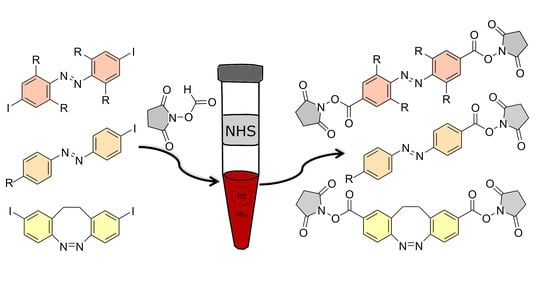
Active Ester Functionalized Azobenzenes as Versatile Building Blocks
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. General Procedures
4.2.1. General Procedure 1 Carbonylation
4.2.2. General Procedure 2 Carbonylation
4.2.3. General Procedure Condensation Reaction
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhou, H.; Xue, C.; Weis, P.; Suzuki, Y.; Huang, S.; Koynov, K.; Auernhammer, G.K.; Berger, R.; Butt, H.-J.; Wu, S. Photoswitching of Glass Transition Temperatures of Azobenzene-Containing Polymers Induces Reversible Solid-to-Liquid Transitions. Nat. Chem. 2017, 9, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Sasaki, T.; Ichimura, K. Photochemical switching of polarization in ferroelectric liquid-crystal films. Nature 1993, 361, 428–430. [Google Scholar] [CrossRef]
- Yamada, M.; Kondo, M.; Mamiya, J.-I.; Yu, Y.; Kinoshita, M.; Barrett, C.J.; Ikeda, T. Photomobile Polymer Materials: Towards Light-Driven Plastic Motors. Angew. Chem. Int. Ed. 2008, 47, 4986–4988. [Google Scholar] [CrossRef] [PubMed]
- Kizilkan, E.; Strueben, J.; Jin, X.; Schaber, C.F.; Adelung, R.; Staubitz, A.; Gorb, S.N. Influence of the Porosity on the Photoresponse of a Liquid Crystal Elastomer. R. Soc. Open. Sci. 2016, 3, 150700. [Google Scholar] [CrossRef] [PubMed]
- Kizilkan, E.; Strueben, J.; Staubitz, A.; Gorb, S.N. Bioinspired Photocontrollable Microstructured Transport Device. Sci. Robot. 2017, 2, eaak9454. [Google Scholar] [CrossRef]
- Dowds, M.; Bank, D.; Strueben, J.; Soto, D.P.; Sönnichsen, F.D.; Renth, F.; Temps, F.; Staubitz, A. Efficient Reversible Photoisomerisation with Large Solvodynamic Size-Switching of a Main Chain poly(azobenzene-alt-trisiloxane). J. Mater. Chem. C 2020, 8, 1835–1845. [Google Scholar] [CrossRef]
- Archut, A.; Vögtle, F.; De Cola, L.; Azzelini, G.C.; Balzani, V.; Ramanujam, P.S.; Berg, R.H. Azobenzene-Functionalized Cascade Molecules: Photoswitchable Supramolecular Systems. Chem. Eur. J. 1998, 4, 699–706. [Google Scholar] [CrossRef]
- Pötschke, D.; Ballauff, M.; Lindner, P.; Fischer, M.; Vögtle, F. Analysis of the Structure of Dendrimers in Solution by Small-Angle Neutron Scattering Including Contrast Variation. Macromolecules 1999, 32, 4079–4087. [Google Scholar] [CrossRef]
- Beharry, A.A.; Woolley, G.A. Azobenzene Photoswitches for Biomolecules. Chem. Soc. Rev. 2011, 40, 4422–4437. [Google Scholar] [CrossRef]
- Schehr, M.; Ianes, C.; Weisner, J.; Heintze, L.; Muller, M.P.; Pichlo, C.; Charl, J.; Brunstein, E.; Ewert, J.; Lehr, M.; et al. 2-Azo-, 2-Diazocine-thiazols and 2-Azo-imidazoles as Photoswitchable Kinase Inhibitors: Limitations and Pitfalls of the Photoswitchable Inhibitor Approach. Photochem. Photobiol. Sci. 2019, 18, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Heintze, L.; Schmidt, D.; Rodat, T.; Witt, L.; Ewert, J.; Kriegs, M.; Herges, R.; Peifer, C. Photoswitchable Azo- and Diazocine-Functionalized Derivatives of the VEGFR-2 Inhibitor Axitinib. Int. J. Mol. Sci. 2020, 21, 8961. [Google Scholar] [CrossRef]
