An Overview on Total Valorization of Litsea cubeba as a New Woody Oil Plant Resource toward a Zero-Waste Biorefinery
Abstract
:1. Introduction
2. Woody Oil Plant Resources in China
2.1. Camellia oleifera
2.2. Juglans regia
2.3. Paeonia suffruticosa
2.4. Xanthoceras sorbifolium
2.5. Acer truncatum
2.6. Eucommia ulmoide
2.7. Swida wilsoniana
2.8. Litsea cubeba
3. Litsea cubeba Essential Oil and Citral-Based Derivatives
3.1. Extraction of Litsea cubeba Essential Oils
3.2. Bioactivities of Litsea cubeba Essential Oils
3.2.1. Antimicrobial Activity
3.2.2. Antioxidant Activity
3.2.3. Anthelmintic Activity
3.2.4. Other Bioactivities
3.3. Purification of Litsea cubeba Essential Oil and Its Derivatives
3.3.1. Purification of Litsea cubeba Essential Oil
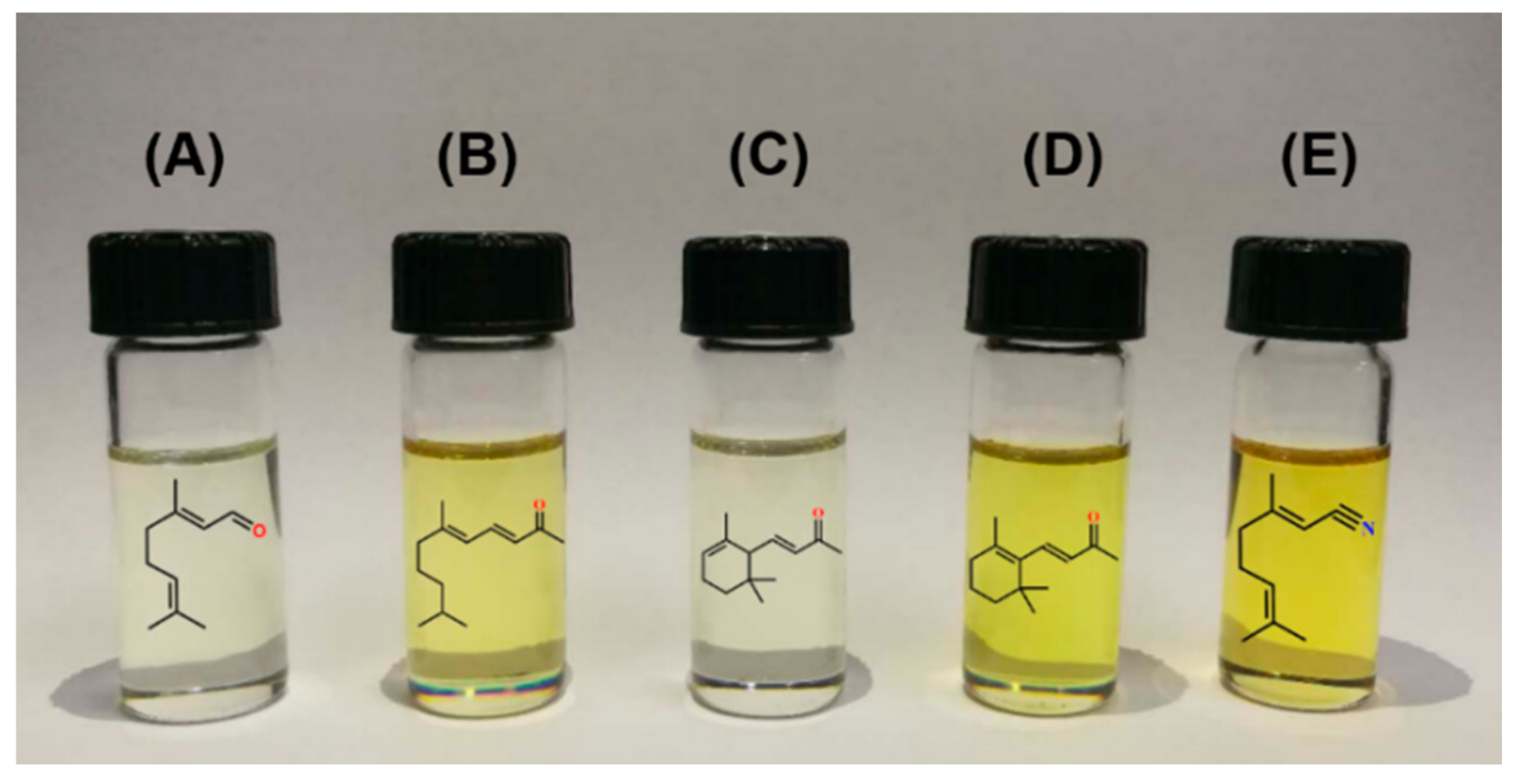
3.3.2. Citral-Derived Fragrances
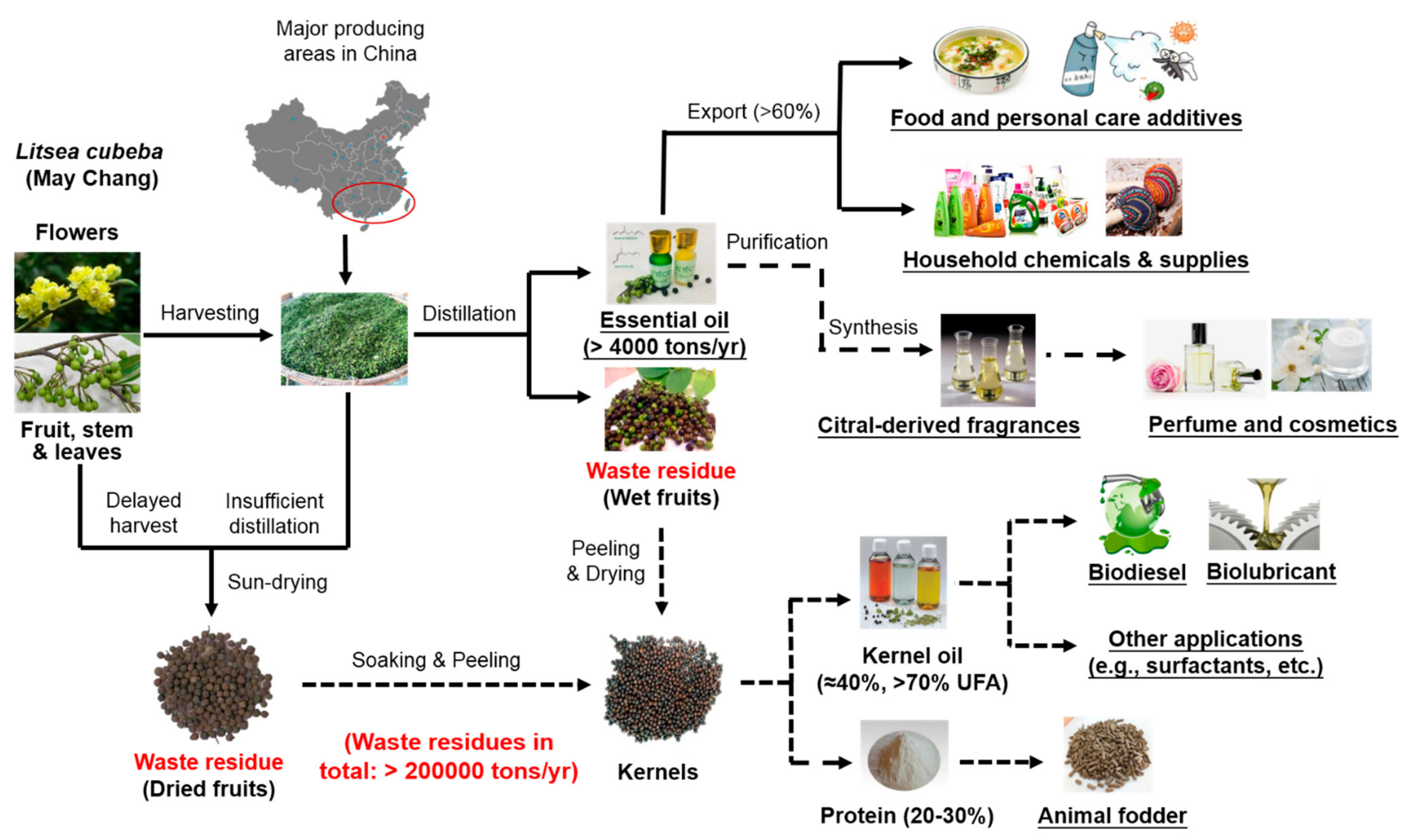
4. Biorefinery after Litsea cubeba Essential Oil Production
4.1. Litsea cubeba Kernel Oil
4.1.1. Purification of Fatty Acids and Derived Green Surfactants
4.1.2. Biodiesel Production from Kernel Oil
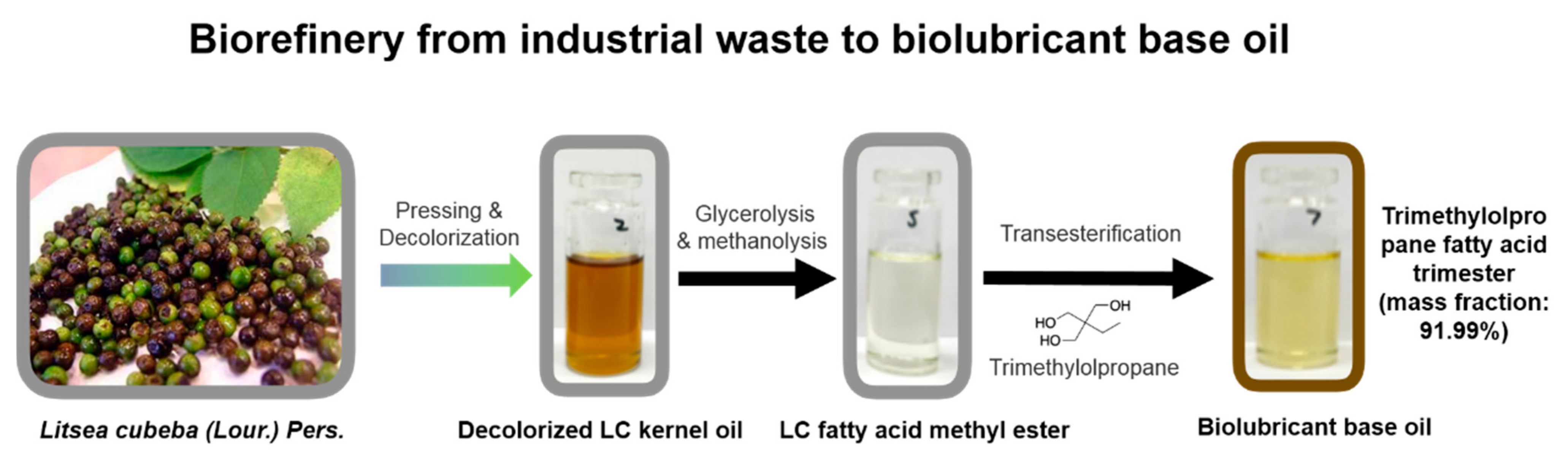
4.1.3. Biolubricant Base Oil
4.2. Litsea cubeba Protein
4.3. Litsea cubeba Pomace
5. Conclusions & Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Dai, Y.; Sun, D.; Yang, X. Research on the status and development proposals for the wood oil industry in China. Cereals Oils 2015, 28, 17–19. [Google Scholar]
- Gatto, A.D.; Mengarelli, C.; Pedretti, E.F.; Duca, D.; Pieri, S.; Mangoni, L.; Signor, M.; Raccuia, S.A.; Melilli, M.G. Adaptability of sunflower (Helianthus annuus L.) high oleic hybrids to different Italian areas for biodiesel production. Ind. Crop. Prod. 2015, 75, 108–117. [Google Scholar] [CrossRef]
- Lauri, P.; Havlík, P.; Kindermann, G.; Forsell, N.; Böttcher, H.; Obersteiner, M. Woody biomass energy potential in 2050. Energy Policy 2014, 66, 19–31. [Google Scholar] [CrossRef]
- Yin, D.; Li, S.; Wu, Q.; Feng, C.; Li, B.; Wang, Q.; Wang, L.; Xu, W. Advances in research of six woody oil crops in China. Chin. Bull. Bot. 2018, 53, 110–125. [Google Scholar]
- Yuan, J.; Wang, C.; Chen, H.; Zhou, H.; Ye, J. Prediction of fatty acid composition in Camellia oleifera oil by near infrared transmittance spectroscopy (NITS). Food Chem. 2013, 138, 1657–1662. [Google Scholar] [CrossRef]
- Xiao, H.; Yao, Z.; Peng, Q.; Ni, F.; Sun, Y.; Zhang, C.X.; Zhong, Z.X. Extraction of squalene from camellia oil by silver ion complexation. Sep. Purif. Technol. 2016, 169, 196–201. [Google Scholar] [CrossRef]
- Gao, P.; Liu, R.J.; Jin, Q.Z.; Wang, X.G. Comparison of solvents for extraction of walnut oils: Lipid yield, lipid compositions, minor-component content, and antioxidant capacity. LWT 2019, 110, 346–352. [Google Scholar] [CrossRef]
- Reíter, R.J.; Manchester, L.C.; Tan, D.X. Melatonin in walnuts: Influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 2005, 21, 920–924. [Google Scholar] [CrossRef]
- Han, J.G.; Liu, Z.; Li, X.Q.; Li, J.; Hu, Y.H. Diversity in seed oil content and fatty acid composition in three tree peony species with potential as sources of omega-3 fatty acids. Hortic. Sci. Biotechnol. 2016, 91, 175–179. [Google Scholar] [CrossRef]
- Li, S.S.; Yuan, R.Y.; Chen, L.G.; Wang, L.S.; Hao, X.H.; Wang, L.J.; Zheng, X.C.; Du, H. Systematic qualitative and quantitative assessment of fatty acids in the seeds of 60 tree peony (Paeonia section Moutan DC.) cultivars by GC–MS. Food Chem. 2015, 173, 133–140. [Google Scholar] [CrossRef]
- Wu, S.H.; Wu, D.G.; Chen, Y.W. Chemical constituents and bioactivities of plants from the genus Paeonia. Chem. Biodivers. 2010, 7, 90–104. [Google Scholar] [CrossRef]
- Zhang, S.; Zu, Y.G.; Fu, Y.J.; Luo, M.; Liu, W.; Li, J.; Efferth, T. Supercritical carbon dioxide extraction of seed oil from yellow horn (Xanthoceras sorbifolia Bunge.) and its anti-oxidant activity. Bioresour. Technol. 2010, 101, 2537–2544. [Google Scholar] [CrossRef]
- Venegas-Calerón, M.; Ruíz-Méndez, M.V.; Martínez-Force, E.; Garcés, R.; Salas, J.J. Characterization of Xanthoceras sorbifolium Bunge seeds: Lipids, proteins and saponins content. Ind. Crop. Prod. 2017, 109, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Liang, Q.; Wang, W.W.; Yuan, F.L.; Liu, X.; Li, D.L.; Yang, K.Q. Characterization of yuanbaofeng (Acer truncatum Bunge) samaras: Oil, fatty acid, and phytosterol content. Ind. Crop. Prod. 2019, 135, 344–351. [Google Scholar] [CrossRef]
- Gu, R.H.; Morcol, T.; Liu, B.; Shi, M.J.; Kennelly, E.J.; Long, C.L. GC–MS, UPLC-QTOF-MS, and bioactivity characterization of Acer truncatum seeds. Ind. Crop. Prod. 2019, 138, 111480. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Liu, Y.L.; Che, L.M. Characterization of a new α—Linolenic acid—Rich oil: Eucommia ulmoides seed oil. Food Sci. 2018, 83, 617–623. [Google Scholar] [CrossRef]
- Xing, Y.F.; He, D.; Wang, Y.; Zeng, W.; Zhang, C.; Lu, Y.; Su, N.; Kong, Y.H.; Xing, X.H. Chemical constituents, biological functions and pharmacological effects for comprehensive utilization of Eucommia ulmoides Oliver. Food Sci. Hum. Wellness 2019, 8, 177–188. [Google Scholar] [CrossRef]
- Xiao, Z.H.; Li, C.Z.; Chen, J.Z.; Liu, R.K.; Wu, X.F. Analysis of oil composition in different parts of Cornus wilsoniana Wanger. fruit. China Oils Fats 2009, 34, 72–74. [Google Scholar]
