Fortification of Acidophilus-bifidus-thermophilus (ABT) Fermented Milk with Heat-Treated Industrial Yeast Enhances Its Selected Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Heat-Treated Yeast
2.2.2. Production of Fermented Milk by ABT
2.2.3. Chemical Analysis
2.2.4. Evaluation of the Viability of Starter Culture
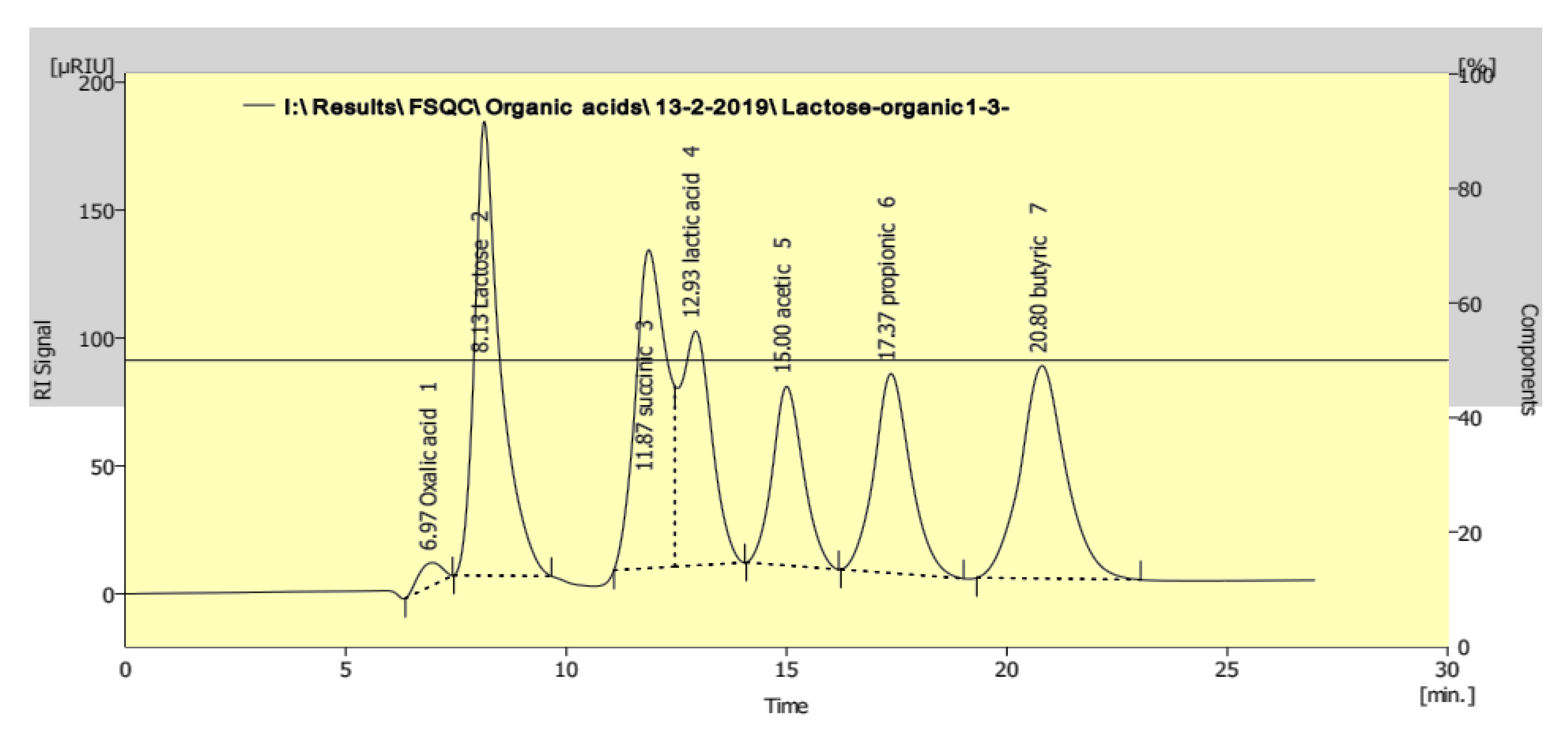
2.2.5. Organic Acid Profile
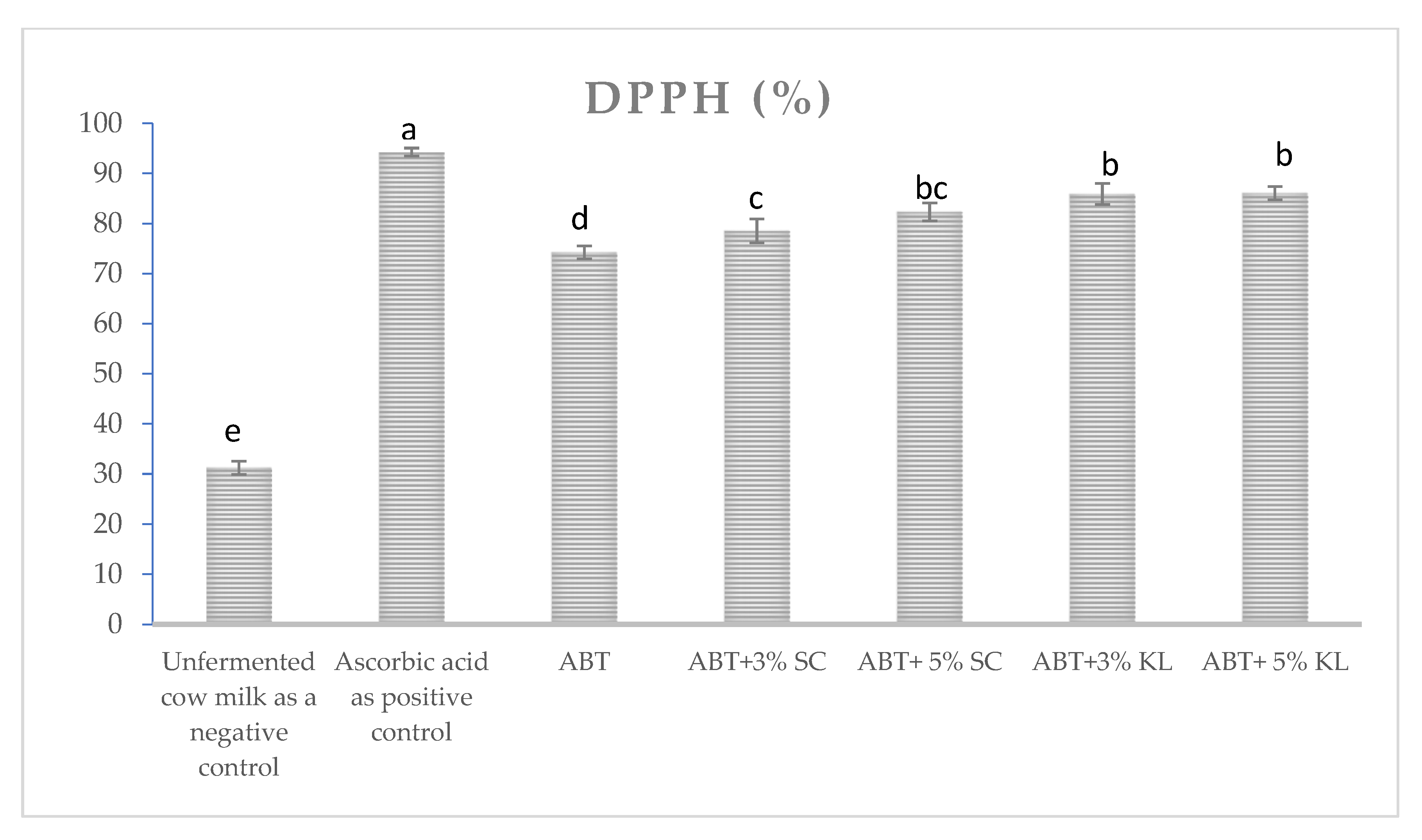
2.2.6. Antioxidant Activity
2.2.7. Proteolytic Activity
2.2.8. Statistical Analysis
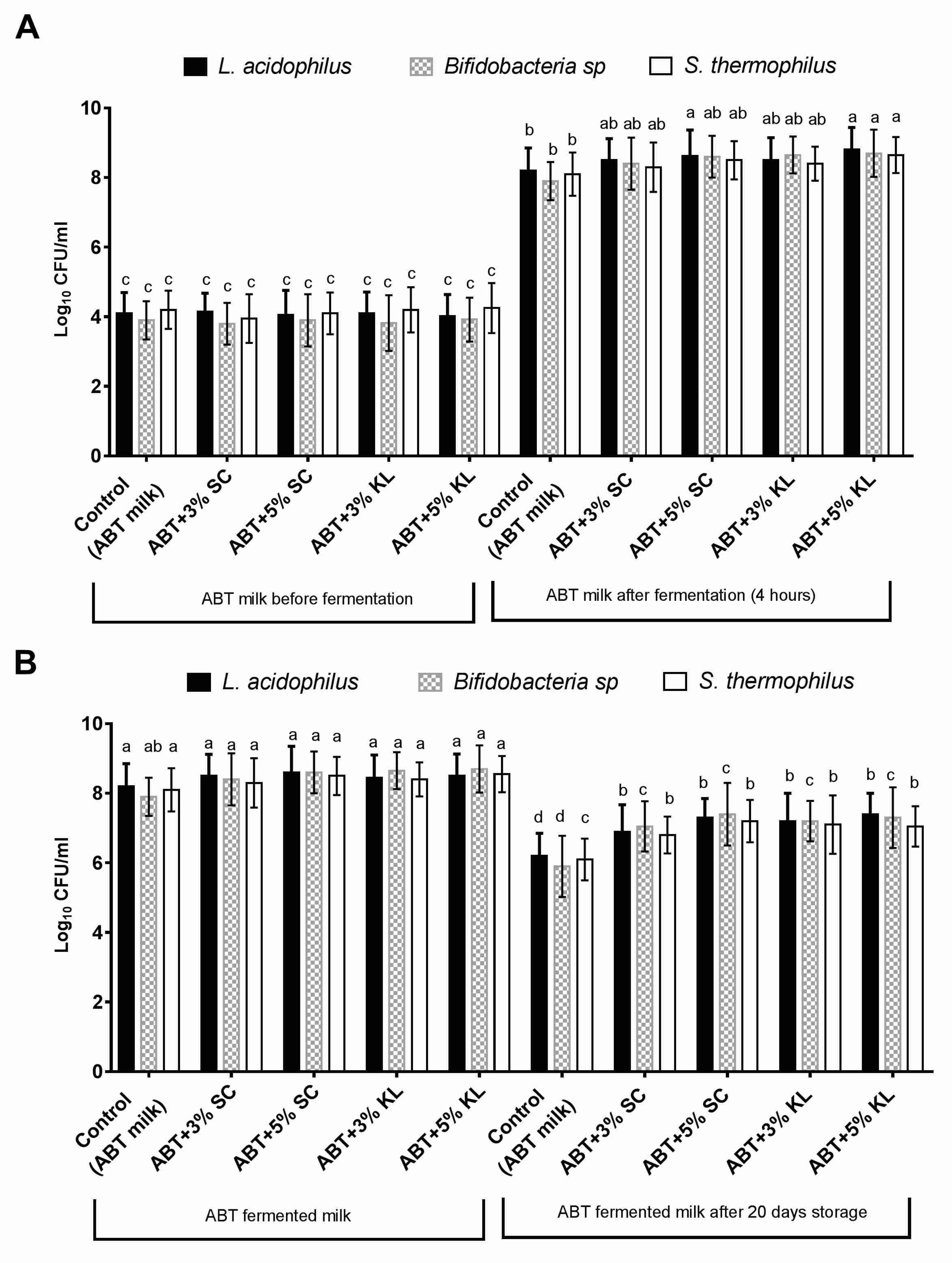
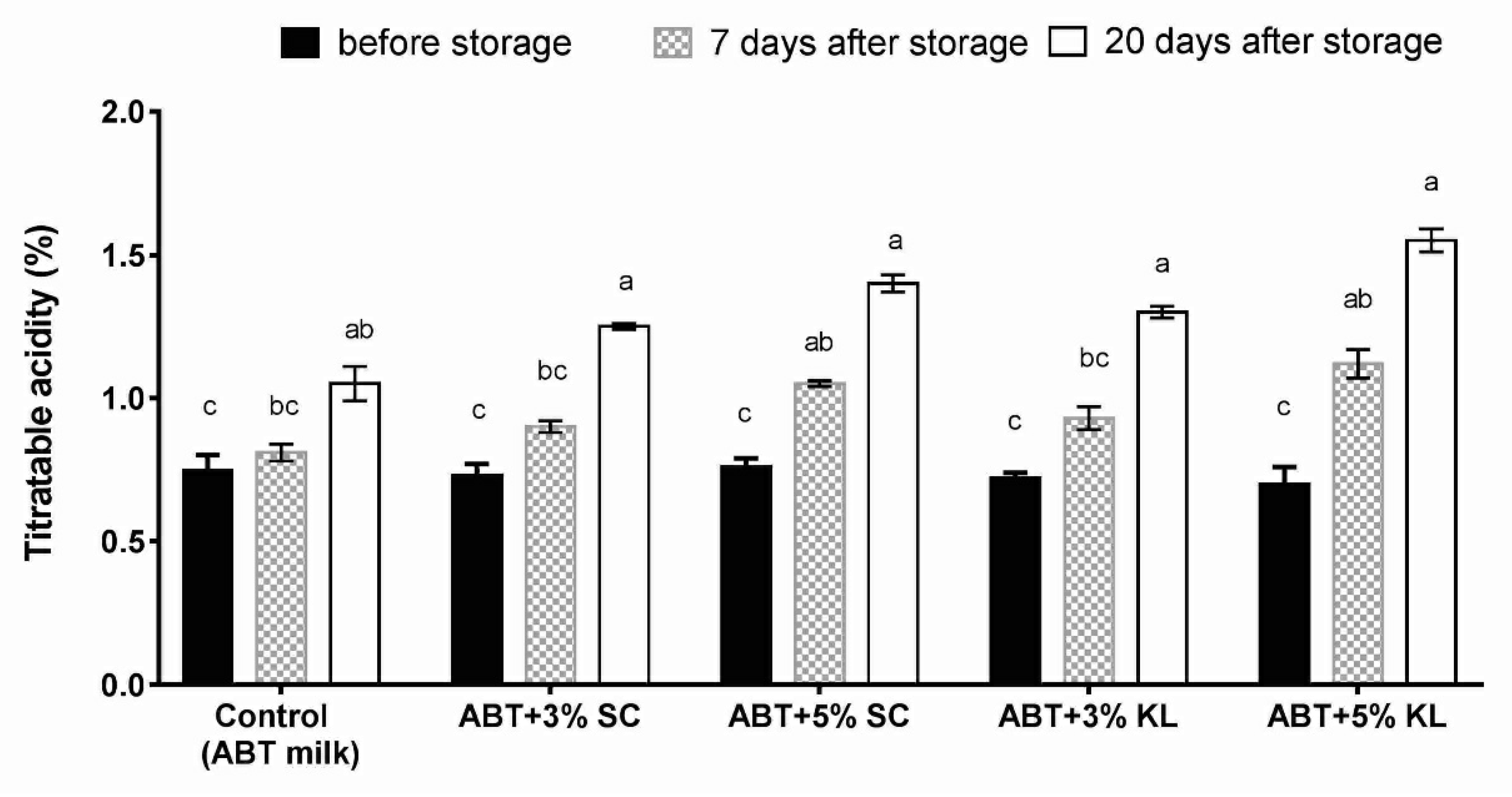
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABT | acidophilus-bifidus-thermophilus |
| NRRL | Northern Regional Research Laboratory |
| MRS | de Man, Rogosa, Sharp |
| ME | malt extract |
| TA | titratable acidity |
| HPLC | high-performance liquid chromatography |
| DPPH | Diphenylpicrylhydrazyl |
| OPA | o-phthaldialdehyde |
| CFU | colony forming unit |
| LAB | lactic acid bacteria |
References
- Yang, Y. Scientific substantiation of functional food health claims in China. J. Nutr. 2008, 138, 1199–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.H.; Ansari, F.A. Probiotics: The friendly bacteria with market potential in global market. Pak. J. Pharm. Sci. 2007, 20, 71–76. [Google Scholar]
- McFarland, L. A review of evidence of health claims for biotherapeutic agents. Microb. Ecol. Health Dis. 2000, 12, 65–76. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Calder, P.C. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Jungersen, M.; Wind, A.; Johansen, E.; Christensen, J.; Stuer-Lauridsen, B.; Eskesen, D. The Science behind the Probiotic Strain Bifidobacterium animalis subsp. lactis BB-12®. Microorganisms 2014, 2, 92–110. [Google Scholar] [CrossRef] [PubMed]
