Resveratrol Butyrate Esters Inhibit Obesity Caused by Perinatal Exposure to Bisphenol A in Female Offspring Rats
Abstract
:1. Introduction
2. Results
2.1. Changes in Body Weight, Abdominal Lipid Weight, Plasma Biochemical Markers, and Adipose Tissues in Female Offspring Rats
2.2. Changes in Intestinal Microbiota and Faecal Concentrations of SCFAs in Female Offspring Rats
3. Discussion
3.1. RBE Suppressed BPA-Induced Obesity
3.2. The Relationship between the Suppression of Obesity by RBE and Intestinal Microbiota
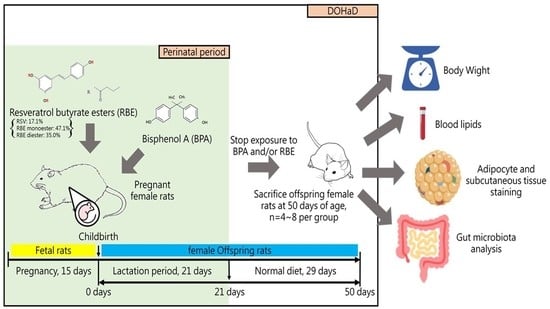
4. Materials and Methods
4.1. Synthesis of Resveratrol Butyrate Ester
4.2. Experimental Animals
4.3. Plasma Biochemical and Hormonal Analysis
4.4. Histopathology
4.5. Metagenomics Analysis
4.6. Quantification of Faecal SCFAs
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pelch, K.; Wignall, J.A.; Goldstone, A.E.; Ross, P.K.; Blain, R.B.; Shapiro, A.J.; Holmgren, S.D.; Hsieh, J.H.; Svoboda, D.; Auerbach, S.S.; et al. A scoping review of the health and toxicological activity of bisphenol A (BPA) structural analogues and functional alternatives. Toxicology 2019, 424, 152235. [Google Scholar] [CrossRef]
- Rahman, M.S.; Adegoke, E.O.; Pang, M.-G. Drivers of owning more BPA. J. Hazard. Mater. 2021, 417, 126076. [Google Scholar] [CrossRef]
- Moon, S.; Yu, S.H.; Lee, C.B.; Park, Y.J.; Yoo, H.J.; Kim, D.S. Effects of bisphenol A on cardiovascular disease: An epidemiological study using National Health and Nutrition Examination Survey 2003–2016 and meta-analysis. Sci. Total Environ. 2021, 763. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B.; Engin, A. The effect of environmental Bisphenol A exposure on breast cancer associated with obesity. Environ. Toxicol. Pharmacol. 2021, 81. [Google Scholar] [CrossRef]
- Lee, I.; Park, Y.J.; Kim, M.J.; Kim, S.; Choi, S.; Park, J.; Cho, Y.H.; Hong, S.; Yoo, J.; Park, H.; et al. Associations of urinary concentrations of phthalate metabolites, bisphenol A, and parabens with obesity and diabetes mellitus in a Korean adult population: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Environ. Int. 2021, 146, 106227. [Google Scholar] [CrossRef] [PubMed]
- Angle, B.M.; Do, R.P.; Ponzi, D.; Stahlhut, R.W.; Drury, B.E.; Nagel, S.C.; Welshons, W.V.; Besch-Williford, C.L.; Palanza, P.; Parmigiani, S.; et al. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): Evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod. Toxicol. 2013, 42, 256–268. [Google Scholar] [CrossRef] [Green Version]
- Rancière, F.; Botton, J.; Slama, R.; Lacroix, M.Z.; Debrauwer, L.; Charles, M.A.; Roussel, R.; Balkau, B.; Magliano, D.J.; Balkau, B.; et al. Exposure to bisphenol a and bisphenol s and incident type 2 diabetes: A case-cohort study in the French cohort D.E.S.I.R. Environ. Health Perspect. 2019, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, L.; Meng, Q.; Diamante, G.; Tsai, B.; Chen, Y.-W.; Mikhail, A.; Luk, H.; Ritz, B.; Allard, P.; Yang, X. Prenatal bisphenol a exposure in mice induces multitissue multiomics disruptions linking to cardiometabolic disorders. Endocrinology 2019, 160, 409–429. [Google Scholar] [CrossRef] [Green Version]
- Churchwell, M.I.; Camacho, L.; Vanlandingham, M.M.; Twaddle, N.C.; Sepehr, E.; Delclos, K.B.; Fisher, J.W.; Doerge, D.R. Comparison of life-stage-dependent internal dosimetry for bisphenol A, ethinyl estradiol, a reference estrogen, and endogenous estradiol to test an estrogenic mode of action in Sprague Dawley rats. Toxicol. Sci. 2014, 139, 4–20. [Google Scholar] [CrossRef] [Green Version]