- Wang, L.B.; Yu, H.Y.; He, X.H.; Liu, R.Y. Influence of fatty acid composition of woody biodiesel plants on the fuel properties. J. Fuel Chem. Technol. 2012, 40, 397–404. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, W.C.; Xu, H.N.; Jiang, X.M. Industrial refining and physicochemical characteristics of fruit oil from Swida wilsoniana. Food Sci. 2012, 33, 163–166. [Google Scholar]
- Qiu, L.Y.; Chen, H.P.; Yan, Y.F.; Li, Y.Y.; Wang, H.; Liao, Z.P.; Huang, Q.R. Sasanquasaponin promotes cellular chloride efflux and elicits cardioprotection via the PKCɛ pathway. Mol. Med. Rep. 2016, 13, 3597–3603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, W.J.; Ko, J.; Huang, W.C.; Cheng, W.Y.; Yang, H.Y. Crude extract of Camellia oleifera pomace ameliorates the progression of non-alcoholic fatty liver disease via decreasing fat accumulation, insulin resistance and inflammation. Br. J. Nutr. 2020, 123, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Bumrungpert, A.; Pavadhgul, P.; Kalpravidh, R.W. Camellia oil-enriched diet attenuates oxidative stress and inflammatory markers in hypercholesterolemic subjects. J. Med. Food 2016, 19, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.P.; Yen, G.C. Antioxidant activity and bioactive compounds of tea seed (Camellia oleifera Abel.) oil. J. Agric. Food Chem. 2006, 54, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Lee, J.; Baek, J.; Jung, K.; Lee, J.; Huh, S.; Kim, S.; Koh, J.; Park, D. Effect of Camellia japonica oil on human type I procollagen production and skin barrier function. J. Ethnopharmacol. 2007, 112, 127–131. [Google Scholar] [CrossRef]
- Shao, P.; Liu, Q.; Fang, Z.X.; Sun, P.L. Chemical composition, thermal stability and antioxidant properties of tea seed oils obtained by different extraction methods: Supercritical fluid extraction yields the best oil quality. Eur. J. Lipid Sci. Technol. 2015, 117, 355–365. [Google Scholar] [CrossRef]
- Mei, L.; Qiao, H.; Ke, F.; Peng, C.; Hou, R.; Wan, X.; Cai, H. One-step synthesis of zirconium dioxide-biochar derived from Camellia oleifera seed shell with enhanced removal capacity for fluoride from water. Appl. Surf. Sci. 2020, 509, 144685. [Google Scholar] [CrossRef]
- Cofrades, S.; Serrano, A.; Ayo, J.; Carballo, J.; Jiménez-Colmenero, F. Characteristics of meat batters with added native and preheated defatted walnut. Food Chem. 2008, 107, 1506–1514. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, L.; Song, W.; Zhang, C.; Hua, Y.; Chen, Y.; Li, X. Separation, identification and molecular binding mechanism of dipeptidyl peptidase IV inhibitory peptides derived from walnut (Juglans regia L.) protein. Food Chem. 2021, 347, 129062. [Google Scholar] [CrossRef]
- Zabihi, M.; Haghighi Asl, A.; Ahmadpour, A. Studies on adsorption of mercury from aqueous solution on activated carbons prepared from walnut shell. J. Hazard. Mater. 2010, 174, 251–256. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.; Zhang, R.; Pan, J.; Qi, D.; Wang, J.; Yang, X. The protective effects of walnut green husk polysaccharide on liver injury, vascular endothelial dysfunction and disorder of gut microbiota in high fructose-induced mice. Int. J. Biol. Macromol. 2020, 162, 92–106. [Google Scholar] [CrossRef]
- Soto-Maldonado, C.; Vergara-Castro, M.; Jara-Quezada, J.; Caballero-Valdés, E.; Müller-Pavez, A.; Zúñiga-Hansen, M.E.; Altamirano, C. Polyphenolic extracts of walnut (Juglans regia) green husk containing juglone inhibit the growth of HL-60 cells and induce apoptosis. Electron. J. Biotechnol. 2019, 39, 1–7. [Google Scholar] [CrossRef]
- Rajaram, S. Health benefits of plant-derived α-linolenic acid. Am. J. Clin. Nutr. 2014, 100, 443–448. [Google Scholar] [CrossRef] [Green Version]
- Lowry, J.R.; Marshall, N.; Wenzel, T.J.; Murray, T.E.; Klegeris, A. The dietary fatty acids α-linolenic acid (ALA) and linoleic acid (LA) selectively inhibit microglial nitric oxide production. Mol. Cell. Neurosci. 2020, 109, 103569. [Google Scholar] [CrossRef]
- Kim, K.B.; Nam, Y.A.; Kim, H.S.; Hayes, A.W.; Lee, B.M. α-Linolenic acid: Nutraceutical, pharmacological and toxicological evaluation. Food Chem. Toxicol. 2014, 70, 163–178. [Google Scholar] [CrossRef]
- Yu, H.; Fan, S.; Bi, Q.; Wang, S.; Hu, X.; Chen, M.; Wang, L. Seed morphology, oil content and fatty acid composition variability assessment in yellow horn (Xanthoceras sorbifolium Bunge) germplasm for optimum biodiesel production. Ind. Crop. Prod. 2017, 97, 425–430. [Google Scholar] [CrossRef]
- Yao, Z.Y.; Qi, J.H.; Yin, L.M. Biodiesel production from Xanthoceras sorbifolia in China: Opportunities and challenges. Renew. Sustain. Energy Rev. 2013, 24, 57–65. [Google Scholar] [CrossRef]
- Fan, H.; Sun, L.; Yang, L.; Zhou, J.; Yin, P.; Li, K.; Xue, Q.; Li, X.; Liu, Y. Assessment of the bioactive phenolic composition of Acer truncatum seed coat as a byproduct of seed oil. Ind. Crop. Prod. 2018, 118, 11–19. [Google Scholar] [CrossRef]
- Wang, X.Y.; Fan, J.S.; Wang, S.Y.; Sun, R.C. A new resource of nervonic acid from purpleblow maple (Acer truncatum) seed oil. For. Prod. J. 2006, 56, 147–150. [Google Scholar]
