Understanding the Impact of Key Wine Components on the Use of a Non-Swelling Ion-Exchange Resin for Wine Protein Fining Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Model Wines
2.2. Experimental Design
2.3. Adsorption Isotherms
3. Results and Discussion
3.1. Impact of pH on Protein Adsorption by Macro-Prep®
3.2. Impact of Ethanol on Protein Adsorption by Macro-Prep®
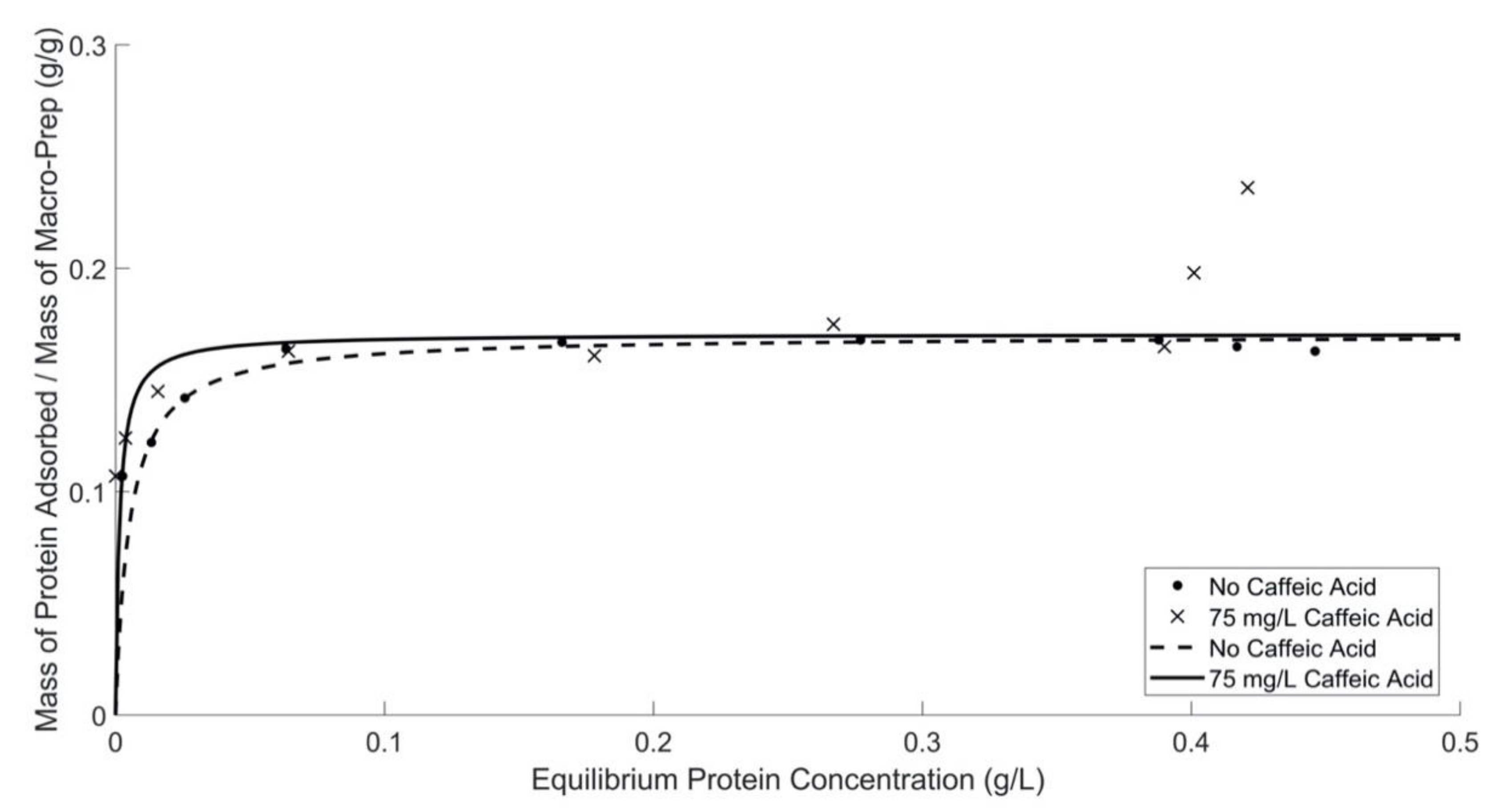
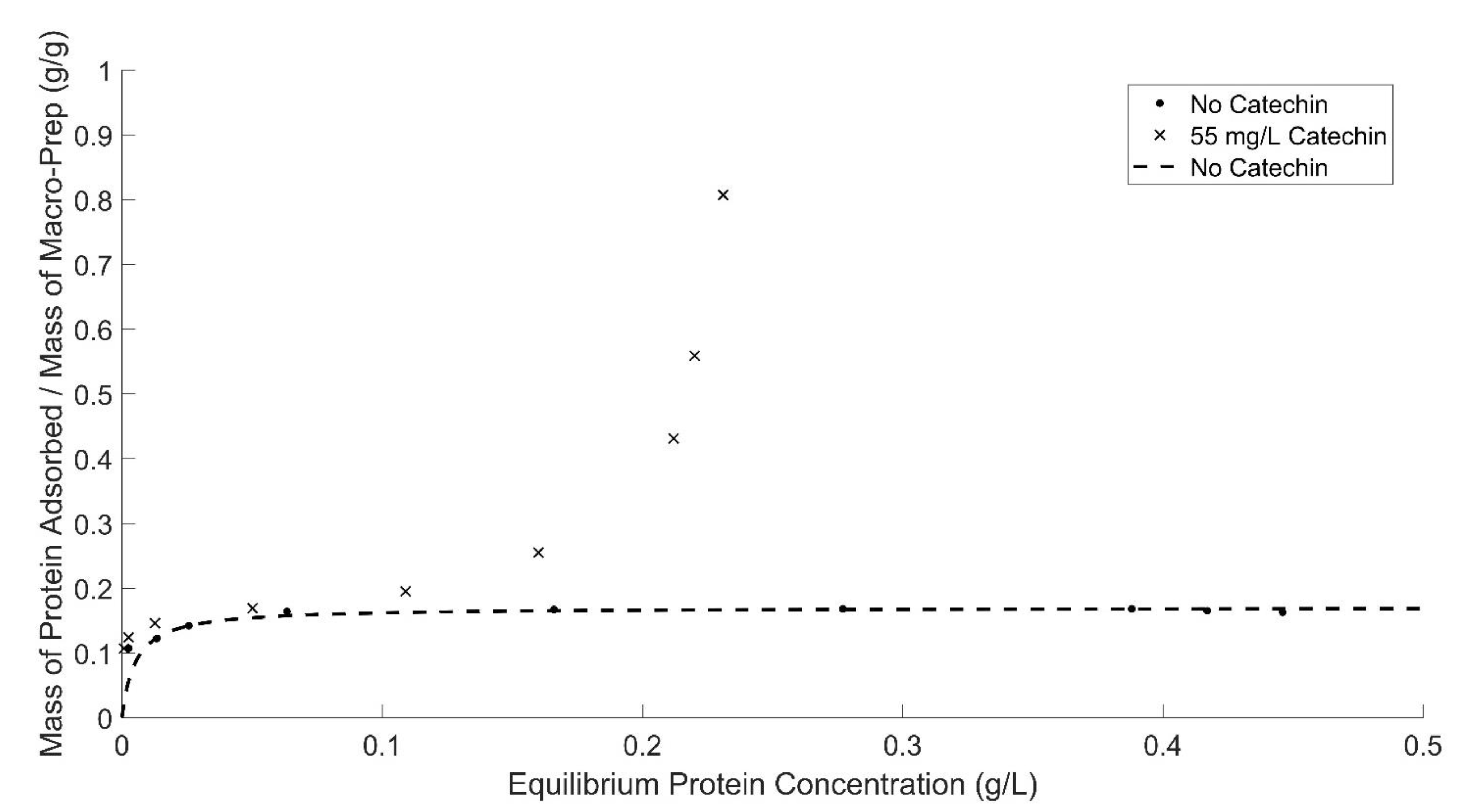
3.3. Impact of Prototypical Phenolic Compounds, Caffeic Acid and Catechin, on Protein Adsorption by Macro-Prep®
3.4. Impact of a Prototypical Polysaccharide on Protein Adsorption by Macro-Prep®
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Saywell, L.G. Clarification of Wine. Ind. Eng. Chem. 1934, 26, 981–982. [Google Scholar] [CrossRef]
- Mcleod, J.R.; Akiyoshi, M.; Berg, H.W. A New Bentonite. Am. J. Enol. Vitic. 1969, 20, 196–197. [Google Scholar]
- O’Neal, R.; Cruss, W.V. Comparative Effects of Casein, Gelatin and Bentonite Fining. Am. J. Enol. Vitic. 1950, 1, 69–72. [Google Scholar]
- Majewski, P.; Barbalet, A.; Waters, E. $1 Billion Hidden Cost of Bentonite Fining. Aust. N. Z. Grape-Grow. Winemak. 2011, 569, 58–62. [Google Scholar]
- Sarmento, M.; Oliveira, J.; Slatner, M.M.; Boulton, R. Kinetics of the adsorption of bovine serum albumin contained in a model wine solution by non-swelling ion-exchange resins. J. Food Eng. 1999, 39, 65–71. [Google Scholar] [CrossRef]
- Ladisch, M.R. Bioseparation Engineering: Principles, Practice, and Economics; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- de Bruijn, J.; Valdebenito, A.; Loyola, C.; Serra, I.; Salazar, F.; López, F. Continuous Stabilization of Chardonnay with Ion-Exchange Resin: Influence on Protein and Phenolic Profiles of Wine. Chil. J. Agric. Res 2000, 69, 54–59. [Google Scholar]
