S.; Gee, D.G. The Role of the Endocannabinoid System and Genetic Variation in Adolescent Brain Devel-opment. Neuropsychopharmacology 2018, 43, 21–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harkany, T.; Guzmán, M.; Galve-Roperh, I.; Berghuis, P.; Devi, L.A.; Mackie, K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol. Sci. 2007, 28, 83–92. [Google Scholar] [CrossRef]
- Bara, A.; Ferland, J.-M.N.; Rompala, G.; Szutorisz, H.; Hurd, Y.L. Cannabis and synaptic reprogramming of the developing brain. Nat. Rev. Neurosci. 2021, 1–16. [Google Scholar] [CrossRef]
- Ahmed, K.T.; Amin, R.; Shah, P.; Ali, D.W. Motor neuron development in zebrafish is altered by brief (5-hr) exposures to THC (∆9-tetrahydrocannabinol) or CBD (cannabidiol) during gastrulation. Sci. Rep. 2018, 8, 10518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalterio, S.L. Perinatal or adult exposure to cannabinoids alters male reproductive functions in mice. Pharmacol. Biochem. Behav. 1980, 12, 143–153. [Google Scholar] [CrossRef]
- Navarro, M.; De Fonseca, F.; Hernández, M.; Ramos, J.; Fernández-Ruiz, J. Motor behavior and nigrostriatal dopaminergic activity in adult rats perinatally exposed to cannabinoids. Pharmacol. Biochem. Behav. 1994, 47, 47–58. [Google Scholar] [CrossRef]
- de Salas-Quiroga, A.; Díaz-Alonso, J.; García-Rincón, D.; Remmers, F.; Vega, D.; Gómez-Cañas, M.; Lutz, B.; Guzmán, M.; Galve-Roperh, I. Prenatal exposure to cannabinoids evokes long-lasting functional alterations by targeting CB1 receptors on developing cortical neurons. Proc. Natl. Acad. Sci. USA 2015, 112, 13693–13698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dow-Edwards, D.; Silva, L. Endocannabinoids in brain plasticity: Cortical maturation, HPA axis function and behavior. Brain Res. 2017, 1654, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Parker, L.A. The Endocannabinoid System and the Brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [Green Version]
- Volkow, N.D.; Baler, R.D.; Compton, W.M.; Weiss, S.R. Adverse Health Effects of Marijuana Use. N. Engl. J. Med. 2014, 370, 2219–2227. [Google Scholar] [CrossRef] [Green Version]
- Ananth, P.; Reed-Weston, A.; Wolfe, J. Medical marijuana in pediatric oncology: A review of the evidence and implications for practice. Pediatr. Blood Cancer 2017, 65, e26826. [Google Scholar] [CrossRef]
- Blázquez, C.; Salazar, M.; Carracedo, A.; Lorente, M.; Egia, A.; González-Feria, L.; Haro, A.; Velasco, G.; Guzmán, M. Cannabinoids Inhibit Glioma Cell Invasion by Down-regulating Matrix Metalloproteinase-2 Expression. Cancer Res. 2008, 68, 1945–1952. [Google Scholar] [CrossRef] [Green Version]
- Ngwa, W.; Kumar, R.; Moreau, M.; Dabney, R.; Herman, A. Nanoparticle Drones to Target Lung Cancer with Radiosensitizers and Cannabinoids. Front. Oncol. 2017, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Greish, K.; Mathur, A.; Al Zahrani, R.; Elkaissi, S.; Al Jishi, M.; Nazzal, O.; Taha, S.; Pittalà, V.; Taurin, S. Synthetic cannabinoids nano-micelles for the management of triple negative breast cancer. J. Control. Release 2018, 291, 184–195. [Google Scholar] [CrossRef]
- I Abrams, D.; Vizoso, H.P.; Shade, S.B.; Jay, C.; E Kelly, M.; Benowitz, N.L. Vaporization as a Smokeless Cannabis Delivery System: A Pilot Study. Clin. Pharmacol. Ther. 2007, 82, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Ruchlemer, R.; Amit-Kohn, M.; Raveh, D.; Hanuš, L. Inhaled medicinal cannabis and the immunocompromised patient. Support Care Cancer 2014, 23, 819–822. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, C.A.; Russo, E.B. Practical considerations in medical cannabis administration and dosing. Eur. J. Intern. Med. 2018, 49, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.; Stjepanovi’c, D.; Caulkins, J.; Lynskey, M.; Leung, J.; Campbell, G.; Degenhardt, L. Public health implications of legalizing the production and sale of cannabis for medicinal and recreational use. Lancet 2019, 394, 1580–1590. [Google Scholar] [CrossRef]