- Wegener, M.; Hansen, M.J.; Driessen, A.J.M.; Szymanski, W.; Feringa, B.L. Photocontrol of Antibacterial Activity: Shifting from UV to Red Light Activation. J. Am. Chem. Soc. 2017, 139, 17979–17986. [Google Scholar] [CrossRef]
- Hoorens, M.W.H.; Ourailidou, M.E.; Rodat, T.; van der Wouden, P.E.; Kobauri, P.; Kriegs, M.; Peifer, C.; Feringa, B.L.; Dekker, F.J.; Szymanski, W. Light-Controlled Inhibition of BRAFV600E Kinase. Eur. J. Med. Chem. 2019, 179, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Hull, K.; Morstein, J.; Trauner, D. In Vivo Photopharmacology. Chem. Rev. 2018, 118, 10710–10747. [Google Scholar] [CrossRef]
- Stein, M.; Middendorp, S.J.; Carta, V.; Pejo, E.; Raines, D.E.; Forman, S.A.; Sigel, E.; Trauner, D. Azo-Propofols: Photochromic Potentiators of GABA(A) Receptors. Angew. Chem. Int. Ed. 2012, 51, 10500–10504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velema, W.A.; Szymanski, W.; Feringa, B.L. Photopharmacology: Beyond Proof of Principle. J. Am. Chem. Soc. 2014, 136, 2178–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arkhipova, V.; Fu, H.; Hoorens, M.W.H.; Trinco, G.; Lameijer, L.N.; Marin, E.; Feringa, B.L.; Poelarends, G.J.; Szymanski, W.; Slotboom, D.J.; et al. Structural Aspects of Photopharmacology: Insight into the Binding of Photoswitchable and Photocaged Inhibitors to the Glutamate Transporter Homologue. J. Am. Chem. Soc. 2021, 143, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Kolarski, D.; Miller, S.; Oshima, T.; Nagai, Y.; Aoki, Y.; Kobauri, P.; Srivastava, A.; Sugiyama, A.; Amaike, K.; Sato, A.; et al. Photopharmacological Manipulation of Mammalian CRY1 for Regulation of the Circadian Clock. J. Am. Chem. Soc. 2021, 143, 2078–2087. [Google Scholar] [CrossRef]
- Hansen, M.J.; Hille, J.I.C.; Szymanski, W.; Driessen, A.J.M.; Feringa, B.L. Easily Accessible, Highly Potent, Photocontrolled Modulators of Bacterial Communication. Chem 2019, 5, 1293–1301. [Google Scholar] [CrossRef]
- Volgraf, M.; Gorostiza, P.; Numano, R.; Kramer, R.H.; Isacoff, E.Y.; Trauner, D. Allosteric Control of an Ionotropic Glutamate Receptor with an Optical Switch. Nat. Chem. Biol. 2006, 2, 47–52. [Google Scholar] [CrossRef]
- Banghart, M.; Borges, K.; Isacoff, E.; Trauner, D.; Kramer, R.H. Light-Activated Ion Channels for Remote Control of Neuronal Firing. Nat. Neurosci. 2004, 7, 1381–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velema, W.A.; van der Berg, J.P.; Hansen, M.J.; Szymanski, W.; Driessen, A.J.; Feringa, B.L. Optical Control of Antibacterial Activity. Nat. Chem. 2013, 5, 924–928. [Google Scholar] [CrossRef]
- Zhang, Y.; Erdmann, F.; Fischer, G. Augmented Photoswitching Modulates Immune Signaling. Nat. Chem. Biol. 2009, 5, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Yager, K.G.; Barrett, C.J. Novel Photo-Switching using Azobenzene Functional Materials. J. Photochem. Photobiol. A 2006, 182, 250–261. [Google Scholar] [CrossRef]
