The Perspective and Challenge of Nanomaterials in Oil and Gas Wastewater Treatment
Abstract
:1. Introduction
2. Modification of Membranes with Nanomaterials
3. Photocatalysis Technology
3.1. Influence of Characteristics of Oil-Gas Wastewater on Photocatalysis
3.1.1. Salinity
3.1.2. Organic Composition
3.2. Influence of Photo-Catalysis Operating Conditions
3.2.1. Catalyst Concentration
3.2.2. pH
3.2.3. Temperature
3.2.4. Oxidant
3.3. Limitation of Nano-TiO2 and Enhancement of Its Photocatalysis Activity
3.3.1. Difficult to Recycle
3.3.2. Narrow Light Absorption Range
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, P.S.; Shi, Q.; Yue, L.; Liang, Y. The latest progress of shale gas exploration and development in China. Nat. Gas Explor. Dev. 2017, 40, 38–44. (In Chinese) [Google Scholar]
- Dong, D.Z.; Gao, S.K.; Huang, J.L. A discussion on the shale gas exploration & development prospect in the Sichuan Basin. Nat. Gas Ind. 2014, 34, 1–15. (In Chinese) [Google Scholar]
- Sun, Y.; Wang, D.; Tsang, D.C.; Wang, L.; Ok, Y.S.; Feng, Y. A critical review of risks, characteristics, and treatment strategies for potentially toxic elements in wastewater from shale gas extraction. Environ. Int. 2019, 125, 452–469. [Google Scholar] [CrossRef]
- Kassotis, C.D.; Nagel, S.C.; Stapleton, H.M. Unconventional oil and gas chemicals and wastewater-impacted water samples promote adipogenesis via PPARγ-dependent and independent mechanisms in 3T3-L1 cells. Sci. Total Environ. 2018, 640, 1601–1610. [Google Scholar] [CrossRef]
- RRowen, E.L.; Engle, M.A.; Kraemer, T.F.; Schroeder, K.T.; Hammack, R.W.; Doughten, M.W. Geochemical and isotopic evolution of water produced from Middle Devonian Marcellus shale gas wells, Appalachian basin, Pennsylvania. AAPG Bull. 2015, 99, 181–206. [Google Scholar] [CrossRef]
- Bakke, T.; Klungsøyr, J.; Sanni, S. Environmental impacts of produced water and drilling waste discharges from the Norwegian offshore petroleum industry. Mar. Environ. Res. 2013, 92, 154–169. [Google Scholar] [CrossRef] [Green Version]
- Cozzarelli, I.; Skalak, K.; Kent, D.; Engle, M.; Benthem, A.; Mumford, A.; Haase, K.; Farag, A.; Harper, D.; Nagel, S.; et al. Environmental signatures and effects of an oil and gas wastewater spill in the Williston Basin, North Dakota. Sci. Total Environ. 2017, 579, 1781–1793. [Google Scholar] [CrossRef]
- Atkinson, G.M.; Eaton, D.W.; Ghofrani, H.; Walker, D.; Cheadle, B.; Schultz, R.; Shcherbakov, R.; Tiampo, K.; Gu, J.; Harrington, R.M.; et al. Hydraulic Fracturing and Seismicity in the Western Canada Sedimentary Basin. Seism. Res. Lett. 2016, 87, 631–647. [Google Scholar] [CrossRef]