- Chen, C.H.; Shen, Y.K.; Hsieh, S.C. The investigation of gutta-percha temperature and compaction force change when using the vertical compaction of warm gutta-percha technique. J. Polym. Eng. 2014, 34, 219–223. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, T.; Zhang, J.; Hou, G.; Yu, M.; Liu, L. Mechanism analysis of Eucommia ulmoides gum reducing the rolling resistance and the application study in green tires. Polym. Test. 2020, 87, 106539. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, C.; Sun, R.; Liao, X.; Wu, J.; Xie, M. Sustainable elastomer of triazolinedione-modified Eucommia ulmoides gum with enhanced elasticity and shape memory capability. Polymer 2019, 185, 121904. [Google Scholar] [CrossRef]
- Li, N.; Xu, J.; Xu, T. Preparation, properties and modification mechanism of vulcanized Eucommia ulmoides gum modified asphalt. Constr. Build. Mater. 2021, 274, 121992. [Google Scholar] [CrossRef]
- Chen, W.; Wu, F.W.; Zhang, J.H. Potential production of non-food biofuels in China. Renew. Energy 2016, 85, 939–944. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, X.; Liu, K.; Li, Q.; Zhang, L.; Yang, X.; Zhang, Z.; Li, C.; Luo, Y.; He, Z.; et al. Hypolipidemic activity in Sprague–Dawley rats and constituents of a novel natural vegetable oil from Cornus Wilsoniana fruits. Food Sci. 2012, 77, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Wu, S.X.; Hao, Z.J.; Xiao, Z.H. Study on the functional effect of the oils from Swida wilsoniana seeds. Sci. Technol. Food Ind. 2011, 32, 403–405. [Google Scholar]
- Zhang, A.H.; Tang, J.; Lie, J.L.; He, Y.D.; Xiao, Z.H. Study on the preparation of biohydrocarbon fuel by catalytic hydrogenation of Swida wilsoniana pyrolysis products. Adv. Mater. Sci. Eng. 2020, 2020, 3569125. [Google Scholar] [CrossRef]
- Ding, R.; Zhong, S.A.; Li, N.; Yang, J.J. Transesterification of Swida wilsoniana oil with methanol to biodiesel catalyzed by Lipozyme TL IM in MgCl2-saturated solution. J. Fuel Chem. Technol. 2010, 38, 287–291. [Google Scholar] [CrossRef]
- Saikiaa, A.K.; Chetiaa, D.; D’Arrigob, M.; Smerigliob, A.; Stranoc, T.; Ruberto, G. Screening of fruit and leaf essential oils of Litsea cubeba Pers. from north-east India–chemical composition and antimicrobial activity. J. Essent. Oil Res. 2013, 25, 330–338. [Google Scholar] [CrossRef]
- Li, Y.; Fabiano-Tixier, A.S.; Vian, M.A.; Chemat, F. Solvent-free microwave extraction of bioactive compounds provides a tool for green analytical chemistry. TrAC Trends Anal. Chem. 2013, 47, 1–11. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Vian, M.A. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Da Silva, R.P.; Rocha-Santos, T.A.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Liu, S.; Zhao, Z.; Gao, W.; Ma, Y.; Wang, X.; Yan, S.; Luo, D. Ultrasound pre-treatment combined with microwave-assisted hydrodistillation of essential oils from Perilla frutescens (L.) Britt. leaves and its chemical composition and biological activity. Ind. Crop. Prod. 2020, 143, 111908. [Google Scholar] [CrossRef]
- Liu, X.G.; Chen, M.M.; Chen, X.H.; Xie, B.P. Study on Extracting Citral from Litsea cubeba fruits by microwave radiation and determination of citral. Chem. Ind. For. Prod. 2001, 21, 87–90. [Google Scholar]
- Yang, G.; Wang, G.; Li, X.; Zhang, M. Study on new extraction technology and chemical composition of Litsea Cubeba essential oil. Open Mater. Sci. J. 2011, 5, 93–99. [Google Scholar] [CrossRef]
- Du, H.; Zhu, H.; Chen, A. Study on Extracting Litsea cubeba oil by ultrasonic-assisted vacuum distillation. Shandong Agric. Sci. 2018, 50, 133–138. [Google Scholar]
- Peng, B.; Wang, W.Q.; Liu, H.J.; Zhang, N. Optimizing process of extraction of Litsea cubeba oil and citral. Appl. Chem. Ind. 2013, 42, 1786–1788. [Google Scholar]
- Xie, Y.F.; Liang, Y.L.; Wang, F.X.; Xiong, W.J.; Xu, Y.; Liu, X.Y. Study on enzymatic extraction of Litsea cubeba Pers oil. Food Res. Dev. 2013, 34, 57–59. [Google Scholar]
- Zhang, D.Q.; Lu, F.J.; Tai, J.X. Study on extracting Litsea cubeba oil with supercritical CO2 fluid technique. Food Ferment. Ind. 2000, 26, 54–57. [Google Scholar]
- Guo, Y.; Li, Y.; Li, Z.; Jiang, L.; Cao, X.; Gao, W.; Wang, J.; Luo, D.; Chen, F. Deep eutectic solvent-homogenate based microwave-assisted hydrodistillation of essential oil from Litsea cubeba (Lour.) Pers. Fruits and its chemical composition and biological activity. J. Chromatogr. A 2021, 1646, 462089. [Google Scholar]
- Zarena, A.S.; Sachindra, N.M.; Sankar, K.U. Optimisation of ethanol modified supercritical carbon dioxide on the extract yield and antioxidant activity from Garcinia mangostana L. Food Chem. 2012, 130, 203–208. [Google Scholar] [CrossRef]
- Zougagh, M.; Valcárcel, M.; Rıos, A. Supercritical fluid extraction: A critical review of its analytical usefulness. TrAC Trends Anal. Chem. 2004, 23, 399–405. [Google Scholar] [CrossRef]
- Möse, J.R.; Möse, G. Onkolyseversuche mit apathogenen, anaeroben sporenbildnern am Ehrlich-Tumor der Maus. Arzneim 1959, 63, 63–74. [Google Scholar]
- Dai, J.; Li, C.; Cui, H.; Lin, L. Unraveling the anti-bacterial mechanism of Litsea cubeba essential oil against E. coli O157:H7 and its application in vegetable juices. Int. J. Food Microbiol. 2021, 338, 108989. [Google Scholar] [CrossRef]
- Hu, W.; Li, C.; Dai, J.; Cui, H.; Lin, L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind. Crop. Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