- Lancia, F.; Ryabchun, A.; Katsonis, N. Life-Like Motion Driven by Artificial Molecular Machines. Nat. Rev. Chem. 2019, 3, 536–551. [Google Scholar] [CrossRef]
- Iamsaard, S.; Asshoff, S.J.; Matt, B.; Kudernac, T.; Cornelissen, J.J.; Fletcher, S.P.; Katsonis, N. Conversion of Light into Macroscopic Helical Motion. Nat. Chem. 2014, 6, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Koshima, H.; Ojima, N.; Uchimoto, H. Mechanical Motion of Azobenzene Crystals upon Photoirradiation. J. Am. Chem. Soc. 2009, 131, 6890–6891. [Google Scholar] [CrossRef]
- Gelebart, A.H.; Mulder, D.J.; Varga, M.; Konya, A.; Vantomme, G.; Meijer, E.W.; Selinger, R.L.B.; Broer, D.J. Making Waves in a Photoactive Polymer Film. Nature 2017, 546, 632–636. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, T.; Tsutsumi, O. Optical Switching and Image Storage by Means of Azobenzene Liquid-Crystal Films. Science 1995, 268. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.F.; Hashimoto, K.; Fujishima, A. Photoelectrochemical Information Storage Using an Azobenzene Derivative. Nature 1990, 347, 658–660. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hasegawa, M.; Kanazawa, A.; Sihiono, T.; Ikeda, T. Holographic Gratings and Holographic Image Storage via Photochemical Phase Transitions of Polymer Azobenzene Liquid-Crystal Films. J. Mater. Chem. 2000, 10, 337–342. [Google Scholar] [CrossRef]
- Åstrand, P.-O.; Ramanujam, P.S.; Hvilsted, S.; Bak, K.L.; Sauer, S.P.A. Ab Initio Calculation of the Electronic Spectrum of Azobenzene Dyes and Its Impact on the Design of Optical Data Storage Materials. J. Am. Chem. Soc. 2000, 122, 3482–3487. [Google Scholar] [CrossRef]
- Hartley, G.S. The Cis-form of Azobenzene. Nature 1937, 140, 281. [Google Scholar] [CrossRef]
- Bandara, H.M.; Burdette, S.C. Photoisomerization in Different Classes of Azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J. A Refinement of the Crystal Structure of Azobenzene. Acta Cryst. 1966, 21, 146–152. [Google Scholar] [CrossRef]
- Mostad, A.; Rømming, C. A Refinement of the Crystal Structure of cis-Azobenzene. Acta Chem. Scand. 1971, 25, 3561–3568. [Google Scholar] [CrossRef]
- Monti, S.; Orlandi, G.; Palmieri, P. Features of the Photochemically Active State Surfaces of Azobenzenes. Chem. Phys. 1982, 71, 87–99. [Google Scholar] [CrossRef]
- Brash, D.E.; Rudolph, J.A.; Simon, J.A.; Lin, A.; McKenna, G.J.; Baden, H.P.; Halperin, A.J.; Pontén, J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 1991, 88, 10124–10128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forman, J.; Dietrich, M.; Todd Monroe, W. Photobiological and Thermal Effects of Photoactivating UVA Light Doses on Cell Cultures. Photochem. Photobiol. Sci. 2007, 6, 649–658. [Google Scholar] [CrossRef]