- Doerge, D.R.; Twaddle, N.C.; Vanlandingham, M.; Fisher, J.W. Pharmacokinetics of bisphenol A in neonatal and adult Sprague-Dawley rats. Toxicol. Appl. Pharmacol. 2010, 247, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Doerge, D.R.; Twaddle, N.C.; Vanlandingham, M.; Fisher, J.W. Pharmacokinetics of Bisphenol A in neonatal and adult CD-1 mice: Inter-species comparisons with Sprague-Dawley rats and rhesus monkeys. Toxicol. Lett. 2011, 207, 298–305. [Google Scholar] [CrossRef]
- Doerge, D.R.; Twaddle, N.C.; Vanlandingham, M.; Fisher, J.W. Pharmacokinetics of bisphenol A in serum and adipose tissue following intravenous administration to adult female CD-1 mice. Toxicol. Lett. 2012, 211, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Thayer, K.A.; Doerge, D.R.; Hunt, D.; Schurman, S.H.; Twaddle, N.C.; Churchwell, M.I.; Garantziotis, S.; Kissling, G.E.; Easterling, M.R.; Bucher, J.R.; et al. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ. Int. 2015, 83, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Völkel, W.; Colnot, T.; Csanády, G.A.; Filser, J.G.; Dekant, W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem. Res. Toxicol. 2002, 15, 1281–1287. [Google Scholar] [CrossRef]
- Völkel, W.; Kiranoglu, M.; Fromme, H. Determination of free and total bisphenol A in human urine to assess daily uptake as a basis for a valid risk assessment. Toxicol. Lett. 2008, 179, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Teeguarden, J.G.; Calafat, A.M.; Ye, X.; Doerge, D.R.; Churchwell, M.I.; Gunawan, R.; Graham, M.K. Twenty-four hour human urine and serum profiles of bisphenol A during high-dietary exposure. Toxicol. Sci. 2011, 123, 48–57. [Google Scholar] [CrossRef]
- Teeguarden, J.G.; Twaddle, N.C.; Churchwell, M.I.; Yang, X.; Fisher, J.W.; Seryak, L.M.; Doerge, D.R. 24-h human urine and serum profiles of bisphenol A: Evidence against sublingual absorption following ingestion in soup. Toxicol. Appl. Pharmacol. 2015, 288, 131–142. [Google Scholar] [CrossRef]
- Report on the two-phase public consultation on the draft EFSA scientific opinion on bisphenol A (BPA). EFSA Support. Publ. 2017, 12, 740E. [CrossRef] [Green Version]
- Adegoke, E.O.; Rahman, M.S.; Pang, M.G. Bisphenols Threaten Male Reproductive Health via Testicular Cells. Front. Endocrinol. 2020, 11, 624. [Google Scholar] [CrossRef]
- Kitamura, S.; Suzuki, T.; Sanoh, S.; Kohta, R.; Jinno, N.; Sugihara, K.; Yoshihara, S.; Fujimoto, N.; Watanabe, H.; Ohta, S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. 2005, 84, 249–259. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kwon, W.S.; Yoon, S.J.; Park, Y.J.; Ryu, B.Y.; Pang, M.G. A novel approach to assessing bisphenol-A hazards using an in vitro model system. BMC Genom. 2016, 17, 1–12. [Google Scholar] [CrossRef]