- Munirasu, S.; Haija, M.A.; Banat, F. Use of membrane technology for oil field and refinery produced water treatment—A review. Process. Saf. Environ. Prot. 2016, 100, 183–202. [Google Scholar] [CrossRef]
- Aharghi, E.A.; Bonakdarpour, B.; Pakzadeh, M. Treatment of hypersaline produced water employing a moderately halophilic bacterial consortium in a membrane bioreactor: Effect of salt concentration on organic removal performance, mixed liquor characteristics and membrane fouling. Bioresour. Technol. 2014, 164, 203–213. [Google Scholar] [CrossRef]
- Mou, S.Y.; Shi, S.L.; Fang, K. Application of nano materials and technologies in oil exploration and development. Oilfield Chem. 2019, 36, 564–570. (In Chinese) [Google Scholar]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S. Membrane technology enhancement in oil-water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Xiao, C.; Zhao, J.; Liu, X.; Ji, D.; Xu, H. One-step preparation of tubular nanofibers and micro/nanospheres covered membrane with 3D micro/nano structure for highly efficient emulsified oil/water separation. J. Taiwan Inst. Chem. Eng. 2021, 122, 210–221. [Google Scholar] [CrossRef]
- Raji, Y.O.; Othman, M.H.D.; Nordin, N.A.H.S.M.; Kurniawan, T.A.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Bin Adam, M.R.; Alftessi, S.A.; Farag, T.M. Wettability Improvement of Ceramic Membrane by Intercalating Nano-Al2O3 for Oil and Water Separation. Surf. Interfaces 2021, 25, 101178. [Google Scholar] [CrossRef]
- Velayi, E.; Norouzbeigi, R. A mesh membrane coated with dual-scale superhydrophobic nano zincoxide: Efficient oil-water separation. Surf. Coat. Technol. 2020, 385, 125394. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.-L. Three-channel stainless steel hollow fiber membrane with inner layer modified by nano-TiO2coating method for the separation of oil-in-water emulsions. Sep. Purif. Technol. 2019, 222, 75–84. [Google Scholar] [CrossRef]
- Fanga, X.; Li, J.; Ren, B.; Huang, Y.; Wang, D.; Liao, Z.; Li, Q.; Wang, L.; Dionysiou, D.D. ultrafiltration membrane with in situ formed nano-silver within the inner pores for simultaneous separation and catalysis. J. Membr. Sci. 2019, 579, 190–198. [Google Scholar] [CrossRef]
- Pan, J.; Xiao, C.; Huang, Q.; Liu, H.; Zhang, T. ECTFE hybrid porous membrane with hierarchical micro/nano-structural surface for efficient oil/water separation. J. Membr. Sci. 2017, 524, 623–630. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, S.; Hu, Y.; Sun, S. Membrane technology in wastewater treatment enhanced by functional nanomaterials. J. Clean. Prod. 2018, 197, 339–348. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, Z.; Xie, A.; Meng, M.; Cui, Y.; Liu, S.; Lu, J.; Zhou, S.; Yan, Y.; Dong, H. Bio-inspired fabrication of superhydrophilic nanocomposite membrane based on surface modification of SiO2 anchored by polydopamine towards effective oil-water emulsions separation. Sep. Purif. Technol. 2018, 209, 434–442. [Google Scholar] [CrossRef]