- Sarmento, M.R.; Oliveiraz, J.C.; Slatner, M.; Boulton, R.B. Effect of Ion-Exchange Adsorption on the Protein Profiles of White Wines. Food Sci. Technol. Int. 2001, 7, 217–224. [Google Scholar] [CrossRef]
- Boulton, R.; Singleton, V.; Bisson, L.; Kunkee, R. Principles and Practices of Winemaking; Chapman & Hall: London, UK, 1996. [Google Scholar]
- Sarmento, M.R.; Oliveira, J.; Boulton, R.B. Selection of low swelling materials for protein adsorption from white wines. Int. J. Food Sci. Technol. 2000, 35, 41–47. [Google Scholar] [CrossRef]
- Geankoplis, C.J. Transport. In Processes and Unit Operations, 3rd ed.; Prentice-Hall: Hoboken, NJ, USA, 1993. [Google Scholar]
- Bartolomé, B.; Estrella, I.; Hernández, M. Interaction of Low Molecular Weight Phenolics with Proteins (BSA). J. Food Sci. 2000, 65, 617–621. [Google Scholar] [CrossRef]
- Li, S.; Huang, K.; Zhong, M.; Guo, J.; Wang, W.-Z.; Zhu, R. Comparative studies on the interaction of caffeic acid, chlorogenic acid and ferulic acid with bovine serum albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Trnkova, L.; Bousova, I.; Kubicek, V.; Drsata, J. Binding of naturally occurring hydroxycinnamic acids to bovine serum albumin. Nat. Sci. 2010, 02, 563–570. [Google Scholar] [CrossRef] [Green Version]
- Sarni-Manchado, P.; Deleris, A.; Avallone, S.; Cheynier, V.; Moutounet, M. Analysis and Characterization of Wine Condensed Tannins Precipitated by Proteins Used as Fining Agent in Enology. Am. J. Enol. Vitic. 1999, 50, 81–86. [Google Scholar]
- Simon, C.; Barathieu, K.; Laguerre, M.; Schmitter, J.-M.; Fouquet, E.; Pianet, I.; Dufourc, E.J. Three-Dimensional Structure and Dynamics of Wine Tannin−Saliva Protein Complexes. A Multitechnique Approach. Biochemistry 2003, 42, 10385–10395. [Google Scholar] [CrossRef]