- Ondee, T.; Pongpirul, K.; Visitchanakun, P.; Saisorn, W.; Kanacharoen, S.; Wongsaroj, L.; Kullapanich, C.; Ngamwongsatit, N.; Settachaimongkon, S.; Somboonna, N.; et al. Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An overview of beneficial effects. In Lactic Acid Bacteria: Genetics, Metabolism and Applications; Springer: Dordrecht, the Netherlands, 2002; pp. 279–289. [Google Scholar]
- Chatterjee, S.; Kar, P.; Das, T.; Ray, S.; Ganguly, S.; Rajendiran, C.; Mitra, M. Randomised Placebo-controlled Double Blind Multicentric Trial on Efficacy and Safety of Lactobacillus acidophilus LA-5® and Bifidobacterium BB-12® for Prevention of Antibiotic-Associated Diarrhoea. J. Assoc. Phys. India 2013, 61, 708–712. [Google Scholar]
- Czerucka, D.; Rampal, P. Experimental effects of Saccharomyces boulardii on diarrheal pathogens. Microbes Infect. Inst. Pasteur 2002, 4, 733–739. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Araújo, E.A.; dos Santos Pires, A.C.; Pinto, M.S.; Jan, G.; Carvalho, A.F. Probiotics in dairy fermented products. Rijeja Intech 2012, 3, 129–148. [Google Scholar]
- EL-Deib, S.M.; Abd Rabo, F.R.; Badran, S.M.; Abd El-Fattah, A.M.; Elshaghabee, F.F. The growth behavior and enhancement of probiotic viability in bio-yoghurt. Int. Dairy J. 2012, 22, 44. [Google Scholar] [CrossRef]
- Antunes, A.C.; Cazetto, T.F.; Abolini, H.M. Viability of probiotic micro-organisms during storage, post acidification and sensory analysis of fat-free yogurts with added whey protein concentrate. Int. J. Dairy Technol. 2005, 58, 169–173. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Saarela, M.; Sondergaard, A.K.; Mistry, V.V.; Shah, N.P. Production and maintenance of viability of probiotic micro-organisms in dairy products. In Probiotic Dairy Products; Tamime, A.Y., Ed.; Blackwell Publishing: Oxford, UK; Hoboken, NJ, USA, 2005; pp. 39–72. [Google Scholar]