- Chen, L.; Li, N.; Wen, Z.; Zhang, L.; Chen, Q.; Chen, L.; Si, P.; Feng, J.; Li, Y.; Lou, J.; et al. Graphene oxide based membrane intercalated by nanoparticles for high performance nanofiltration application. Chem. Eng. J. 2018, 347, 12–18. [Google Scholar] [CrossRef]
- Song, Z.; Fathizadeh, M.; Huang, Y.; Chu, K.H.; Yoon, Y.; Wang, L.; Yu, M. TiO2 nanofiltration membranes prepared by molecular layer deposition for water purification. J. Membr. Sci. 2016, 510, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Weschenfelder, S.E.; Louvisse, A.M.T.; Borges, C.P.; Meabe, E.; Izquierdo, J.; Campos, J.C. Evaluation of ceramic membranes for oilfield produced water treatment aiming reinjection in offshore units. J. Pet. Sci. Eng. 2015, 131, 51–57. [Google Scholar] [CrossRef]
- Weschenfelder, S.E.; Borges, C.P.; Campos, J.C. Oilfield produced water treatment by ceramic membranes: Bench and pilot scale evaluation. J. Membr. Sci. 2015, 495, 242–251. [Google Scholar] [CrossRef]
- Da, X.; Chen, X.; Sun, B.; Wen, J.; Qiu, M.; Fan, Y. Preparation of zirconia nanofiltration membranes through an aqueous sol–gel process modified by glycerol for the treatment of wastewater with high salinity. J. Membr. Sci. 2016, 504, 29–39. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, T.; Chen, X.; Qiu, M.; Fan, Y. Fabrication of TiO2-doped ZrO2 nanofiltration membranes by using a modified colloidal sol-gel process and its application in simulative radioactive effluent. J. Membr. Sci. 2016, 514, 476–486. [Google Scholar] [CrossRef]
- Riley, S.M.; Oliveira, J.; Regnery, J.; Cath, T.Y. Hybrid membrane bio-systems for sustainable treatment of oil and gas produced water and fracturing flowback water. Sep. Purif. Technol. 2016, 171, 297–311. [Google Scholar] [CrossRef] [Green Version]
- Al-Sabahi, J.; Bora, T.; Claereboudt, M.; Al-Abri, M.; Dutta, J. Visible light photocatalytic degradation of HPAM polymer in oil produced water using supported zinc oxide nanorods. Chem. Eng. J. 2018, 351, 56–64. [Google Scholar] [CrossRef]
- Grzechulska, J.; Hamerski, M.; Morawski, A.W. Photocatalytic decomposition of oil in water. Water Res. 2000, 34, 1638–1644. [Google Scholar] [CrossRef]
- Bessa, E.; Sant’Anna, G.L., Jr.; Dezotti, M. Photocatalytic/H2O2 treatment of oil field produced waters. Appl. Catal. B Environ. 2001, 29, 125–134. [Google Scholar] [CrossRef]