- Beausoleil, C.; Emond, C.; Cravedi, J.P.; Antignac, J.P.; Applanat, M.; Appenzeller, B.M.R.; Beaudouin, R.; Belzunces, L.P.; Canivenc-Lavier, M.C.; Chevalier, N.; et al. Regulatory identification of BPA as an endocrine disruptor: Context and methodology. Mol. Cell. Endocrinol. 2018, 475, 4–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tain, Y.-L.; Chan, J.Y.H.; Lee, C.-T.; Hsu, C.-N. Maternal melatonin therapy attenuates methyl-donor diet-induced programmed hypertension in male adult rat offspring. Nutrients 2018, 10, 1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tain, Y.-L.; Lin, Y.-J.; Chan, J.Y.H.; Lee, C.-T.; Hsu, C.-N. Maternal melatonin or agomelatine therapy prevents programmed hypertension in male offspring of mother exposed to continuous light. Biol. Reprod. 2017, 97, 636–643. [Google Scholar] [CrossRef]
- Sergeyev, O.V.; Nikitin, A.I. Developmental origins of health and disease (DOHaD) and paternal origins of health and disease (POHaD). Multigenerational inheritance. Obstet. Gynecol. Reprod. 2019, 13, 326–336. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Lin, Y.-J.; Sheen, J.-M.; Yu, H.-R.; Tiao, M.-M.; Chen, C.-C.; Tsai, C.-C.; Huang, L.-T.; Hsu, C.-N. High Fat Diets Sex-Specifically Affect the Renal Transcriptome and Program Obesity, Kidney Injury, and Hypertension in the Offspring. Nutrients 2017, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Mhaouty-Kodja, S.; Belzunces, L.P.; Canivenc, M.-C.; Schroeder, H.; Chevrier, C.; Pasquier, E. Impairment of learning and memory performances induced by BPA: Evidences from the literature of a MoA mediated through an ED. Mol. Cell. Endocrinol. 2018, 475, 54–73. [Google Scholar] [CrossRef]
- Malaisé, Y.; Menard, S.; Cartier, C.; Gaultier, E.; Lasserre, F.; Lencina, C.; Harkat, C.; Geoffre, N.; Lakhal, L.; Castan, I.; et al. Gut dysbiosis and impairment of immune system homeostasis in perinatally-exposed mice to Bisphenol A precede obese phenotype development. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Reddivari, L.; Veeramachaneni, D.N.R.; Walters, W.A.; Lozupone, C.; Palmer, J.; Hewage, M.K.; Bhatnagar, R.; Amir, A.; Kennett, M.J.; Knight, R.; et al. Perinatal bisphenol a exposure induces chronic inflammation in rabbit offspring via modulation of gut bacteria and their metabolites. mSystems 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Janesick, A.; Blumberg, B. Obesogens, stem cells and the developmental programming of obesity. Int. J. Androl. 2012, 35, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.H.; Shioda, T.; Soto, A.M.; vomSaal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Kundakovic, M.; Gudsnuk, K.; Franks, B.; Madrid, J.; Miller, R.L.; Perera, F.P.; Champagne, F.A. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol a exposure. Proc. Natl. Acad. Sci. USA 2013, 110, 9956–9961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Susiarjo, M.; Sasson, I.; Mesaros, C.; Bartolomei, M.S. Bisphenol A Exposure Disrupts Genomic Imprinting in the Mouse. PLoS Genet. 2013, 9, e1003401. [Google Scholar] [CrossRef] [Green Version]
- Somm, E.; Schwitzgebel, V.M.; Toulotte, A.; Cederroth, C.R.; Combescure, C.; Nef, S.; Aubert, M.L.; Hüppi, P.S. Perinatal exposure to bisphenol A alters early adipogenesis in the rat. Environ. Health Perspect. 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darby, J.R.T.; Mohd Dollah, M.H.B.; Regnault, T.R.H.; Williams, M.T.; Morrison, J.L. Systematic review: Impact of resveratrol exposure during pregnancy on maternal and fetal outcomes in animal models of human pregnancy complications—Are we ready for the clinic? Pharmacol. Res. 2019, 144, 264–278. [Google Scholar] [CrossRef]