- Gogoi, R.; Loying, R.; Sarma, N.; Munda, S.; Pandey, S.K.; Lal, M. A comparative study on antioxidant, anti-inflammatory, genotoxicity, anti-microbial activities and chemical composition of fruit and leaf essential oils of Litsea cubeba Pers. from North-east India. Ind. Crop. Prod. 2018, 125, 131–139. [Google Scholar] [CrossRef]
- Yu, A.; Wang, W.; Hu, H.; Wang, L.; Su, J. Synergistic antibacterial and antioxidant effects of Litsea cubeba essential oil and D-borneol. Food Sci. Technol. 2019, 44, 286–291. [Google Scholar]
- Wang, H.; Liu, Y. Chemical composition and antibacterial activity of essential oils from different parts of Litsea cubeba. Chem. Biodivers. 2010, 7, 229–235. [Google Scholar] [CrossRef]
- Meng, Y.; Cui, H.; Zhao, Q.; Li, M.; Bian, C.; Bai, R.; Ma, Y. Antimicrobial effects and mechanism of action of essential oils against dominant spoilage bacteria isolated from large yellow croaker (Larimichthys crocea) during chilled storage. J. Fish. China 2018, 42, 1140–1153. [Google Scholar]
- Niu, B.; Jin, C.; Liang, J.; Shang, R.; Hao, B.; Wang, X.; Yang, Z.; Liu, Y. Chemical constituents and antibacterial activities in vitro of three plant essential oils. Prog. Vet. Med. 2019, 40, 18–23. [Google Scholar]
- Liu, T.T.; Yang, T.S. Antimicrobial impact of the components of essential oil of Litsea cubeba from Taiwan and antimicrobial activity of the oil in food systems. Int. J. Food Microbiol. 2012, 156, 68–75. [Google Scholar] [CrossRef]
- Gogoi, P.; Baruah, P.; Nath, S.C. Antifungal activity of the essential oil of Litsea cubeba Pers. J. Essent. Oil Res. 1997, 9, 213–215. [Google Scholar] [CrossRef]
- Li, Y.; Kong, W.; Li, M.; Liu, H.; Zhao, X.; Yang, S.; Yang, M. Litsea cubeba essential oil as the potential natural fumigant: Inhibition of Aspergillus flavus and AFB1 production in licorice. Ind. Crop. Prod. 2016, 80, 186–193. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Gao, M.; Wu, L.; Wang, Y.; Chen, Y. Analysis of chemical constituents and antimicrobial activity of essential oils in three species from May Chang tree. Nat. Prod. Res. Dev. 2019, 31, 1847–1856. [Google Scholar]
- He, H.; Zhu, Y.; Wang, Z.; Liu, P.; Zhan, M.; Zeng, X. Inhibitory effects of ten plant essential oils on three pathogens of cherry tomatoes. Sci. Technol. Food Ind. 2016, 37, 153–157. [Google Scholar]
- Huang, Y.; Li, H.; Xu, X.; Li, Y.; Dong, T. In vitro susceptibility study of Litsea cubeba oil and its combination with ketoconazole, itraconazole and fluconazole against Malassezia. J. Clin. Dermatol. 2016, 45, 565–568. [Google Scholar]
- You, L.; Lin, N.; Guo, H.; Liao, X.; Wei, Q. On Diversity of endophytic fungi isolated from Litsea Cubeba. J. Southwest China Norm. Univ. 2013, 38, 121–126. [Google Scholar]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Zhang, R.; Yao, W. Major components in Lilac and Litsea cubeba essential oils kill Penicillium roqueforti through mitochondrial apoptosis pathway. Ind. Crop. Prod. 2020, 149, 112349. [Google Scholar] [CrossRef]
- Thielmann, J.; Theobald, M.; Wutz, A.; Krolo, T.; Buergy, A.; Niederhofer, J.; Welle, F.; Muranyi, P. Litsea cubeba fruit essential oil and its major constituent citral as volatile agents in an antimicrobial packaging material. Food Microbiol. 2021, 96, 103725. [Google Scholar] [CrossRef]
- Hwang, J.K.; Choi, E.M.; Lee, J.H. Antioxidant activity of Litsea cubeba. Fitoterapia 2005, 76, 684–686. [Google Scholar] [CrossRef]
- She, Q.H.; Li, W.S.; Jiang, Y.Y.; Wu, Y.C.; Zhou, Y.H.; Zhang, L. Chemical composition, antimicrobial activity and antioxidant activity of Litsea cubeba essential oils in different months. Nat. Prod. Res. 2020, 34, 3285–3288. [Google Scholar] [CrossRef]
- Wang, Y.; Cen, C.; Chen, J.; Zhou, C.; Fu, L. Nano-emulsification improves physical properties and bioactivities of Litsea cubeba essential oil. LWT 2021, 137, 110361. [Google Scholar] [CrossRef]
- Chen, F.; Peng, Y.; Yan, X.; Lin, H.; Zeng, D.; Zhang, Y. Chemical components and bioactivity of essential oil derived from Litsea cubeba against Aedes albopictus. Chin. J. Biol. Control 2012, 28, 521–527. [Google Scholar]
- Seo, S.M.; Kim, J.; Lee, S.G.; Shin, C.H.; Shin, S.C.; Park, I.K. Fumigant antitermitic activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica), caraway (Carum carvi), dill (Anethum graveolens), geranium (Pelargonium graveolens), and litsea (Litsea cubeba) oils against Japanese termite (Reticulitermes speratus Kolbe). J. Agric. Food Chem. 2009, 57, 6596–6602. [Google Scholar]
- Noosidum, A.; Prabaripai, A.; Chareonviriyaphap, T.; Chandrapatya, A. Excito-repellency properties of essential oils from Melaleuca leucadendron L., Litsea cubeba (Lour.) Persoon, and Litsea salicifolia (Nees) on Aedes aegypti (L.) mosquitoes. J. Vector Ecol. 2008, 33, 305–312. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, M.; Yang, Z. Repellent activity screening of 12 essential oils against Aedes albopictus Skuse: Repellent liquid preparation of Mentha arvensis and Litsea cubeba oils and bioassay on hand skin. Ind. Crop. Prod. 2019, 128, 464–470. [Google Scholar] [CrossRef]