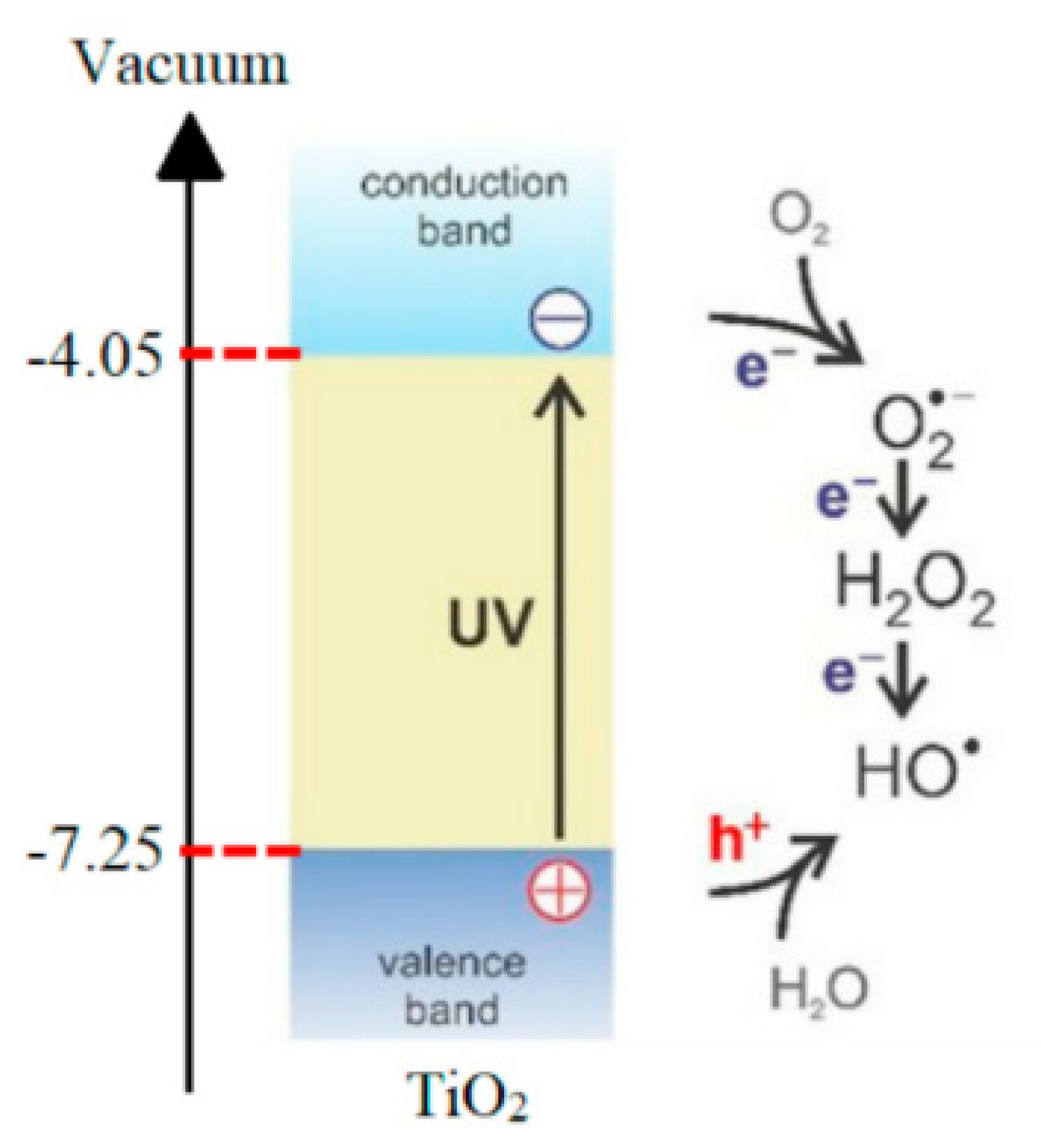
- Fujisawa, J.-I.; Eda, T.; Hanaya, M. Comparative study of conduction-band and valence-band edges of TiO2, SrTiO3, and BaTiO3 by ionization potential measurements. Chem. Phys. Lett. 2017, 685, 23–26. [Google Scholar] [CrossRef]
- Ng, K.H. Adoption of TiO2-photocatalysis for palm oil mill effluent (POME) treatment: Strengths, weaknesses, opportunities, threats (SWOT) and its practicality against traditional treatment in Malaysia. Chemosphere 2021, 270, 129378. [Google Scholar] [CrossRef]
- Labuz, P.; Gryboś, J.; Pietrzyk, P.; Sobanska, K.; Macyk, W.; Sojka, Z. Photo-generation of reactive oxygen species over ultrafine TiO2 particles functionalized with rutineligand induced sensitization and crystallization effect. Res. Chem. Intermed. 2019, 45, 5782–5800. [Google Scholar] [CrossRef] [Green Version]
- Ziolli, R.L.; Jardim, W.F. Photocatalytic decomposition of seawater-soluble crude-oil fractions using high surface area colloid nanoparticles of TiO2. J. Photochem. Photobiol. A Chem. 2002, 147, 205–212. [Google Scholar] [CrossRef]
- Saien, J.; Nejati, H. Enhanced photocatalytic degradation of pollutants in petroleum refinery wastewater under mild conditions. J. Hazard. Mater. 2007, 148, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.P.; Meng, Y.F.; Li, G.; Zhou, H. Photocatalytic Oxidation Degradation Behavior on Fracturing Wastewater in Oilfield. Adv. Mater. Res. 2011, 396–398, 1918–1922. [Google Scholar] [CrossRef]
- Ni, L.; Li, Y.; Zhang, C.; Li, L.; Zhang, W.; Wang, D. Novel floating photocatalysts based on polyurethane composite foams modified with silver/titanium dioxide/graphene ternary nanoparticles for the visible-light-mediated remediation of diesel-polluted surface water. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Younesi, H.; Zinatizadeh, A.A. Preparation, characterization and photocatalytic application of TiO2/Fe-ZSM-5 nanocomposite for the treatment of petroleum refinery wastewater: Optimization of process parameters by response surface methodology. Chemosphere 2016, 159, 552–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahrezaei, F.; Mansouri, Y.; Zinatizadeh, A.A.L.; Akhbari, A. Process modeling and kinetic evaluation of petroleum refinery wastewater treatment in a photocatalytic reactor using TiO2 nanoparticles. Powder Technol. 2012, 221, 203–212. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Zhang, J.; Louangsouphom, B.; Song, J.; Wang, X.; Zhao, J. Synthesis of expanded graphite C/C composites (EGC) based Ni-N-TiO2 floating photocatalysts for in situ adsorption synergistic photocatalytic degradation of diesel oil. J. Photochem. Photobiol. A Chem. 2017, 347, 105–115. [Google Scholar] [CrossRef]