- Cruz, A.G.; Faria, J.F.; Walter, E.M.; Andrade, R.R.; Cavalcanti, R.N.; Oliveira, C.F.; Granato, D. Processing optimization of probiotic yogurt containing glucose oxidase using response surface methodology. J. Dairy Sci. 2010, 93, 5059–5068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamann, W.T.; Marth, E.H. Survival of Streptococcus thermophilus and Lactobacillus bulgaricus in commercial and experimental yogurts. J. Food Prot. 1983, 47, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Kailasapathy, K.; Harmstorf, I.; Phillips, M. Survival of Lactobacillus acidophilus and Bifidobacterium animalis ssp. lactis in stirred fruit yogurts. LWT Food Sci. Technol. 2008, 41, 1317–1322. [Google Scholar] [CrossRef]
- Donkor, O.N.; Nilmini, S.I.; Stolic, P.; Vasiljevic, T.; Shah, N.P. Survival and activity of selected probiotic organisms in set type yoghurt during cold storage. Int. Dairy J. 2007, 17, 657–665. [Google Scholar] [CrossRef]
- Sarao, L.K.; Arora, M. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 2017, 2, 344–371. [Google Scholar] [CrossRef]
- Karaolis, C.; Botsaris, G.; Pantelides, I.; Tsaltas, D. Potential application of Saccharomyces boulardii as a probiotic in goat’s yoghurt: Survival and organoleptic effects. Int. J. Food Sci. Technol. 2013, 48, 1445–1452. [Google Scholar] [CrossRef]
- Niamah, A.K. Physicochemical and microbial characteristics of yogurt with Added Saccharomyces boulardii. Curr. Res. Nutr. Food Sci. J. 2017, 5, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Lourens-Hattingh, A.; Viljoen, B.C. Growth and survival of a probiotic yeast in dairy products. Food Res. Int. 2001, 34, 791–796. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Method of Examination of Dairy Products, 16th ed.; American Public Health Association: Washington DC, USA, 1992. [Google Scholar]