- Dong, Q.; Svoboda, K.; Tiersch, T.R.; Todd Monroe, W. Photobiological effects of UVA and UVB light in zebrafish embryos: Evidence for a competent photorepair system. J. Photochem. Photobiol. B 2007, 88, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Beharry, A.A.; Sadovski, O.; Woolley, G.A. Azobenzene Photoswitching without Ultraviolet Light. J. Am. Chem. Soc. 2011, 133, 19684–19687. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; McCormick, T.M.; Schmidt, S.K.; Seferos, D.S.; Woolley, G.A. Robust visible light photoswitching with ortho-thiol substituted azobenzenes. Chem. Commun. 2013, 49, 10314–10316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanta, S.; Beharry, A.A.; Sadovski, O.; McCormick, T.M.; Babalhavaeji, A.; Tropepe, V.; Woolley, G.A. Photoswitching Azo Compounds in Vivo with Red Light. J. Am. Chem. Soc. 2013, 135, 9777–9784. [Google Scholar] [CrossRef]
- Rullo, A.; Reiner, A.; Reiter, A.; Trauner, D.; Isacoff, E.Y.; Woolley, G.A. Long wavelength optical control of glutamate receptor ion channels using a tetra-ortho-substituted azobenzene derivative. Chem. Commun. 2014, 50, 14613–14615. [Google Scholar] [CrossRef] [Green Version]
- Konrad, D.B.; Frank, J.A.; Trauner, D. Synthesis of Redshifted Azobenzene Photoswitches by Late-Stage Functionalization. Chem. Eur. J. 2016, 22, 4364–4368. [Google Scholar] [CrossRef]
- Hansen, M.J.; Lerch, M.M.; Szymanski, W.; Feringa, B.L. Direct and Versatile Synthesis of Red-Shifted Azobenzenes. Angew. Chem. Int. Ed. 2016, 55, 13514–13518. [Google Scholar] [CrossRef]
- Dong, M.; Babalhavaeji, A.; Collins, C.V.; Jarrah, K.; Sadovski, O.; Dai, Q.; Woolley, G.A. Near-Infrared Photoswitching of Azobenzenes under Physiological Conditions. J. Am. Chem. Soc. 2017, 139, 13483–13486. [Google Scholar] [CrossRef] [PubMed]
- Bléger, D.; Schwarz, J.; Brouwer, A.M.; Hecht, S. o-Fluoroazobenzenes as Readily Synthesized Photoswitches Offering Nearly Quantitative Two-Way Isomerization with Visible Light. J. Am. Chem. Soc. 2012, 134, 20597–20600. [Google Scholar] [CrossRef] [PubMed]
- Lameijer, L.N.; Budzak, S.; Simeth, N.A.; Hansen, M.J.; Feringa, B.L.; Jacquemin, D.; Szymanski, W. General Principles for the Design of Visible-Light-Responsive Photoswitches: Tetra-ortho-Chloro-Azobenzenes. Angew. Chem. Int. Ed. 2020, 59, 21663–21670. [Google Scholar] [CrossRef]
- Yang, Y.; Hughes, R.P.; Aprahamian, I. Near-Infrared Light Activated Azo-BF2 Switches. J. Am. Chem. Soc. 2014, 136, 13190–13193. [Google Scholar] [CrossRef]
- Siewertsen, R.; Neumann, H.; Buchheim-Stehn, B.; Herges, R.; Näther, C.; Renth, F.; Temps, F. Highly Efficient Reversible Z-E Photoisomerization of a Bridged Azobenzene with Visible Light through Resolved S1(nπ*) Absorption Bands. J. Am. Chem. Soc. 2009, 131, 15594–15595. [Google Scholar] [CrossRef]
- Lentes, P.; Stadler, E.; Röhricht, F.; Brahms, A.; Gröbner, J.; Sönnichsen, F.D.; Gescheidt, G.; Herges, R. Nitrogen Bridged Diazocines: Photochromes Switching within the Near-Infrared Region with High Quantum Yields in Organic Solvents and in Water. J. Am. Chem. Soc. 2019, 141, 13592–13600. [Google Scholar] [CrossRef]