- Brame, J.A.; Hong, S.W.; Lee, J.; Lee, S.-H.; Alvarez, P.J. Photocatalytic pre-treatment with food-grade TiO2 increases the bioavailability and bioremediation potential of weathered oil from the Deepwater Horizon oil spill in the Gulf of Mexico. Chemosphere 2013, 90, 2315–2319. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A.; Bielan, Z.; Wysocka, I.; Strychalska, J.; Janczarek, M.; Klimczuk, T. Magnetic semiconductor photocatalysts for the degradation of recalcitrant chemicals from flow back water. J. Environ. Manag. 2017, 195, 157–165. [Google Scholar] [CrossRef]
- Alizadeh Fard, M.; Aminzadeh, B.; Vahidi, H. Degradation of petroleum aromatic hydrocarbons using TiO2 nanopowder film. Environ. Technol. 2013, 34, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Ang, T.C.; Cheng, J.X.; Chen, F.Z.; Fu, J.M.; Sheng, G.Y.; Ye, H.P. Photo-electrocatalytic degradation of oilfield wastewater with high Content of chlorine. Res. Environ. Sci. 2006, 1, 30–34. (In Chinese) [Google Scholar]
- Wang, Y.L.; Li, T.; Zhang, X.F. Development and application of fracturing waste liquid treatment technology in shale gas exploitation. Environ. Prot. Oil Gas Fields 2012, 22, 53–56. (In Chinese) [Google Scholar]
- Fang, Q.Y.; He, H.J.; Wang, Y.H. Research progress of drilling wastewater and acid fracturing wastewater treatment technology. Oilfield Chem. 2002, 19, 387–390. (In Chinese) [Google Scholar]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Cheng, C.K.; Deraman, M.R.; Ng, K.H.; Khan, M.R. Preparation of titania doped argentum photocatalyst and its photoactivity towards palm oil mill effluent degradation. J. Clean. Prod. 2016, 112, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.K.; Rizauddin Derahman, M.; Khan, M.R. Evaluation of the photocatalytic degradation of pre-treated palm oil mill effluent (POME) over Pt-loaded titania. J. Environ. Chem. Eng. 2015, 3, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.Y.; Wang, B.H.; Cheng, Y. Study on the treatment of oily wastewater by photocatalytic oxidation. Chem. Ind. Eng. Prog. 2003, 22, 67–70. (In Chinese) [Google Scholar]
- Zhang, J.; Wang, X.; Song, J.; Huang, J.; Louangsouphom, B.; Zhao, J. Floating photocatalysts based on loading Bi/N-doped TiO2 on expanded graphite C/C (EGC) composites for the visible light degradation of diesel. RSC Adv. 2015, 5, 71931. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Cazoir, D.; Fine, L.; Ferronato, C.; Chovelon, J.-M. Hydrocarbon removal from bilgewater by a combination of air-stripping and photocatalysis. J. Hazard. Mater. 2012, 235–236, 159–168. [Google Scholar] [CrossRef]