- Huang, X.; Li, X.; Xie, M.; Huang, Z.; Huang, Y.; Wu, G.; Peng, Z.; Sun, Y.; Ming, Q.; Liu, Y.; et al. Resveratrol: Review on its discovery, anti-leukemia effects and pharmacokinetics. Chem. Biol. Interact. 2019, 306, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.-L.; Chang, S.K.C.; Liao, J.-X.; Chen, Y.-W.; Huang, H.-T.; Li, Y.-L.; Hou, C.-Y. Synthesis of Short-Chain-Fatty-Acid Resveratrol Esters and Their Antioxidant Properties. Antioxidants 2021, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.-L.; Jheng, L.-C.; Chang, S.K.C.; Chen, Y.-W.; Huang, L.-T.; Liao, J.-X.; Hou, C.-Y. Synthesis and Characterization of Novel Resveratrol Butyrate Esters That Have the Ability to Prevent Fat Accumulation in a Liver Cell Culture Model. Molecules 2020, 25, 4199. [Google Scholar] [CrossRef]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Hu, X.-P.; Yin, F.-W.; Zhou, D.-Y.; Xie, H.-K.; Zhu, B.-W.; Ma, X.-C.; Tian, X.-G.; Wang, C.; Shahidi, F. Stability of resveratrol esters with caprylic acid during simulated in vitro gastrointestinal digestion. Food Chem. 2019, 276, 675–679. [Google Scholar] [CrossRef]
- Wilkins, A.T.; Reimer, R.A. Obesity, early life gut microbiota, and antibiotics. Microorganisms 2021, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Xu, M.; Qiao, G.; Li, C.; Lin, L.; Zheng, G. Smilax china L. polyphenols alleviates obesity and inflammation by modulating gut microbiota in high fat/high sucrose diet-fed C57BL/6J mice. J. Funct. Foods 2021, 77, 104332. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Z.H.; Zhu, C.X.; Quan, Y.S.; Yang, Z.Y.; Wu, S.; Luo, W.W.; Tan, B.; Wang, X.Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Lzzo, A.A. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef] [Green Version]
- Strakovsky, R.S.; Wang, H.; Engeseth, N.J.; Flaws, J.A.; Helferich, W.G.; Pan, Y.X.; Lezmi, S. Developmental bisphenol A (BPA) exposure leads to sex-specific modification of hepatic gene expression and epigenome at birth that may exacerbate high-fat diet-induced hepatic steatosis. Toxicol. Appl. Pharmacol. 2015, 284, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Lejonklou, M.H.; Dunder, L.; Bladin, E.; Pettersson, V.; Rönn, M.; Lind, L.; Waldén, T.B.; Lind, P.M. Effects of low-dose developmental bisphenol a exposure on metabolic parameters and gene expression in male and female fischer 344 rat offspring. Environ. Health Perspect. 2017, 125. [Google Scholar] [CrossRef]
- Yang, T.C.; Peterson, K.E.; Meeker, J.D.; Sánchez, B.N.; Zhang, Z.; Cantoral, A.; Solano, M.; Tellez-Rojo, M.M. Bisphenol A and phthalates in utero and in childhood: Association with child BMI z-score and adiposity. Environ. Res. 2017, 156, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Rubin, B.S.; Paranjpe, M.; DaFonte, T.; Schaeberle, C.; Soto, A.M.; Obin, M.; Greenberg, A.S. Perinatal BPA exposure alters body weight and composition in a dose specific and sex specific manner: The addition of peripubertal exposure exacerbates adverse effects in female mice. Reprod. Toxicol. 2017, 68, 130–144. [Google Scholar] [CrossRef] [Green Version]
- vomSaal, F.S.; Nagel, S.C.; Coe, B.L.; Angle, B.M.; Taylor, J.A. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol. Cell. Endocrinol. 2012, 354, 74–84. [Google Scholar] [CrossRef] [Green Version]