- Anderson, G.W.; Zimmerman, J.E.; Callahan, F.M. N-Hydroxysuccinimide Esters in Peptide Synthesis. J. Am. Chem. Soc. 1963, 85, 3039. [Google Scholar] [CrossRef]
- Anderson, G.W.; Zimmerman, J.E.; Callahan, F.M. The Use of Esters of N-Hydroxysuccinimide in Peptide Synthesis. J. Am. Chem. Soc. 1964, 86, 1839–1842. [Google Scholar] [CrossRef]
- Anderson, G.W.; Callahan, F.M.; Zimmerman, J.E. Synthesis of N-Hydroxysuccinimide Esters of Acyl Peptides by the Mixed Anhydride Method. J. Am. Chem. Soc. 1967, 89, 178. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Khoshdel, E.; Haddleton, D.M. Bioconjugation onto Biological Surfaces with Fluorescently Labeled Polymers. Chem. Commun. 2007, 1722–1724. [Google Scholar] [CrossRef]
- Koniev, O.; Wagner, A. Developments and Recent Advancements in the Field of Endogenous Amino Acid Selective Bond Forming Reactions for Bioconjugation. Chem. Soc. Rev. 2015, 44, 5495–5551. [Google Scholar] [CrossRef] [Green Version]
- A, S.; Xu, Q.; Zhou, D.; Gao, Y.; Vasquez, J.M.; Greiser, U.; Wang, W.; Liu, W.; Wang, W. Hyperbranched PEG-Based Multi-NHS Polymer and Bioconjugation with BSA. Polym. Chem. 2017, 8, 1283–1287. [Google Scholar] [CrossRef]
- Stephanopoulos, N.; Francis, M.B. Choosing an Effective Protein Bioconjugation Strategy. Nat. Chem. Biol. 2011, 7, 876–884. [Google Scholar] [CrossRef]
- Luo, Y.; Prestwich, G.D. Hyaluronic Acid-N-hydroxysuccinimide: A Useful Intermediate for Bioconjugation. Bioconjugate Chem. 2001, 12, 1085–1088. [Google Scholar] [CrossRef]
- Fissi, A.; Ciardelli, F. Photoresponsive Polymers: Azobenzene-Containg Poly-(L-Lysine). Biopolymers 1987, 26, 1993–2007. [Google Scholar] [CrossRef]
- Fang, L.; Han, G.; Zhang, J.; Zhang, H.; Zhang, H. Synthesis of Well-Defined Easily Crosslinkable Azobenzene Side-Chain Liquid Crystalline Polymers via Reversible Addition–Fragmentation Chain Transfer Polymerization and Photomechanical Properties of their Post-Crosslinked Fibers. Eur. Polym. J. 2015, 69, 592–604. [Google Scholar] [CrossRef]
- Gallot, B.; Fafiotte, M. Poly(L-lysine) Containing Azobenzene Units in the Side Chains: Influence of the Degree of Substitution on Liquid Crystalline Structure and Thermotropic Behaviour. Liq. Cryst. 1997, 23, 137–146. [Google Scholar] [CrossRef]
- Guo, C.; Gao, J.; Ma, S.; Zhang, H. Efficient Preparation of Chemically Crosslinked Recyclable Photodeformable Azobenzene Polymer Fibers with High Processability and Reconstruction Ability via a Facile Post-Crosslinking Method. Eur. Polym. J. 2020, 139, 109998. [Google Scholar] [CrossRef]
- Han, G.; Zhang, H.; Chen, J.; Sun, Q.; Zhang, Y.; Zhang, H. Easily Crosslinkable Side-Chain Azobenzene Polymers for Fast and Persistent Fixation of Surface Relief Gratings. New J. Chem. 2015, 39, 1410–1420. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Kuang, Y.; Xu, B. Enzymatic Formation of a Photoresponsive Supramolecular Hydrogel. Chem. Commun. 2010, 46, 5364–5366. [Google Scholar] [CrossRef]