- Süle, J.; Kõrösi, T.; Hucker, A.; Varga, L. Evaluation of culture media for selective enumeration of bifidobacteria and lactic acid bacteria. Braz. J. Microbiol. 2014, 45, 1023–1030. [Google Scholar] [CrossRef] [Green Version]
- Dave, R.I.; Shah, N.P. Evaluation of media for selective enumeration of Streptococcus thermophilus, Lactobacillus delbrueckii spp. bulgaricus, Lactobacillus acidophilus and bifidobacteria. J. Dairy Sci. 1996, 79, 1529–1536. [Google Scholar] [CrossRef]
- Jordano, R.; Serrano, C.E.; Torres, M.; Salmeron, J. Comparison of three M17 media for the enumeration of Streptococcus thermophilus in fermented dairy products. J. Food Prot. 1992, 55, 999–1002. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Bockelmann, W.; Meske, D.; de Vrese, M.; Walte, H.G.; Schrezenmeir, J.; Heller, K.J. Ethanol production by selected intestinal microorganisms and lactic acid bacteria growing under different nutritional conditions. Front. Microbiol. 2016, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Maksoud, A.A.; Anankanbil, S.; Zhou, Y.; Abd El-Ghany, I.H.; El-Beltagi, H.S.; Banerjee, C.; Petersen, S.V.; Guo, Z. Grafting phenolics onto milk protein via conjugated polymerization for delivery of multiple functionalities: Synthesis and characterization. Food Chem. 2019, 301, 125298. [Google Scholar] [CrossRef]
- Hébert, E.M.; Raya, R.R.; De Giori, G.S. Nutritional requirements of Lactobacillus delbrueckii subsp. lactis in a chemically defined medium. Curr. Microbiol. 2004, 49, 341–345. [Google Scholar]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Laursen, R.R.; Ouwehand, A.C. The production and delivery of probiotics: A review of a practical approach. Microorganisms 2019, 7, 83. [Google Scholar] [CrossRef] [Green Version]