- Wang, S.; Cao, M.W.; Ding, L.M. Fracturing Fluid Waste liquid Disposal by Using Nanometer TiO2 in He Nan Oil Field. Drill. Fluid Completion Fluid 2006, 23, 65–68. (In Chinese) [Google Scholar]
- Mascolo, G.; Comparelli, R.; Curri, M.L. Photocatalytic degradation of methyl red by TiO2: Comparison of the efficiency of immobilized nanoparticles versus conventional suspended catalyst. J. Hazard. Mater. 2007, 142, 130–137. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, K. Preparation of granulated N-doped TiO2/diatomite composite and its applications of visible light degradation and disinfection. Powder Technol. 2016, 303, 176–191. [Google Scholar] [CrossRef]
- Yang, T.; Ma, Z.; Yang, Q. Formation and performance of Kaolin/MnO2 bi-layer composite dynamic membrane for oily wastewater treatment: Effect of solution conditions. Desalination 2011, 270, 50–56. [Google Scholar] [CrossRef]
- Ren, W.; Ai, Z.; Jia, F. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl. Catal. B Environ. 2007, 69, 138–144. [Google Scholar] [CrossRef]
- Zheng, S.L.; Xue, Y.L.; Wang, Y.F. Study on photocatalytic performance of F-doped nano-TiO2/diatomite composite. J. Synth. Cryst. 2016, 45, 2180–2184. (In Chinese) [Google Scholar]
- Shao, G.N.; Imran, S.M.; Jeon, S.J. Sol-gel synthesis of vanadium doped titania: Effect of the synthetic routes and investigation of their photocatalytic properties in the presence of natural sunlight. Appl. Surf. Sci. 2015, 351, 1213–1223. [Google Scholar] [CrossRef]
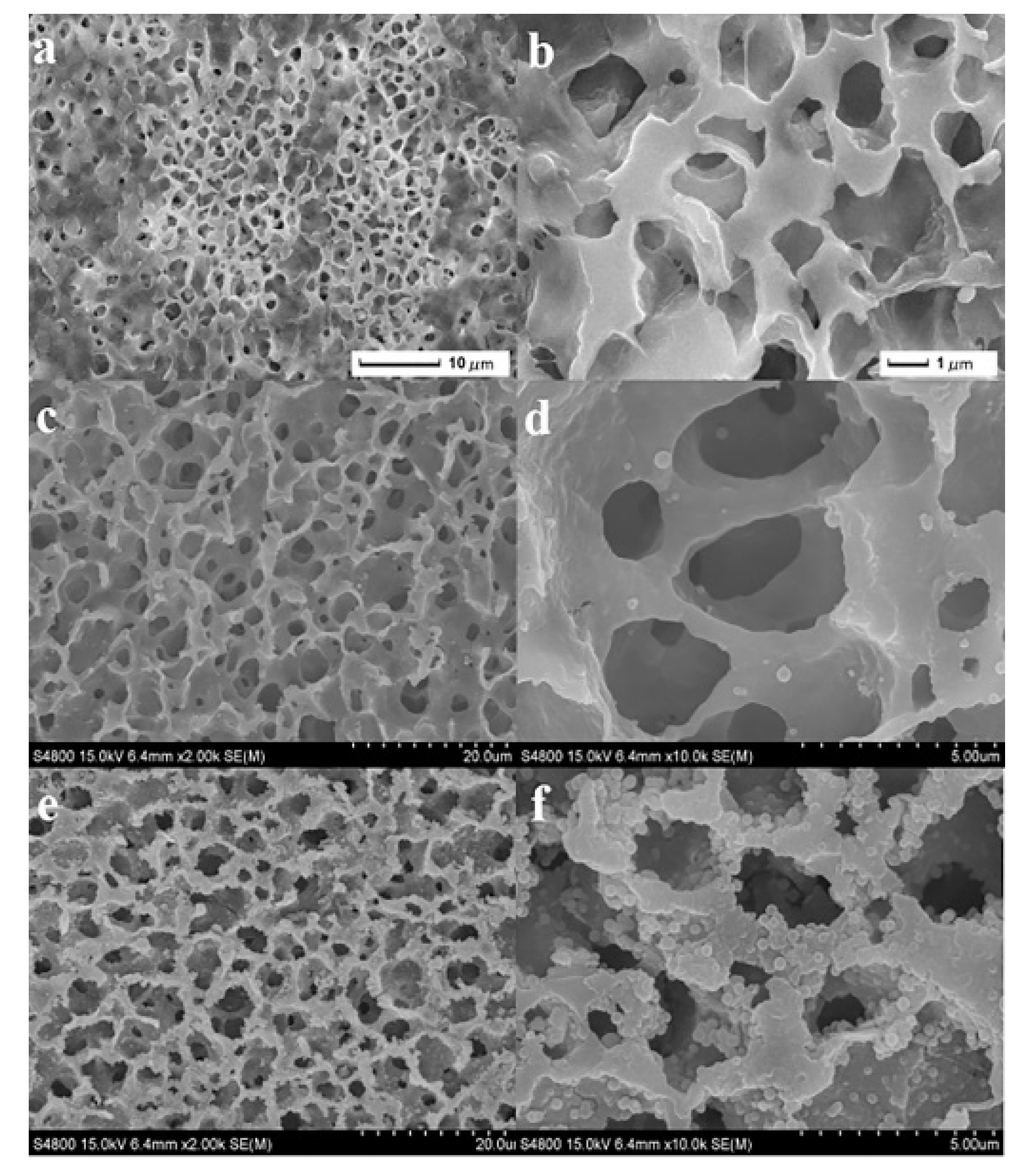
| Separation Membrane | Pore Size | Application |
|---|---|---|
| MF | 0.1~10 μm | Filter out most suspended solids, bacteria and other impurities. Viruses and ions can pass through |
| UF | 2~100 nm | Filter out most proteins and other macromolecules and viruses |
| NF | 0.5~1 nm | Filter out divalent and multivalent ions, such as SO42−, Ca2+, Mg2+ |
| RO | Only water can pass through |
| Nano-Materials | Preparation Method of Modified Membrane | Performance | Ref. |
|---|---|---|---|
| Tubular nanofibers and micro/nano spheres | Electrostatic spraying | 3D hierarchical micro/nano structure; Super hydrophilic; The morphology of the covered micro/nano spheres can be easily controlled; High separation efficiency | [14] |
| nano-Al2O3 | Impregnation method | The oil repellent and hydrophilic properties are enhanced; With an increase in surface roughness, the membrane fouling decreases | [15] |
| nano-ZnO | Chemical deposition | It exhibits good separation performance for highly corrosive aqueous solutions and light oil/heavy oil mixtures | [16] |