- Li, X.; Wen, R.; Zhang, Y.; Zhu, L.; Zhang, B.; Zhang, H. Photoresponsive Side-Chain Liquid Crystalline Polymers with an Easily Cross-Linkable Azobenzene Mesogen. J. Mater. Chem. 2009, 19, 236–245. [Google Scholar] [CrossRef]
- Lv, J.-a.; Wang, W.; Wu, W.; Yu, Y. A Reactive Azobenzene Liquid-Crystalline Block Copolymer as a Promising Material for Practical Application of Light-Driven Soft Actuators. J. Mater. Chem. C 2015, 3, 6621–6626. [Google Scholar] [CrossRef]
- Pang, X.; Xu, B.; Qing, X.; Wei, J.; Yu, Y. Photo-Induced Bending Behavior of Post-Crosslinked Liquid Crystalline Polymer/Polyurethane Blend Films. Macromol. Rapid. Commun. 2018, 39, 1700237. [Google Scholar] [CrossRef]
- Rastogi, S.K.; Anderson, H.E.; Lamas, J.; Barret, S.; Cantu, T.; Zauscher, S.; Brittain, W.J.; Betancourt, T. Enhanced Release of Molecules upon Ultraviolet (UV) Light Irradiation from Photoresponsive Hydrogels Prepared from Bifunctional Azobenzene and Four-Arm Poly(ethylene glycol). ACS Appl. Mater. Interfaces 2018, 10, 30071–30080. [Google Scholar] [CrossRef]
- Wang, C.; Fadeev, M.; Zhang, J.; Vazquez-Gonzalez, M.; Davidson-Rozenfeld, G.; Tian, H.; Willner, I. Shape-Memory and Self-Healing Functions of DNA-Based Carboxymethyl Cellulose Hydrogels Driven by Chemical or Light Triggers. Chem. Sci. 2018, 9, 7145–7152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barré, A.; Ţînţaş, M.-L.; Levacher, V.; Papamicaël, C.; Gembus, V. An Overview of the Synthesis of Highly Versatile N-Hydroxysuccinimide Esters. Synthesis 2016, 49, 472–483. [Google Scholar] [CrossRef]
- Patel, N.; Davies, M.C.; Hartshorne, M.; Heaton, R.J.; Roberts, C.J.; Tendler, S.J.B.; Williams, P.M. Immobilization of Protein Molecules onto Homogeneous and Mixed Carboxylate-Terminated Self-Assembled Monolayers. Langmuir 1997, 13, 6485–6490. [Google Scholar] [CrossRef]
- Zhan, N.; Palui, G.; Merkl, J.-P.; Mattoussi, H. Bio-Orthogonal Coupling as a Means of Quantifying the Ligand Density on Hydrophilic Quantum Dots. J. Am. Chem. Soc. 2016, 138, 3190–3201. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Doung, T.T.; McKee, S.P.; Thompson, W.J. N,N′-Dissuccinimidyl Carbonate: A Useful Reagent for Alkoxycarbonylation of Amines. Tetrahedron Lett. 1992, 33, 2781–2784. [Google Scholar] [CrossRef]
- Schulze, A.; Giannis, A. IBX-Mediated Conversion of Primary Alcohols and Aldehydes to N-Hydroxysuccinimide Esters. Adv. Synth. Catal. 2004, 346, 252–256. [Google Scholar] [CrossRef]
- Tan, B.; Toda, N.; Barbas, C.F., 3rd. Organocatalytic Amidation and Esterification of Aldehydes with Activating Reagents by a Cross-Coupling Strategy. Angew. Chem. Int. Ed. 2012, 51, 12538–12541. [Google Scholar] [CrossRef]
- Barré, A.; Ţînţaş, M.-L.; Alix, F.; Gembus, V.; Papamicaël, C.; Levacher, V. Palladium-Catalyzed Carbonylation of (Hetero)Aryl, Alkenyl and Allyl Halides by Means of N-Hydroxysuccinimidyl Formate as CO Surrogate. J. Org. Chem. 2015, 80, 6537–6544. [Google Scholar] [CrossRef]