- Hassanein, S.M.; Soliman, N.K. Effect of probiotic (Saccharomyces cerevisiae) adding to diets on intestinal microflora and performance of Hy-Line layers hens. J. Am. Sci. 2010, 6, 159–169. [Google Scholar]
- Lourens-Hattingh, A.; Viljoen, B.C. Yogurt as probiotic carrier food. Int. Dairy J. 2001, 11, 1–17. [Google Scholar] [CrossRef]
- Oliveira, R.P.S.; Florence, A.C.R.; Silva, R.C.; Perego, P.; Converti, A.; Gioielli, L.A.; Oliveira, M.N. Effect of different prebiotics on the fermentation kinetics, probiotic survival and fatty acids profiles in nonfat symbiotic fermented milk. Int. J. Food Microbiol. 2009, 128, 467–472. [Google Scholar] [CrossRef]
- Varga, L.; Süle, J.; Nagy, P. Short communication: Viability of culture organisms in honey-enriched acidophilus-bifidus-thermophilus (ABT)-type fermented camel milk. J. Dairy Sci. 2014, 97, 6814–6818. [Google Scholar] [CrossRef] [PubMed]
- Medina, L.; Jordano, R. Survival of constitutive microflora in commercially fermented milk containing bifidobacteria during refrigerated storage. J. Food Prot. 1994, 56, 731–733. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Cruz, A.G.; Faria, J.F.; Shah, N.P. Probiotic dairy products as functional foods. Compr. Rev. Food Sci. Food Saf. 2010, 9, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Teixidó, N.; Usall, J.; Atarés, E.; Viñas, I. The effect of nitrogen and carbon sources on growth of the biocontrol agent Pantoea agglomerans strain CPA-2. Lett. Appl. Microbiol. 2002, 35, 117–120. [Google Scholar] [CrossRef]
- Mohamed, M.S.M.; Elshaghabee, F.M.F.; Alharbi, S.A.; El-Hussein, A. The prospective beneficial effects of red laser exposure on Lactocaseibacillus casei fermentation of skim milk. Biology 2020, 9, 256. [Google Scholar] [CrossRef] [PubMed]