- Rubin, B.S.; Soto, A.M. Bisphenol A: Perinatal exposure and body weight. Mol. Cell. Endocrinol. 2009, 304, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Desai, M.; Ferrini, M.G.; Han, G.; Jellyman, J.K.; Ross, M.G. In vivo maternal and in vitro BPA exposure effects on hypothalamic neurogenesis and appetite regulators. Environ. Res. 2018. [Google Scholar] [CrossRef]
- Murata, M.; Kang, J.-H. Bisphenol A (BPA) and cell signaling pathways. Biotechnol. Adv. 2018, 36, 311–327. [Google Scholar] [CrossRef]
- Pu, Y.; Gingrich, J.D.; Steibel, J.P.; Veiga-Lopez, A. Sex-Specific Modulation of Fetal Adipogenesis by Gestational Bisphenol A and Bisphenol S Exposure. Endocrinology 2017, 158, 3844–3858. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.Y.; Yu, H.R.; Tsai, C.C.; Huang, L.T.; Chen, C.C.; Sheen, J.M.; Tiao, M.M.; Tain, Y.L.; Lin, I.C.; Lai, Y.J.; et al. Resveratrol intake during pregnancy and lactation re-programs adiposity and ameliorates leptin resistance in male progeny induced by maternal high-fat/high sucrose plus postnatal high-fat/high sucrose diets via fat metabolism regulation. Lipids Health Dis. 2020, 19, 1–13. [Google Scholar] [CrossRef]
- Franco, J.G.; Dias-Rocha, C.P.; Fernandes, T.P.; Albuquerque Maia, L.; Lisboa, P.C.; Moura, E.G.; Pazos-Moura, C.C.; Trevenzoli, I.H. Resveratrol treatment rescues hyperleptinemia and improves hypothalamic leptin signaling programmed by maternal high-fat diet in rats. Eur. J. Nutr. 2016, 55, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; McCrory, J.L.; Kearcher, K.; Vickers, A.; Frear, B.; Gilleland, D.L.; Bonner, D.E.; Thomas, J.M.; Donley, D.A.; Lively, M.W.; et al. Resveratrol Enhances Exercise-Induced Cellular and Functional Adaptations of Skeletal Muscle in Older Men and Women. Journals Gerontol.-Ser. A Biol. Sci. Med. Sci. 2017, 72, 1595–1606. [Google Scholar] [CrossRef] [Green Version]
- Bennett, B.T.; Mohamed, J.S.; Alway, S.E. Effects of resveratrol on the recovery of muscle mass following disuse in the plantaris muscle of aged rats. PLoS ONE 2013, 8, e83518. [Google Scholar] [CrossRef] [Green Version]
- Salomão, R.A.S.; DePaula, T.G.; Zanella, B.T.T.; Carvalho, P.L.P.F.; daSilva Duran, B.O.; Valente, J.S.; deAlmeida Fantinatti, B.E.; Fernandes, A.A.; Barros, M.M.; Mareco, E.A.; et al. The combination of resveratrol and exercise enhances muscle growth characteristics in pacu (Piaractus mesopotamicus). Comp. Biochem. Physiol.-Part A Mol. Integr. Physiol. 2019, 235, 46–55. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Nelson, A.J. HDL and cardiovascular disease. Pathology 2019, 51, 142–147. [Google Scholar] [CrossRef]
- Hirayama, S.; Miida, T. Small dense LDL: An emerging risk factor for cardiovascular disease. Clin. Chim. Acta 2012, 414, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Gingrich, J.; Ticiani, E.; Veiga-Lopez, A. Placenta Disrupted: Endocrine Disrupting Chemicals and Pregnancy. Trends Endocrinol. Metab. 2020, 31, 508–524. [Google Scholar] [CrossRef]
- Jimoh, A.; Tanko, Y.; Ayo, J.O.; Ahmed, A.; Mohammed, A. Resveratrol increases serum adiponectin level and decreases leptin and insulin level in an experimental model of hypercholesterolemia. Pathophysiology 2018, 25, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Rönn, M.; Lind, L.; Örberg, J.; Kullberg, J.; Söderberg, S.; Larsson, A.; Johansson, L.; Ahlström, H.; Lind, P.M. Bisphenol A is related to circulating levels of adiponectin, leptin and ghrelin, but not to fat mass or fat distribution in humans. Chemosphere 2014, 112, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Rangwala, S.M.; Rhoades, B.; Shapiro, J.S.; Rich, A.S.; Kim, J.K.; Shulman, G.I.; Kaestner, K.H.; Lazar, M.A. Genetic modulation of PPARgamma phosphorylation regulates insulin sensitivity. Dev. Cell 2003, 5, 657–663. [Google Scholar] [CrossRef] [Green Version]