- Ueda, T.; Konishi, H.; Manabe, K. Trichlorophenyl Formate: Highly Reactive and Easily Accessible Crystalline CO Surrogate for Palladium-Catalyzed Carbonylation of Aryl/Alkenyl Halides and Triflates. Org. Lett. 2012, 14, 5370–5373. [Google Scholar] [CrossRef]
- Keiper, S.; Vyle, J.S. Reversible Photocontrol of Deoxyribozyme-Catalyzed RNA Cleavage under Multiple-Turnover Conditions. Angew. Chem. Int. Ed. 2006, 45, 3306–3309. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Haldar, S.; Franses, J.; Kim, J.-M.; Thompson, D.H. Synthesis and Self-assembly Properties of Acylated Cyclodextrins and Nitrilotriacetic Acid (NTA)-modified Inclusion Ligands for Interfacial Protein Crystallization. Supramol. Chem. 2006, 17, 101–111. [Google Scholar] [CrossRef]
- Hu, M.; Li, L.; Wu, H.; Su, Y.; Yang, P.Y.; Uttamchandani, M.; Xu, Q.H.; Yao, S.Q. Multicolor, one- and two-photon imaging of enzymatic activities in live cells with fluorescently Quenched Activity-Based Probes (qABPs). J. Am. Chem Soc. 2011, 133, 12009–12020. [Google Scholar] [CrossRef]
- Tuuttila, T.; Lipsonen, J.; Huuskonen, J.; Rissanen, K. Chiral Donor–π-Acceptor Azobenzene Dyes. Dyes Pigm. 2009, 80, 34–40. [Google Scholar] [CrossRef]
- Zhao, D.; Ouyang, D.; Jiang, M.; Liao, Y.; Peng, H.; Xie, X. Photomodulated Electro-optical Response in Self-Supporting Liquid Crystalline Physical Gels. Langmuir 2018, 34, 7519–7526. [Google Scholar] [CrossRef] [PubMed]
- Fatas, P.; Longo, E.; Rastrelli, F.; Crisma, M.; Toniolo, C.; Jimenez, A.I.; Cativiela, C.; Moretto, A. Bis(azobenzene)-Based Photoswitchable, Prochiral, Calpha-Tetrasubstituted Alpha-Amino Acids for Nanomaterials Applications. Chem. Eur. J. 2011, 17, 12606–12611. [Google Scholar] [CrossRef] [PubMed]
- Velema, W.A.; Hansen, M.J.; Lerch, M.M.; Driessen, A.J.; Szymanski, W.; Feringa, B.L. Ciprofloxacin-Photoswitch Conjugates: A Facile Strategy for Photopharmacology. Bioconjug. Chem. 2015, 26, 2592–2597. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xie, Y.; Shao, H.; Jiang, X. Using Azobenzene-Embedded Self-Assembled Monolayers to Photochemically Control Cell Adhesion Reversibly. Angew. Chem. Int. Ed. 2009, 48, 4406–4408. [Google Scholar] [CrossRef] [PubMed]
- Tarn, D.; Ferris, D.P.; Barnes, J.C.; Ambrogio, M.W.; Stoddart, J.F.; Zink, J.I. A reversible light-operated nanovalve on mesoporous silica nanoparticles. Nanoscale 2014, 6, 3335–3343. [Google Scholar] [CrossRef] [Green Version]
- Hamon, F.; Blaszkiewicz, C.; Buchotte, M.; Banaszak-Léonard, E.; Bricout, H.; Tilloy, S.; Monflier, E.; Cézard, C.; Bouteiller, L.; Len, C.; et al. Synthesis and characterization of a new photoinduced switchable beta-cyclodextrin dimer. Beilstein J. Org. Chem. 2014, 10, 2874–2885. [Google Scholar] [CrossRef] [Green Version]
- Samanta, S.; Qin, C.; Lough, A.J.; Woolley, G.A. Bidirectional Photocontrol of Peptide Conformation with a Bridged Azobenzene Derivative. Angew. Chem. Int. Ed. 2012, 51, 6452–6455. [Google Scholar] [CrossRef] [PubMed]