- Murillo, G.; Sun, J.; Ali, S.S.; Yan, Y.; Bartocci, P.; He, Y. Evaluation of the kinematic viscosity in biodiesel production with waste vegetable oil, ultrasonic irradiation and enzymatic catalysis: A comparative study in two-reactors. Fuel 2018, 227, 448–456. [Google Scholar] [CrossRef]
- Abu-Taraboush, H.M.; Al-Dagal, M.M.; Al-Royli, M.A. Growth, Viability, and Proteolytic Activity of Bifidobacteria in Whole Camel Milk. J. Dairy Sci. 1998, 81, 354–361. [Google Scholar] [CrossRef]
- Guo, C.; Yuan, Y.; Yue, T.; Hatab, S.; Wang, Z. Binding mechanism of patulin to heat-treated yeast cell. Lett. Appl. Microbiol. 2012, 55, 453–459. [Google Scholar] [CrossRef]
- EL-Deib, S.M.; Abd Rabo, F.R.; Badran, S.M.; Abd El-Fattah, A.M.; Elshaghabee, F.M.F. In vitro model for assessment of the health benefits of some microbial strains. Int. J. Probiotics Prebiotics 2010, 5, 157. [Google Scholar]
- Ibrahim, H.R.; Isono, H.; Miyata, T. Potential antioxidant bioactive peptides from camel milk proteins. Anim. Nutr. 2018, 4, 273–280. [Google Scholar] [CrossRef]
| Treatments * | Storage Period, Days | Concentration of Organic Acids (µg/mL) | |
|---|---|---|---|
| Lactic Acid | Acetic Acid | ||
| Control Unfermented Milk | 0 | ND | ND |
| 15 | ND | ND | |
| ABT | 0 | 75.25 ± 1.30 d,** | 285.60 ± 0.80 d |
| 20 | 120.45 ± 0.65 c | 457.80 ± 0.70 b | |
| ABT+3% SC | 0 | 85.30 ± 0.45 d | 290.78 ± 0.60 c |
| 20 | 135.40 ± 0.85 b | 479.60 ± 0.85 a | |
| ABT+5% SC | 0 | 86.35 ± 0.58 d | 295.85 ± 0.25 c |
| 20 | 145.67 ± 0.77 a | 488.32 ± 0.33 a | |
| ABT+3% KL | 0 | 85.35 ± 1.20 d | 293.25 ± 0.72 c |
| 20 | 138.30 ± 0.52 b | 475.05 ± 1.06 a | |
| ABT+5% KL | 0 | 88.45 ± 1.33 d | 289.90 ± 0.38 c |