| nano-TiO2 | Impregnation method | The separation rate of oil-water emulsion is high. It shows excellent anti-pollution performance and recyclability | [17] |
| nano-Ag | In situ co-mixed reduction method | It can effectively reduce and separate macromolecular pollutants | [18] |
| nano-SiO2 | Thermally induced phase separation | The modified film has super hydrophilic and superhydrophobic properties; It has high oil-water separation efficiency | [19] |
| Wastewater Type | Catalyst | Light Source | Major Funding | Ref. |
|---|---|---|---|---|
| Bilge water | TiO2/KOH | 370W UV lamp | The oil is completely decomposed | [30] |
| Oil produce water | P25 | 125 UV lamp | DOC removal 90% in 7 days | [35] |
| Refinery wastewater | P25 | 400W UV lamp | COD removal 90% in 240 min | [36] |
| Fracturing wastewater | Bentonite loading TiO2-Ag2O | Visible light | COD removal 58.1% 180 min | [37] |
| Diesel-polluted surface water | Silver/titanium dioxide/graphene ternary nanoparticles | Visible light (500-W halogen tungsten lamp with a UV cutoff filter) | Diesel oil removal efficiency was 75% in 16 h | [38] |
| Petroleum refinery wastewater (PRWW) | TiO2/Fe-ZSM-5 | 8W UV lamp | COD removal 80% in 240 min | [39] |
| Petroleum refinery wastewater | P25 | 400W UV lamp | TCOD removal 83% in 120 min | [40] |
| Diesel oil | Ni-N-TiO2/PEGC | Visible light (500W xenon lamp with the UV cutoff filter) | Diesel oil removal efficiency was 95.9% in 5 h | [41] |
| Weathered oil | Food-grade TiO2 | 4W UV lamp | DOC increase 60% in 24 h | [42] |
| Fracturing wastewater | Fe3O4@SiO2/TiO2_P25 | UV | COD removal 40% in180 min | [43] |
| Oil and gas produce water | ZnO2 | Visible light (a solar simulator fitted with IR filter) | TOC removal 20% in 7 h | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Ruan, W.; Wang, W.; Zhang, X.; Liu, Y.; Liu, J. The Perspective and Challenge of Nanomaterials in Oil and Gas Wastewater Treatment. Molecules 2021, 26, 3945. https://doi.org/10.3390/molecules26133945
Liu X, Ruan W, Wang W, Zhang X, Liu Y, Liu J. The Perspective and Challenge of Nanomaterials in Oil and Gas Wastewater Treatment. Molecules. 2021; 26(13):3945. https://doi.org/10.3390/molecules26133945
Chicago/Turabian StyleLiu, Xiaoying, Wenlin Ruan, Wei Wang, Xianming Zhang, Yunqi Liu, and Jingcheng Liu. 2021. "The Perspective and Challenge of Nanomaterials in Oil and Gas Wastewater Treatment" Molecules 26, no. 13: 3945. https://doi.org/10.3390/molecules26133945
APA StyleLiu, X., Ruan, W., Wang, W., Zhang, X., Liu, Y., & Liu, J. (2021). The Perspective and Challenge of Nanomaterials in Oil and Gas Wastewater Treatment. Molecules, 26(13), 3945. https://doi.org/10.3390/molecules26133945