- Achaerandio, I.; Pachova, V.; Güell, C.; López, F. Protein Adsorption by Bentonite-123. Am. J. Enol. Vitic. 2001, 52, 122–126. [Google Scholar]
- Blade, W.H.; Boulton, R. Adsorption of Protein by Bentonite in a Model Wine Solution. Am. J. Enol. Vitic. 1988, 39, 193–199. [Google Scholar]
- Thibaud, C.; Coupé, A.; Livage, J. Interactions of Bovine Serum Albumin and Lysozyme with Sodium Silicate Solutions. Colloids Surf. B. 2003, 29, 189–196. [Google Scholar]
- Lucchetta, M.; Pocock, K.F.; Waters, E.J.; Marangon, M. Use of Zirconium Dioxide during Fermentation as an Alternative to Protein Fining with Bentonite for White Wines. Am. J. Enol. Vitic. 2013, 64, 400–404. [Google Scholar] [CrossRef]




| Min | Mid | Max | |
|---|---|---|---|
| pH | 3.3 | 3.6 | 3.9 |
| Ethanol (v/v %) | 10 | 12 | 14 |
| Caffeic acid (mg/L) | 0 | 75 | |
| Catechin (mg/L) | 0 | 55 | |
| Arabinogalactan (mg/L) | 0 | 100 |
| Model Wine pH 1 | Model Wine pH 2 | |||||
|---|---|---|---|---|---|---|
| 3.3 | 3.6 | 3.9 | 3.3 | 3.6 | 3.9 | |
| Langmuir constant, KL (g/L) | 0.0041 | 0.0057 | 0.022 | 0.0035 | 0.0051 | 0.0047 |
| Langmuir capacity, (qm) (g/g) | 0.17 | 0.16 | 0.16 | 0.17 | 0.17 | 0.17 |
| Langmuir correlation coefficient | 0.97 | 0.98 | 0.89 | 0.98 | 0.99 | 0.96 |
| Ethanol Concentration (v/v %) 1 | Ethanol Concentration (v/v %) 2 | |||||
|---|---|---|---|---|---|---|
| 10% | 12% | 14% | 10% | 12% | 14% | |
| Langmuir constant, KL (g/L) | 0.033 | 0.0060 | 0.0017 | 0.0014 | 0.0051 | 0.0013 |
| Langmuir capacity, (qm) (g/g) | 0.18 | 0.16 | 0.16 | 0.16 | 0.17 | 0.17 |
| Langmuir correlation coefficient | 0.97 | 0.99 | 0.97 | 0.98 | 0.99 | 0.94 |