- Paris, M.; Tran, N. The existence of ”Nederwiet”, a new fact in the history of cannabis. Ann. Pharm. Françaises 1998, 56, 264–267. [Google Scholar]
- Buisman-Pijlman, F.; Rigter, S.M.; Hoek, J.; Goldschmidt, H.M.J.; Niesink, R.J.M. Strong increase in total delta-THC in cannabis preparations sold in Dutch coffee shops. Addict. Biol. 2005, 10, 171–180. [Google Scholar] [CrossRef]
- Giaginis, C.; Lakiotaki, E.; Korkolopoulou, P.; Konstantopoulos, K.; Patsouris, E.; Theocharis, S. Endocannabinoid system: A promising therapeutic target for the treatment of haematological malignancies? Curr. Med. Chem. 2016, 23, 1. [Google Scholar] [CrossRef]


| Apparatus | Effect | Number of Patients | Exposure | Ref. |
|---|---|---|---|---|
| Respiratory tract | Chronic obstructive lung disease | 878 | More than 50 cigarettes | [28] |
| Pneumothorax | 3 | Daily | [29] | |
| Emphysema | 399 | Dose-response | [30] | |
| Large lung bullae | 4 | High exposure | [31] | |
| Bullous lung bullae | 10 | Regular chronic exposure | [32] | |
| Cardiovascular system | Hypertension, Tachyarrythmia | 1 | Infrequent use | [33] |
| Atrial fibrillation | 6 | [34] | ||
| Liver | Fibrosis | 204 | Daily | [35] |
| Cognitive alteration | Cognitive defect | 102 | Long-term use | [36] |
| Executive function | 55 | 3 times/week | [37] | |
| Dependence | Cognitive deficiency, psychoses, and depressive alterations | 2152 | Frequent or heavy use | [38] |
| Apparatus | Number of Patients | Exposure | Ref. | |
|---|---|---|---|---|
| Cancer | Lung | 49,321 | More than 50 times | [45] |
| Head and Neck | 6 | Habitual use | [46] | |
| Transitional cell carcinoma | 52 | Habitual use | [47] | |
| Glioma | 133,811 | Once a month | [57] | |
| Head and Neck | 173 | Dose-response | [58] | |
| Testicular germ cell tumors | 49,343 | More than 50 times | [59] |
| Diseases and Cells | Study | Mechanism | Ref. |
|---|---|---|---|
| Acute lymphoblastic leukemia (MOLM-13, Jurkat cells) | In vitro | Apoptosis | [149] |
| Acute Myeloid leukemia with lymphoid differentiation pattern | In vitroEx vivo | H2O2 mediated mechanism | [150] |
| Lymphoma (EL-4 cells) | In vitro | Oxidative stress | [151] |
| Acute promyelocytic leukemia (CEM cells, HL60 cells), Erythroblastic leukemia (HEL-92) | In vitro | Apoptosis | [152] |
| B-ALL (RS;11, Reh cells), T-ALL (MOLT-3 cells, Jurkat cells), Chronic myeloid leukemia (K562 cells) | In vitro | Mitochondria changes, endoplasmic reticulum stress | [153] |
| T cell leukemia (Jurkat cells) | In vitro | AKT phosphorylation | [154] |
| Cutaneous T cell lymphoma (My-La and HuT-70 cells) | In vitro | Apoptosis | [155] |
| Mantle cell lymphoma cells | In vitro | Apoptosis | [156] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irrera, N.; Bitto, A.; Sant’Antonio, E.; Lauro, R.; Musolino, C.; Allegra, A. Pros and Cons of the Cannabinoid System in Cancer: Focus on Hematological Malignancies. Molecules 2021, 26, 3866. https://doi.org/10.3390/molecules26133866
Irrera N, Bitto A, Sant’Antonio E, Lauro R, Musolino C, Allegra A. Pros and Cons of the Cannabinoid System in Cancer: Focus on Hematological Malignancies. Molecules. 2021; 26(13):3866. https://doi.org/10.3390/molecules26133866
Chicago/Turabian StyleIrrera, Natasha, Alessandra Bitto, Emanuela Sant’Antonio, Rita Lauro, Caterina Musolino, and Alessandro Allegra. 2021. "Pros and Cons of the Cannabinoid System in Cancer: Focus on Hematological Malignancies" Molecules 26, no. 13: 3866. https://doi.org/10.3390/molecules26133866
APA StyleIrrera, N., Bitto, A., Sant’Antonio, E., Lauro, R., Musolino, C., & Allegra, A. (2021). Pros and Cons of the Cannabinoid System in Cancer: Focus on Hematological Malignancies. Molecules, 26(13), 3866. https://doi.org/10.3390/molecules26133866