- Masuno, H.; Kidani, T.; Sekiya, K.; Sakayama, K.; Shiosaka, T.; Yamamoto, H.; Honda, K. Bisphenol A in combination with insulin can accelerate the conversion of 3T3-L1 fibroblasts to adipocytes. J. Lipid Res. 2002. [Google Scholar] [CrossRef]
- Li, S.-W.; Yu, H.-R.; Sheen, J.-M.; Tiao, M.-M.; Tain, Y.-L.; Lin, I.-C.; Lin, Y.-J.; Chang, K.-A.; Tsai, C.-C.; Huang, L.-T. A Maternal High-Fat Diet during Pregnancy and Lactation, in Addition to a Postnatal High-Fat Diet, Leads to Metabolic Syndrome with Spatial Learning and Memory Deficits: Beneficial Effects of Resveratrol. Oncotarget 2017, 8, 111998–112013. [Google Scholar] [CrossRef] [Green Version]
- Bridgeman, S.C.; Northrop, W.; Melton, P.E.; Ellison, G.C.; Newsholme, P.; Mamotte, C.D.S. Butyrate generated by gut microbiota and its therapeutic role in metabolic syndrome. Pharmacol. Res. 2020, 160, 105174. [Google Scholar] [CrossRef]
- Li, T.; Gao, J.; Du, M.; Mao, X. Milk fat globule membrane supplementation modulates the gut microbiota and attenuates metabolic endotoxemia in high-fat diet-fed mice. J. Funct. Foods 2018, 47, 56–65. [Google Scholar] [CrossRef]
- Serino, M.; Luche, E.; Gres, S.; Baylac, A.; Bergé, M.; Cenac, C.; Waget, A.; Klopp, P.; Iacovoni, J.; Klopp, C.; et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 2012. [Google Scholar] [CrossRef]
- Wang, P.; Gao, J.; Ke, W.; Wang, J.; Li, D.; Liu, R.; Jia, Y.; Wang, X.; Chen, X.; Chen, F.; et al. Resveratrol reduces obesity in high-fat diet-fed mice via modulating the composition and metabolic function of the gut microbiota. Free Radic. Biol. Med. 2020, 156, 83–98. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Lin, Y.-J.; Tain, Y.-L. Maternal Exposure to Bisphenol A Combined with High-Fat Diet-Induced Programmed Hypertension in Adult Male Rat Offspring: Effects of Resveratrol. Int. J. Mol. Sci. 2019, 20, 4382. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Huang, G.; Nagy, T.; Guo, T.L. Bisphenol A alteration of type 1 diabetes in non-obese diabetic (NOD) female mice is dependent on window of exposure. Arch. Toxicol. 2019, 93, 1083–1093. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Carnielli, V.P.; Ksiazyk, J.; Luna, M.S.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; Singhal, A.; Wabitsch, M. Factors affecting early-life intestinal microbiota development. Nutrition 2020, 78, 110812. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Huang, L.-T.; Sheen, J.-M.; Hou, C.-Y.; Yeh, Y.-T.; Chiang, C.-P.; Lin, I.-C.; Tiao, M.-M.; Tsai, C.-C.; Lin, Y.-J.; et al. Resveratrol treatment improves the altered metabolism and related dysbiosis of gut programed by prenatal high-fat diet and postnatal high-fat diet exposure. J. Nutr. Biochem. 2020, 75, 108260. [Google Scholar] [CrossRef]
- Zha, C.; Xiao, H.; Song, B.; Zheng, C.; Yang, X.; Wang, W.; Wang, L. Resveratrol promotes mammary cell proliferation and antioxidation capacity during pregnancy and lactation in mice. J. Appl. Microbiol. 2020. [Google Scholar] [CrossRef]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017.