- Yang, K.; Wang, C.F.; You, C.X.; Geng, Z.F.; Sun, R.Q.; Guo, S.S.; Du, S.S.; Liu, Z.L.; Deng, Z.W. Bioactivity of essential oil of Litsea cubeba from China and its main compounds against two stored product insects. J. Asia Pac. Entomol. 2014, 17, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Park, I.K.; Kim, J.; Lee, S.G.; Shin, S.C. Nematicidal Activity of Plant Essential Oils and Components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica) and litsea (Litsea cubeba) essential oils against pine wood nematode (Bursaphelenchus Xylophilus). J. Nematol. 2007, 39, 275–279. [Google Scholar]
- Luo, Q.; Wang, Z.; Wang, X.; Lu, Y. Fumigation activity of five plant oils against adults of Liposcelis Entomophila (Enderlein). J. Henan Univ. Technol. Nat. Sci. Ed. 2016, 37, 82–86. [Google Scholar]
- Zhang, H.J.; Zheng, L.H.; Zhao, K.; Gao, H.R.; Luo, S.; Chen, Y.; Wang, T. Separation and identification of insecticidal constituents of Litsea cubeba. Sci. Technol. Cereals Oils Foods 2016, 24, 65–68. [Google Scholar]
- Jiang, Z.; Akhtar, Y.; Bradbury, R.; Zhang, X.; Isman, M.B. Comparative toxicity of essential oils of Litsea pungens and Litsea cubeba and blends of their major constituents against the cabbage looper, Trichoplusia ni. J. Agric. Food Chem. 2009, 57, 4833–4837. [Google Scholar] [CrossRef] [PubMed]
- Pumnuan, J.; Chandrapatya, A.; Insung, A. Acaricidal activities of plant essential oils from three plants on the mushroom mite, Luciaphorus perniciosus Rack (Acari: Pygmephoridae). Pak. J. Zool. 2010, 42, 247–252. [Google Scholar]
- Amer, A.; Mehlhorn, H. Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol. Res. 2006, 99, 478–490. [Google Scholar] [CrossRef]
- Chen, H.C.; Chang, W.T.; Hseu, Y.C.; Chen, H.Y.; Chuang, H.C.; Lin, C.C.; Lee, M.S.; Lin, M.K. Immunosuppressive Effect of Litsea cubeba L. Essential Oil on Dendritic Cell and Contact Hypersensitivity Responses. Int. J. Mol. Sci. 2016, 17, 1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.; Wang, Q.; Ja, Y. Empirical study on antiasthmatic effect of citral from aqueous extract of fruit of Litsea cubeba tree. Chin. J. Clin. Pharmacol. Ther. 2006, 11, 197–201. [Google Scholar]
- Tang, J.; Wan, J.; Shi, C.; Fang, J.; Wang, W. Technology of supercritical CO2 extraction from Fructus Litseae and its analgesic activity. Cent. South Pharm. 2008, 6, 301–303. [Google Scholar]
- Ho, C.L.; Ou, J.P.; Liu, Y.C.; Hung, C.P.; Tsai, M.C.; Liao, P.C.; Wang, E.I.C.; Chen, Y.L.; Su, Y.C. Compositions and in vitro anticancer activities of the leaf and fruit oils of Litsea cubeba from Taiwan. Nat. Prod. Commun. 2010, 5, 617–620. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.M.; Hwang, J.K. Effects of methanolic extract and fractions from Litsea cubeba bark on the production of inflammatory mediators in RAW264.7 cells. Fitoterapia 2004, 75, 141–148. [Google Scholar] [CrossRef]
- Zhong, Y.M.; Zheng, Q.M.; Wang, L.; Zeng, X.L.; Cao, L.Q. Study on the anti-inflammatory, anti-cancer activities and fresh-keeping effect of Litsea cubeba oil. Guangdong Agric. Sci. 2014, 41, 100–105. [Google Scholar]
- Huang, X.W. Chemical Composition and Tyrosinase Inhibition Activity and Antioxidant Activities of Three Essential Oils. Master’s Thesis, Huazhong University of Science and Technology, Hubei, China, 2013. [Google Scholar]
- Chen, C.J.; Tseng, Y.H.; Chu, F.H.; Wen, T.Y.; Cheng, W.W.; Chen, Y.T.; Tsao, N.W.; Wang, S.Y. Neuropharmacological activities of fruit essential oil from Litsea cubeba Persoon. J. Wood Sci. 2012, 58, 538–543. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Sivamaruthi, B.S.; Wongwan, J.; Thiwan, K.; Rungseevijitprapa, W.; Klunklin, A.; Kunaviktikul, W. Effects of Litsea cubeba (Lour.) Persoon essential oil aromatherapy on mood states and salivary cortisol levels in healthy volunteers. Evid. Based Complement. Altern. Med. 2020, 2020, 4389239. [Google Scholar] [CrossRef]
- Gnatta, J.R.; Kurebayashi, L.F.S.; Turrini, R.N.T.; Silva, M.J.P. Aromatherapy and nursing: Historical and theoretical conception. Rev. Esc. Enferm. USP 2016, 50, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, M.; Xie, B. Extraction of citral from Litsea Cubeba fruits and determination of citral. Fine Chem. 2001, 18, 398–400. [Google Scholar]
- Peng, X.L.; Cai, B.L.; Fu, H.J. Study on separation and purification of citral in Litsea Cubeba essential oil. China For. Prod. Ind. 2018, 45, 17–22. [Google Scholar]
- Fu, H.J.; Peng, X.L.; Kuang, C.T.; Zong, H.Y. Separation of citral from Litsea cubeba oil by phase transfer catalysis. Food Mach. 2016, 9, 161–165. [Google Scholar]
- Zhong, C.Y.; Zhou, Z.M.; Huang, H.Y.; Li, Y.J. Process improvement of refining citral from Litsea cubeba oil. Guangxi For. Sci. 2013, 42, 387–389. [Google Scholar]
- Cheng, J.; Shi, B.J.; Jiang, H.F.; Zhu, Y.Y.; Zhang, W.M. Study on purifying high quality citral from Litsea cubeba oil. Sci. Technol. Food Ind. 2015, 36, 231–234. [Google Scholar]
- Huang, M.; Zhong, Z.S. Process for purification of Litsea cubeba oil by molecular distillation. Food Sci. Technol. 2005, 8, 52–54. [Google Scholar]
- Hu, T.; Wang, Y.; Pi, S.F.; Sun, H.Z.; Li, J.L. Process conditions of synthesis ionone from citral. Food Mach. 2014, 30, 224–227. [Google Scholar]
- Xing, K.; You, K.; Yin, D.; Yuan, Z.; Mao, L. A simple and efficient approach for synthesis of pseudoionone from citral and acetone catalyzed by powder LiOH·H2O. Catal. Commun. 2009, 11, 236–239. [Google Scholar] [CrossRef]