| 20 | 143.40 ± 1.05 a | 482.85 ± 1.25 a | |
| Treatments | Before Storage | After 7 Days | After 20 Days Cold Storage |
|---|---|---|---|
| ABT | 3.15 ± 0.07 f,** | 3.50 ± 0.07 e | 3.84 ± 0.12 c,d |
| ABT + 3% SC | 3.31 ± 0.11 f | 3.68 ± 0.06 d,e | 3.92 ± 0.04 b,c |
| ABT + 5% SC | 3.51 ± 0.05 f | 3.72 ± 0.03 d,e | 4.21 ± 0.15 b |
| ABT + 3% KL | 3.42 ± 0.20 f,g | 3.81 ± 0.15 c,d | 4.13 ± 0.23 b |
| ABT + 5% KL | 3.71 ± 0.06 d,e | 3.92 ± 0.25 b,c | 4.45 ± 0.13 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshaghabee, F.M.F.; Abd El-Maksoud, A.A.; Alharbi, S.A.; Alfarraj, S.; Mohamed, M.S.M. Fortification of Acidophilus-bifidus-thermophilus (ABT) Fermented Milk with Heat-Treated Industrial Yeast Enhances Its Selected Properties. Molecules 2021, 26, 3876. https://doi.org/10.3390/molecules26133876
Elshaghabee FMF, Abd El-Maksoud AA, Alharbi SA, Alfarraj S, Mohamed MSM. Fortification of Acidophilus-bifidus-thermophilus (ABT) Fermented Milk with Heat-Treated Industrial Yeast Enhances Its Selected Properties. Molecules. 2021; 26(13):3876. https://doi.org/10.3390/molecules26133876
Chicago/Turabian StyleElshaghabee, Fouad M. F., Ahmed A. Abd El-Maksoud, Sulaiman Ali Alharbi, Saleh Alfarraj, and Mahmoud S. M. Mohamed. 2021. "Fortification of Acidophilus-bifidus-thermophilus (ABT) Fermented Milk with Heat-Treated Industrial Yeast Enhances Its Selected Properties" Molecules 26, no. 13: 3876. https://doi.org/10.3390/molecules26133876
APA StyleElshaghabee, F. M. F., Abd El-Maksoud, A. A., Alharbi, S. A., Alfarraj, S., & Mohamed, M. S. M. (2021). Fortification of Acidophilus-bifidus-thermophilus (ABT) Fermented Milk with Heat-Treated Industrial Yeast Enhances Its Selected Properties. Molecules, 26(13), 3876. https://doi.org/10.3390/molecules26133876