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Finegold, S.M.; Summanen, P.H.; Downes, J.; Corbett, K.; Komoriya, T. Detection of Clostridium perfringens toxin genes in the gut microbiota of autistic children. Anaerobe 2017, 45, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Cree, B.A.C.; Spencer, C.M.; Varrin-Doyer, M.; Baranzini, S.E.; Zamvil, S.S. Gut microbiome analysis in neuromyelitis optica reveals overabundance of Clostridium perfringens. Ann. Neurol. 2016. [Google Scholar] [CrossRef]
- Cao, S.-Y.; Zhao, C.-N.; Xu, X.-Y.; Tang, G.-Y.; Corke, H.; Gan, R.-Y.; Li, H.-B. Dietary plants, gut microbiota, and obesity: Effects and mechanisms. Trends Food Sci. Technol. 2019, 92, 194–204. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef]
- Overby, H.B.; Ferguson, J.F. Gut Microbiota-Derived Short-Chain Fatty Acids Facilitate Microbiota: Host Cross talk and Modulate Obesity and Hypertension. Curr. Hypertens. Rep. 2021, 23. [Google Scholar] [CrossRef]
- Mojsak, P.; Rey-Stolle, F.; Parfieniuk, E.; Kretowski, A.; Ciborowski, M. The role of gut microbiota (GM) and GM-related metabolites in diabetes and obesity. A review of analytical methods used to measure GM-related metabolites in fecal samples with a focus on metabolites’ derivatization step. J. Pharm. Biomed. Anal. 2020, 191, 113617. [Google Scholar] [CrossRef]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tain, Y.-L.; Chan, S.H.H.; Chan, J.Y.H. Biochemical basis for pharmacological intervention as a reprogramming strategy against hypertension and kidney disease of developmental origin. Biochem. Pharmacol. 2018, 153, 82–90. [Google Scholar] [CrossRef]
- Otto, G.M.; Franklin, C.L.; Clifford, C.B. Chapter 4—Biology and Diseases of Rats. In Laboratory Animal Medicine: Third Edition; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 151–207. ISBN 9780124095274. [Google Scholar]
- Wei, J.; Sun, X.; Chen, Y.; Li, Y.; Song, L.; Zhou, Z.; Xu, B.; Lin, Y.; Xu, S. Perinatal exposure to bisphenol A exacerbates nonalcoholic steatohepatitis-like phenotype in male rat offspring fed on a high-fat diet. J. Endocrinol. 2014, 222, 313–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.-E.; Lin, Y.-J.; Lin, I.-C.; Yu, H.-R.; Sheen, J.-M.; Tsai, C.-C.; Huang, L.-T.; Tain, Y.-L. Resveratrol prevents combined prenatal NG-nitro-L-arginine-methyl ester (L-NAME) treatment plus postnatal high-fat diet induced programmed hypertension in adult rat offspring: Interplay between nutrient-sensing signals, oxidative stress and gut. J. Nutr. Biochem. 2019, 70, 28–37. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Lin, Y.-J.; Lu, P.-C.; Tain, Y.-L. Maternal resveratrol therapy protects male rat offspring against programmed hypertension induced by TCDD and dexamethasone exposures: Is it relevant to aryl hydrocarbon receptor? Int. J. Mol. Sci. 2018, 19, 2459. [Google Scholar] [CrossRef] [Green Version]
- abcamFree Fatty Acid Quantification Assay Kit ab ab65341, Protocol Book Let. Available online: https://www.abcam.com/Free-Fatty-Acid-Assay-Kit-Quantification-ab65341.html?pageNumber=2 (accessed on 29 June 2021).
- abcamCholesterol/Cholesteryl Ester Quantitation Assay Kit ab65359, Protocol Book Let. Available online: Cholesterol/CholesterylEhttps://www.abcam.com/ps/products/65/ab65359/documents/ab65359Cholesterol-CholesterylEsterQuantificationkitprotocolv5(website).pdf (accessed on 2 April 2021).
- abcamHDL and LDL/VLDL Cholesterol Assay Kit ab65390, Protocol Book Let. Available online: https://www.abcam.com/ps/products/65/ab65390/documents/HDL-and-LDL-VLDL-Cholesterol-assay-protocol%20(website).pdf (accessed on 29 June 2021).
- ThermoFisher ScientificRat Leptin ELISA Kit KRC2281, Protocol Book Let. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/MAN0004044_KRC2281_Rt_Leptin_ELISA_PI.pdf (accessed on 2 April 2021).