- Huang, Z.L.; Lu, H.F.; Wei, G.F.; Huang, H.; Wei, L.B. Synthesis of lemonile from citral under microwave assisted solvent-free condition with phase transfer catalysis of polyethylene glycol 600. Fine Chem. 2008, 25, 576–579. [Google Scholar]
- Liu, X.G.; Chen, M.M.; Xiong, Y.F.; Huang, X.G. Synthesis of irone. Flavour Fragr. Cosmet. 2001, 2, 5–7. [Google Scholar]
- Nik Norulaini, N.A.; Setianto, W.B.; Zaidul, I.S.M.; Nawi, A.H.; Azizi, C.Y.M.; MohdOmar, A.K. Effects of supercritical carbon dioxide extraction parameters on virgin coconut oil yield and medium-chain triglyceride content. Food Chem. 2009, 116, 193–197. [Google Scholar] [CrossRef]
- Liu, R.; Tang, S.; Xiao, Z.; Zhang, A.; Li, P.; Sun, F.; Li, H.; Li, C.; Xia, L. The impact of processing techniques on composition of Litsea cubeba kernel oil. Hunan For. Sci. Technol. 2017, 44, 68–71. [Google Scholar]
- Li, X.; Zhou, J.; Ren, T.; Yang, G. Extraction technology and chemical composition of Litsea cubeba kernel oil. J. For. Eng. 2016, 1, 54–58. [Google Scholar]
- He, Y.; Xiao, Z.; Du, G.; Zhang, A. The extraction of Litsea cubeba oil by microwave-assisted water extraction method. Hunan For. Sci. Technol. 2018, 45, 51–56. [Google Scholar]
- Zhu, H.; Sun, J.; Peng, L.; Lai, C.; Zhu, C. Optimization of microwave-assisted extraction of Litsea cubeba kernel oil by response surface methodology. Guihaia 2017, 37, 1074–1082. [Google Scholar]
- Chen, T.B.; Yuan, X.Y.; Zhang, M. Study on Extraction Technology of Litsea cubeba Kernel Oil by Supercritical Carbon Dioxide. Biomass Chem. Eng. 2009, 43, 5–8. [Google Scholar]
- Zhuang, X.; Zhang, Z.; Wang, Y.; Li, Y. The effect of alternative solvents to n-hexane on the green extraction of Litsea cubeba kernel oils as new oil sources. Ind. Crop. Prod. 2018, 126, 340–346. [Google Scholar] [CrossRef]
- Zhou, J.; Ren, T.; Li, X.; Yang, G. Preparation of high purity lauric acid from Litsea cubeba kernel oil. China Oils Fats 2016, 41, 64–67. [Google Scholar]
- Zhou, J.; Li, X.; Yang, G.; Qian, W.; Li, R. The extraction, purification of Litsea Cubeba kernel oil and its application in the synthesis of surfactant. China For. Prod. Ind. 2013, 40, 13–16. [Google Scholar]
- Yuan, L.; Yuan, X.Y.; Jia, G.; Yao, K.; Hu, T. Study on acetal types degradable surfactants synthesized by Litsea Cubeba kernel oil. J. Hunan Univ. Sci. Eng. 2012, 33, 49–52. [Google Scholar]
- Chemat, F.; Vian, M.A.; Ravi, H.K. Toward petroleum-free with plant-based chemistry. Curr. Opin. Green Sustain. Chem. 2021, 28, 100450. [Google Scholar] [CrossRef]
- Wang, F.; Chang, Z.; Duan, P.; Yan, W.; Xu, Y.; Zhang, L.; Miao, J.; Fan, Y. Hydrothermal liquefaction of Litsea cubeba seed to produce bio-oils. Bioresour. Technol. 2013, 149, 509–515. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, K.; Ma, P. Preparation of biodiesel from Litsea cubeba kernel oil. Chem. Eng. Oil Gas 2015, 44, 14–17. [Google Scholar]
- Li, D.; Feng, W.; Chen, C.; Chen, S.; Fan, G.; Liao, S.; Wu, G.; Wang, Z. Transesterification of Litsea cubeba kernel oil to biodiesel over zinc supported on zirconia heterogeneous catalysts. Renew. Energy 2021, 177, 13–22. [Google Scholar] [CrossRef]
- Schneider, M.P. Plant-oil-based lubricants and hydraulic fluids. J. Sci. Food Agric. 2006, 86, 1769–1780. [Google Scholar] [CrossRef]
- Cai, Z.; Zhuang, X.; Yang, X.; Huang, F.; Wang, Y.; Li, Y. Litsea cubeba kernel oil as a promising new medium-chain saturated fatty acid feedstock for biolubricant base oil synthesis. Ind. Crop. Prod. 2021, 167, 113564. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, C.; Wang, Y.; He, J.; Zhang, Z. Optimization of protein alkali extraction technology from Litsea cubeba and its functional properties. China Oils Fats 2019, 44, 52–57. [Google Scholar]
- Zhang, X.; Yao, Y.; Li, C.; Huang, X.; Jang, G.; Zhou, W.; Dai, Q. Study on Nutrient and Energy Utilization Rate of Rooster and Duck on Litsea Cubeba Residue. Chin. J. Anim. Sci. 2020, 56, 131–134. [Google Scholar]
- Luo, Y.; Li, H.; He, F.; Huan, C.; Li, J.; Yi, K.; Sun, A. Effects of Litsea cubeba Residue on Rumen Fermentation Characteristics of Hu Sheep in Vitro. J. Henan Agric. Sci. 2020, 49, 142–147. [Google Scholar]
- Lin, B.; Sun, L.N.; Xin, H.L.; Nian, H.; Song, H.T.; Jiang, Y.P.; Wei, Z.Q.; Qin, L.P.; Han, T. Anti-inflammatory constituents from the root of Litsea cubeba in LPS-induced RAW 264.7 macrophages. Pharm. Biol. 2016, 54, 1741–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, W.; Shen, H.; Lin, B.; Han, P.; Li, C.H.; Zhang, Q.Y.; Ye, B.Z.; Rahman, K.; Xin, H.L.; Qin, L.P.; et al. Docking study and ntiosteoporosis effects of a dibenzylbutane lignan isolated from Litsea cubeba targeting Cathepsin K and MEK1. Med. Chem. Res. 2018, 27, 2062–2070. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, J.F.; Lv, W.W.; Zhao, Q.C.; Shi, G.B. Antibacterial, antifungal and cytotoxic isoquinoline alkaloids from Litsea cubeba. Molecules 2012, 17, 12950–12960. [Google Scholar] [CrossRef]
| Woody Oil Plants | Family | Chemical Composition of Seed Oil | References | ||||
|---|---|---|---|---|---|---|---|
| Seed Oil Content (%) | Total Unsaturated Fatty Acids (%) | Unique Bioactive Substances | Other Bioactive Substances | ||||
| Camellia oleifera |  | Theaceae | 1–58 | 85–93 | Camellin | Squalene, tea saponin, lignans, etc. | [5,6] |