- Trads, J.B.; Hull, K.; Matsuura, B.S.; Laprell, L.; Fehrentz, T.; Gorldt, N.; Kozek, K.A.; Weaver, C.D.; Klocker, N.; Barber, D.M.; et al. Sign Inversion in Photopharmacology: Incorporation of Cyclic Azobenzenes in Photoswitchable Potassium Channel Blockers and Openers. Angew. Chem. Int. Ed. 2019, 58, 15421–15428. [Google Scholar] [CrossRef]
- Reynders, M.; Matsuura, B.S.; Bérouti, M.; Simoneschi, D.; Marzio, A.; Pagano, M.; Trauner, D. PHOTACs Enable Optical Control of Protein Degradation. Sci. Adv. 2020, 6, eaay5064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preußke, N.; Moormann, W.; Bamberg, K.; Lipfert, M.; Herges, R.; Sönnichsen, F.D. Visible-Light-Driven Photocontrol of the Trp-Cage Protein Fold by a Diazocine Cross-Linker. Org. Biomol. Chem. 2020, 18, 2650–2660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walther, M.; Kipke, W.; Schultzke, S.; Ghosh, S.; Staubitz, A. Modification of Azobenzenes by Cross-Coupling Reactions. Synthesis 2021, 53, 1213–1228. [Google Scholar] [CrossRef]
- Maier, M.S.; Hüll, K.; Reynders, M.; Matsuura, B.S.; Leippe, P.; Ko, T.; Schäffer, L.; Trauner, D. Oxidative Approach Enables Efficient Access to Cyclic Azobenzenes. J. Am. Chem. Soc. 2019, 141, 17295–17304. [Google Scholar] [CrossRef] [PubMed]
- Thapaliya, E.R.; Zhao, J.; Ellis-Davies, G.C.R. Locked-Azobenzene: Testing the Scope of a Unique Photoswitchable Scaffold for Cell Physiology. ACS Chem. Neurosci. 2019, 10, 2481–2488. [Google Scholar] [CrossRef]
- Fawcett, W.R. Acidity and Basicity Scales for Polar Solvents. J. Phys. Chem. 1993, 97, 9540–9546. [Google Scholar] [CrossRef]
- Magtaan, J.K.; Devocelle, M.; Kelleher, F. Regeneration of Aged DMF for Use in Solid-Phase Peptide Synthesis. J. Pep. Sci. 2019, 25, e3139. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Eleya, N.; Staubitz, A. Cross-coupling strategy for the synthesis of diazocines. Org. Lett. 2020, 22, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.K.; Mitchell, M.J.; Bruce, D.; Lough, A.J.; Yan, H. Synthesis of cyclic azobenzene analogues. Tetrahedron 2012, 68, 8670–8676. [Google Scholar] [CrossRef]
- The ACS Style Guide: Effective Communication of Scientific Information, 3rd ed.; Coghill, A.M.; Garson, L.R. (Eds.) American Chemical Society: Washington, DC, USA, 2006. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schultzke, S.; Walther, M.; Staubitz, A. Active Ester Functionalized Azobenzenes as Versatile Building Blocks. Molecules 2021, 26, 3916. https://doi.org/10.3390/molecules26133916
Schultzke S, Walther M, Staubitz A. Active Ester Functionalized Azobenzenes as Versatile Building Blocks. Molecules. 2021; 26(13):3916. https://doi.org/10.3390/molecules26133916
Chicago/Turabian StyleSchultzke, Sven, Melanie Walther, and Anne Staubitz. 2021. "Active Ester Functionalized Azobenzenes as Versatile Building Blocks" Molecules 26, no. 13: 3916. https://doi.org/10.3390/molecules26133916
APA StyleSchultzke, S., Walther, M., & Staubitz, A. (2021). Active Ester Functionalized Azobenzenes as Versatile Building Blocks. Molecules, 26(13), 3916. https://doi.org/10.3390/molecules26133916