| Caffeic Acid (mg/L) | 0 | 75 |
|---|---|---|
| Langmuir constant, KL (g/L) | 0.0051 | 0.0015 |
| Langmuir capacity, (qm) (g/g) | 0.17 | 0.17 |
| Langmuir correlation coefficient | 0.99 | 0.89 |
| Catechin (mg/L) | 0 | 55 |
|---|---|---|
| Langmuir constant KL (g/L) | 0.0051 | 0.00038 |
| Langmuir capacity (qm) (g/g) | 0.17 | 0.15 |
| Langmuir correlation coefficient | 0.99 | 0.94 |
| Arabinogalactan (mg/L) 1 | Arabinogalactan (mg/L) 2 | |||
|---|---|---|---|---|
| 0 | 100 | 0 | 100 | |
| Langmuir constant KL (g/L) | 0.0060 | 0.0038 | 0.0051 | 0.0052 |
| Langmuir capacity (qm) (g/g) | 0.16 | 0.16 | 0.17 | 0.17 |
| Langmuir correlation coefficient | 0.99 | 0.93 | 0.99 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Srinivas, A.; Runnebaum, R.C. Understanding the Impact of Key Wine Components on the Use of a Non-Swelling Ion-Exchange Resin for Wine Protein Fining Treatment. Molecules 2021, 26, 3905. https://doi.org/10.3390/molecules26133905
Sun L, Srinivas A, Runnebaum RC. Understanding the Impact of Key Wine Components on the Use of a Non-Swelling Ion-Exchange Resin for Wine Protein Fining Treatment. Molecules. 2021; 26(13):3905. https://doi.org/10.3390/molecules26133905
Chicago/Turabian StyleSun, Lin, Ananya Srinivas, and Ron C. Runnebaum. 2021. "Understanding the Impact of Key Wine Components on the Use of a Non-Swelling Ion-Exchange Resin for Wine Protein Fining Treatment" Molecules 26, no. 13: 3905. https://doi.org/10.3390/molecules26133905
APA StyleSun, L., Srinivas, A., & Runnebaum, R. C. (2021). Understanding the Impact of Key Wine Components on the Use of a Non-Swelling Ion-Exchange Resin for Wine Protein Fining Treatment. Molecules, 26(13), 3905. https://doi.org/10.3390/molecules26133905