| Juglans regia |  | Juglandaceae | 59–68 | 90–91 | Juglone | Minerals, melatonin, phospholipid, carotene, vitamin, etc. | [7,8] |
| Paeonia suffruticosa |  | Paeonia | 28–31 | 88–93 | Paeonol, paeonoside | Monoterpenes, triterpenes, etc. | [9,10,11] |
| Xanthoceras sorbifolium |  | Sapindaceae | 55–70 | 83–88 | Nervonic acid, saponin | Sterols, phospholipids, etc. | [12,13] |
| Acer truncatum |  | Aceraceae | 38–55 | 90–92 | Nervonic acid | Vitamin E, flavonoids, coumarin, etc. | [14,15] |
| Eucommia ulmoides |  | Eucommia | 32–37 | 90–91 | Aucubin, chlorogenic acid | Iridoids, phenylpropanoids, lignans, etc. | [16,17] |
| Swida wilsoniana |  | Cornaceae | 55–62 | 74–82 | Octacosanol | β-sitosterol, vitamin, etc. | [18,19,20] |
| Methods | Conditions | Experimental Remarks | References |
|---|---|---|---|
| Microwave-assisted extraction | Microwave irradiation time: 10–20 min; Microwave temperature: 60–80 °C; Distillation temperature: 80–100 °C; Distillation time: 90–120 min. | Compared to conventional steam distillation, the yield of essential oil increased by 36.5–37.5% and the treatment time was four times shorter. The citral content was 5% higher and the amount of its loss in purification reduced by 33.3% | [55] |
| Microwave power: 650 W; Extraction time: 40 min; Solid-liquid ratio: 1:4 (g/mL). | Average yield of essential oil was up to 10.29% (g/g). | [56] | |
| Ultrasonic-assisted extraction | Liquid to solid ratio: 3.2:1; Vacuum distillation temperature: 76 °C; Particle size: 80 mesh; Ultrasonic time: 35 min. | The extraction rate under vacuum was 6.94%, which was 33.98% higher than the that of conventional steam distillation. The content of citral was 87.65%. | [57] |
| Liquid to solid ratio: 5:1; Ultrasonic time: 25 min; Ultrasonic temperature: 100 °C. | Compared to hydrodistillation and steam distillation, essential oil yield increased with the ultrasonic time; the optimal ultrasonic-assisted extraction was helpful for obtaining high-purity citral. | [58] | |
| Enzymatic-assisted extraction | Heterologous expressed expansin: 500 mL; Cellulase: 2.5 g; Enzymolysis time: 24 h; Temperature: 42 °C; Centrifuge speed: 3000 r/min. | Enzymatic-assisted extraction could significantly improve the yield of essential oil compared to conventional extraction. The highest yield was obtained using composite enzyme (cellulase and expansin), which was 1/3 higher than that using cellulose alone. | [59] |
| Supercritical CO2 extraction | Particle size: 60–80 mesh; Extraction pressure: 25 MPa; Extraction temperature: 45 °C; Extraction time: 60 min; CO2 flow rate: 1.5 mL/min. | The extraction rate was up to above 30.19% and the essential oil had clear color. | [60] |
| Combined extraction | Microwave power: 600 W; Extraction time: 8 min; Solid-liquid ratio: 1:7 (g/mL); Extraction temperature: 85 °C. | Average yield of essential oil of combined extraction assisted by microwave and ultrasound was up to 14.19% (g/g), which was 3.9% higher than that extracted by microwave solely. | [56] |
| Oxalic acid/choline chloride: 1:1; Water content: 50%; Liquid-solid ratio: 12:5:1 mL/g Homogenate time: 2 min Microwave power: 700 W | Deep eutectic solvent-homogenate based microwave-assisted hydrodistillation was developed to have a quite different major compounds (e.g., m-cymeme, trans-linalool, etc.) under optimal conditions, which showed higher in vitro radical scavenging activity but lower antifungal activity. | [61] |
| Organism | MIC [µg/mL] | Method | References | |
|---|---|---|---|---|
| Bacteria | Gram-Type | |||
| Enterohemorrhagic Escherichia coli (EHEC) | − | 500 | Double dilution | [65] |
| Methicillin-resistant Staphylococcus aureus (MRSA) | + | 500 | Broth micro-dilution | [66] |
| Staphylococcus aureus | + | 80 | Broth micro-dilution | [67] |
| Bacillus cereus | + | 40 | Broth micro-dilution | |
| Bacillus subtilis | + | 40 | Broth micro-dilution | |
| Salmonella typhimurium | − | 20 | Broth micro-dilution | |
| Listeria monocytogenes | + | 2500 | Broth micro-dilution | [49] |
| Salmonella | − | 625 | Double dilution | [68] |
| Shigella | − | 625 | Double dilution | |
| Pseudomonas aeruginosa | − | 620 | Broth micro-dilution | [69] |
| Enterococcus faecalis | + | 600 | Broth micro-dilution | |
| Shewanella putrefaciens | − | 0.5 | Broth micro-dilution | [70] |
| Staphylococcus albus | + | 11.88–23.75 | Broth-dilution | [71] |
| Vibrio parahaemolyticus | − | 750 | Broth-dilution | [72] |
| Fungi | ||||
| Alternaria alternaria | 0.05 | Agar-dilution | [73] | |
| Aspergillus flavus | 0.5 | Agar-dilution | [74] | |
| Aspergillus niger | 5 | Agar-dilution | [73] | |
| Fusarium oxysporum | 0.49 | Agar-dilution | [75] | |
| Fusarium moniliforme | 0.5 | Agar-dilution | [73] | |
| Fusarium solani | 0.5 | Agar-dilution | ||
| Galactomyces candidum | 1.0 | Agar-dilution | [76] | |
| Candida albicans | 700 | Broth micro-dilution | [69] | |
| Lactobacillus plantarum | 1500 | Broth-dilution | [72] | |
| Malassezia furfur | 2367.61 ± 688.29 | Broth micro-dilution | [77] | |
| Species | Morphology | Experimental Remarks | References |
|---|---|---|---|
| Aedes aegypti (L.) mosquitoes | Adult | 24 h direct contact mortality: 2.3–20.4% 24 h non-contact mortality: 0–14.3% | [86] |
| Aedes albopictus | Larva (the fourth-instar) | 24 h LC50: 82.48 µg/mL | [84] |