- Wang, G.; Yu, Y.; Garcia-gutierrez, E.; Jin, X.; He, Y.; Wang, L.; Tian, P.; Liu, Z.; Zhao, J.; Zhang, H.; et al. Lactobacillus acidophilus jcm 1132 strain and its mutant with different bacteriocin-producing behaviour have various in situ effects on the gut microbiota of healthy mice. Microorganisms 2020, 8, 49. [Google Scholar] [CrossRef] [Green Version]





| Groups | CN | R30 | BPA | B+R10 | B+R30 |
|---|---|---|---|---|---|
| Body weight (g) | 171.3 ± 2.7 a | 189.9 ± 8.2 b | 198.1 ± 9.8 b | 184.4 ± 6.3 ab | 172.9 ± 2.0 a |
| Lipid weight (g) * | 2.21 ± 0.34 a | 1.45 ± 0.14 a | 3.91 ± 0.88 b | 2.17 ± 0.43 a | 1.32 ± 0.42 a |
| Relative lipid weight (%) # | 1.29 ± 0.25 a | 0.81 ± 0.04 b | 2.20 ± 0.30 c | 1.25 ± 0.18 a | 0.91 ± 0.26 a |
| TG (μg/mL) | 79.5 ± 10.6 a | 77.0 ± 10.7 a | 132.0 ± 2.0 b | 105.0 ± 5.0 c | 68.3 ± 5.5 a |
| TC (μg/mL) | 1095.2 ± 59.9 a | 1167.2 ± 33.3 ab | 1260.5 ± 38.7 c | 1281.4 ± 17.2 c | 1209.2 ± 9.2 bc |
| HDL (μg/mL) | 143.5 ± 15.8 ab | 144.1 ± 12.5 ab | 73.7 ± 2.6 c | 164.2 ± 5.3 a | 134.2 ± 23.3 b |
| LDL (μg/mL) | 667.1 ± 26.7 a | 498.3 ± 5.3 b | 870.2 ± 40.3 c | 536.8 ± 7.9 d | 482.8 ± 22.6 a |
| Leptin (pg/mg) | 350.9 ± 31.6 a | 419.4 ± 6.0 b | 437.6 ± 17.9 b | 344.2 ± 12.3 a | 355.5 ± 11.2 a |
| SCFAs | CN | R30 | BPA | B+R10 | B+R30 |
|---|---|---|---|---|---|
| (μmol/g Faeces) | |||||
| Acetic acid | 21.01 ± 2.03 a | 28.51 ± 3.54 c | 31.67 ± 2.93 bc | 23.65 ± 2.64 ab | 17.75 ± 1.15 a |
| Propanoic acid | 3.50 ± 0.30 ab | 4.30 ± 0.58 ab | 3.62 ± 0.21 ab | 3.71 ± 0.64 a | 2.60 ± 0.25 b |
| Butanoic acid | 2.16 ± 0.61 a | 2.30 ± 1.26 a | 1.88 ± 0.96 a | 2.34 ± 0.29 a | 2.80 ± 1.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, M.-K.; Tain, Y.-L.; Chen, Y.-W.; Hsu, W.-H.; Yeh, Y.-T.; Chang, S.K.C.; Liao, J.-X.; Hou, C.-Y. Resveratrol Butyrate Esters Inhibit Obesity Caused by Perinatal Exposure to Bisphenol A in Female Offspring Rats. Molecules 2021, 26, 4010. https://doi.org/10.3390/molecules26134010
Shih M-K, Tain Y-L, Chen Y-W, Hsu W-H, Yeh Y-T, Chang SKC, Liao J-X, Hou C-Y. Resveratrol Butyrate Esters Inhibit Obesity Caused by Perinatal Exposure to Bisphenol A in Female Offspring Rats. Molecules. 2021; 26(13):4010. https://doi.org/10.3390/molecules26134010
Chicago/Turabian StyleShih, Ming-Kuei, You-Lin Tain, Yu-Wei Chen, Wei-Hsuan Hsu, Yao-Tsung Yeh, Sam K. C. Chang, Jin-Xian Liao, and Chih-Yao Hou. 2021. "Resveratrol Butyrate Esters Inhibit Obesity Caused by Perinatal Exposure to Bisphenol A in Female Offspring Rats" Molecules 26, no. 13: 4010. https://doi.org/10.3390/molecules26134010
APA StyleShih, M.-K., Tain, Y.-L., Chen, Y.-W., Hsu, W.-H., Yeh, Y.-T., Chang, S. K. C., Liao, J.-X., & Hou, C.-Y. (2021). Resveratrol Butyrate Esters Inhibit Obesity Caused by Perinatal Exposure to Bisphenol A in Female Offspring Rats. Molecules, 26(13), 4010. https://doi.org/10.3390/molecules26134010