| Pupae | 24 h LC50: 122.92 µg/mL | ||
| Adult | 73.94 percentage repellency at 20 min (2.0 µL) | [87] | |
| Lasioderma serricorne | Adult | Contact toxicity 24 h LC50: 27.33 µg/adult Fumigant toxicity 24 h LC50: 22.97 mg/L | [88] |
| Liposcelis bostrychophila | Adult | Contact toxicity 24 h LC50:71.56 µg/cm2 Fumigant toxicity 24 h LC50: 0.73 mg/L | |
| Bursaphelenchus Xylophilus | Adult | 24 h LC50: 0.504 mg/mL | [89] |
| Liposcelis entomophila Enderlein | Adult | 24 h LC50: 6.23 µL/L | [90] |
| Tribolium castaneum | Adult | 1.5 g/cm2 repellent rate (12 h): 81.26% | [91] |
| Trichoplusia ni | Larva (the third-instar) | 24 h LC50: 112.5 µg/larva | [92] |
| Luciaphorus perniciosus | Adult | Contact toxicity 12 h LC50: 0.932 µg/cm2, 99 µg/cm2 showed the highest toxicity causing 97.5 ± 4.1% mortality at 12 h Fumigation 12 h LC50: 0.166 µg/cm3 | [93] |
| Anopheles stephensi | Adult | The protection period: 480 min; 100 percentage repellency | [94] |
| Culex quinquefasciatus | Adult | The protection period: 480 min; 100 percentage repellency |
| Methods | Conditions | Experimental Remarks | References |
|---|---|---|---|
| Sodium sulfite chemical addition method | DMSO as phase transfer catalyst: 5% of citral material; Time: 3.5 h, Temperature: 10 °C. | The citral yield was 73.47% and the purity was 85.49%. | [106] |
| Methylated-β-cyclodextrin (RM-β-CD) as phase transfer catalyst: 0.65% of citral material; Time: 3.3 h, Temperature: 15 °C. | The citral yield was 86.6% and the purity was 96.5%. | [107] | |
| Vacuum distillation | Pretreatment: dehydration, magnetization, filtration, and deoxidation; Vacuum degree: 10 mm Hg, tower kettle temperature: <100 °C; The first fractionation column top temperature <65 °C, reflux ratio 2:1; The second fractionation column top temperature <90 °C, reflux ratio 3:1. | The purity of citral was 97.9%, and the yield was 90.8%. | [108] |
| Molecular distillation | Film scraping speed: 400 r/min; Feeding amount: 1 L Cooling water temperature: 12 °C; Distillation temperature: 55 °C; Distillation pressure: 0.18 kPa; Material flow: 15 mL/min. | The purity of citral was up to 98% and the yield rate was up to 77.2%. | [109] |
| Film scraping speed: 370–390 r/min; Cooling water temperature: 4–5 °C; Distillation temperature: 45 °C; Distillation pressure: 0.15 kPa; Material flow: 1 drop/s. | The content of citral was increased from 79.61% to 95.08%, and the yield of citral was 80.02%. | [110] |
| Methods | Process Conditions | Experimental Remarks | References |
|---|---|---|---|
| Mechanical pressing | Press in a single screw press, collect and filter the crude oil, then store it in a 4 °C refrigerator. | The crude oil yield was 26.2%, which was reduced to 21.2% after simple refining. | [116] |
| Solvent reflux method | Refluxing with petroleum ether (60–90 °C); Particle size of material: less than 0.15 mm Solid–liquid ratio at 1:14 (g:mL); Extraction temperature and time: 80 °C, 2.0 h. | The yield of oil is 26.69%. The content of lauric acid was 49.53%. | [117] |
| Microwave-assisted extraction | Microwave time: 65 min; Extraction temperature: 78 °C; Solid–liquid ratio at 1:14.5; Microwave power: 545 W. | The aqueous extraction rate of kernel oil is 29. 36%. The content of lauric acid, capric acid and oleic acid is >50%, 8.512% and 10.603%, respectively. | [118] |
| Microwave time: 63 min; Extraction temperature: 69 °C; Solid–liquid ratio at 1:16 (g:mL); Microwave power: 337 W. | The extraction rate of kernel oil is 37.42%, which improved by 30.11% compared to n-hexane reflux method. The content of lauric acid is the highest (31.36%). | [119] | |
| Supercritical CO2 extraction | Extraction time: 80 min; Extraction temperature: 45 °C; Extraction pressure: 25 MPa; Flow rate of carbon dioxide: 220 L/h. | The extraction rate is above 84.5%, dehulling may increase the extraction rate. | [120] |
| Alternative solvent extraction | Solid–liquid ratio at 1:20 (g:mL); Heating under reflux for 3 h; Extraction temperature: solvents’ boiling point. | Green solvents were superior to alcoholic solvents with higher oil yields. Alternative solvents to n-hexane extracted more micronutrients (e.g, tocopherol, sterol and phenolic compounds) resulting in better antioxidant activities. | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Yu, Y.; Lan, P.; Wang, Y.; Li, Y. An Overview on Total Valorization of Litsea cubeba as a New Woody Oil Plant Resource toward a Zero-Waste Biorefinery. Molecules 2021, 26, 3948. https://doi.org/10.3390/molecules26133948
Qiu Y, Yu Y, Lan P, Wang Y, Li Y. An Overview on Total Valorization of Litsea cubeba as a New Woody Oil Plant Resource toward a Zero-Waste Biorefinery. Molecules. 2021; 26(13):3948. https://doi.org/10.3390/molecules26133948
Chicago/Turabian StyleQiu, Yufei, Yasi Yu, Ping Lan, Yong Wang, and Ying Li. 2021. "An Overview on Total Valorization of Litsea cubeba as a New Woody Oil Plant Resource toward a Zero-Waste Biorefinery" Molecules 26, no. 13: 3948. https://doi.org/10.3390/molecules26133948
APA StyleQiu, Y., Yu, Y., Lan, P., Wang, Y., & Li, Y. (2021). An Overview on Total Valorization of Litsea cubeba as a New Woody Oil Plant Resource toward a Zero-Waste Biorefinery. Molecules, 26(13), 3948. https://doi.org/10.3390/molecules26133948






