1. Introduction
Emulsions have great potential for use as the delivery systems of lipophilic bioactive compounds, mainly for applications in food and pharmaceutical products. The effectiveness of these delivery systems can be assessed by the degree of protection they offer to the encapsulated compounds, or core material, from environmental stresses, such as those encountered during food processing and later in the gastrointestinal tract.
A critical factor that will determine this effectiveness is the encapsulating material. Emulsions are commonly prepared using synthetic or natural emulsifiers (biopolymers) that can form a single layer surrounding the core material [
1,
2,
3]. However, producing double-layer emulsion systems has been gaining attraction due to their potentially enhanced stability when compared with conventional monolayer emulsions [
4,
5,
6,
7,
8]. In these systems, the primary coating is usually achieved with proteins, whereas the secondary layer can be formed using an oppositely charged polysaccharide [
4,
7]. The predominant stabilizing mechanism of polysaccharides is known to be steric, which, unlike proteins that generate electrostatic forces, forms emulsions with better stability under different environmental stresses. Thus, the addition of polysaccharides to a protein monolayer emulsion leads to the formation of interfacial complexes that reduce the van der Waals attractions between proteins and increase the steric and electrostatic repulsion between droplets, which could result in increased physical stability of the emulsion [
9].
Polysaccharides have been extensively used in the food industry not only as thickening and gelling agents but also for their emulsifying properties. For instance, gum arabic, a polysaccharide derived from the exudate of the
Acacia senegal tree, has been extensively used as an emulsifier for food applications [
5,
10,
11]. The emulsifying properties of gum arabic are conferred by its amphiphilic nature: its hydrophobic side, the polypeptide chain, anchors the molecules to the lipid phase, while the arabinogalactan fraction (hydrophilic side) extends into the aqueous phase. Alternatively, guar gum, a linear nonionic polysaccharide, which consists of a chain of β(1–4)-linked mannopyranosyl units with an α(1–6)-linked D-galactopyranosyl chain every second residue [
12], is commonly used in the food industry as a thickening and stabilizing agent. Resistance to pH changes, ionic strength, and/or harsh temperatures, such as heating and freezing, non-toxicity, and easy digestion, are among the potential advantages of adding polysaccharides as secondary layers for food applications [
6,
13]. Those characteristics have practical significance for encapsulation and preservation during processing and storage, as well as the targeted delivery of highly sensitive or unstable bioactive compounds, such as polyunsaturated fatty acids.
Another important consideration during the production of emulsions is the processing technology selected, which can vary from low to high energy, such as spontaneous emulsification, high-shear mixing [
4], high-pressure homogenization [
14], microfluidization [
2,
3], ultrasonication, and membrane and microchannel emulsification. High-pressure homogenization has been widely used to form mono- and double-layer emulsions. The high shear force generated during the homogenization process entails the rapid movement of the encapsulating materials to the interfacial region and causes violent disruption of the droplets, making most droplets in the nano-size range (<200 nm) [
15].
In this study, we hypothesized that the inclusion of polysaccharides in egg yolk/fish oil emulsions can produce emulsions with better stability. Therefore, we assessed the effect of adding gum guar or gum arabic on the stability and cytotoxicity of egg yolk/fish oil emulsions. The performances of two processing conditions, simple and high-pressure homogenization, were also evaluated.
3. Discussion
In this study, egg yolk was used as the primary emulsifier of fish oil triglycerides or ethyl esters. Gum guar or gum arabic was further added to the primary emulsion to act as a secondary layer. Simple or combined simple/high-pressure homogenization were the two homogenization processes tested. The resulting emulsions were developed to protect highly sensitive lipophilic bioactive compounds, namely, EPA and DHA, from environmental stresses. The effectiveness of the emulsions was given by their stability and their ability to prevent oxidation/degradation of EPA and DHA over 10 days of storage at 45 °C.
It was shown that the rheological properties of oil-in-water emulsions are mostly dependent on the original properties of the biopolymers used as wall materials and their effective volume occupied in the emulsion system [
3,
4,
17]. For instance, gum arabic is commonly used as an emulsifier in beverage industries due to its low viscosity, whereas gum guar is widely used in the food industry as a thickening agent. Gum guar belongs to the galactomannan family, which is known for its high-water-binding capacity that allows them to form viscous emulsions, even at low concentrations. Consequently, the emulsions where gum guar was used as a secondary layer had higher viscosity (
p < 0.05) than those using gum arabic.
The particle sizes and distribution of an emulsion is an important characteristic to evaluate since it is an indicator of the efficiency of the emulsification process and the stability of the emulsions [
4,
18,
19]. The narrower particle distribution observed in gum guar emulsions after secondary homogenization agreed with the observations of Bai et al. [
2], who reported narrower monomodal particle distributions at higher homogenization pressures. On the other hand, the size distribution of emulsions containing gum arabic seemed to depend not only on the processing type but also on the esterification of the fish oil used as the core material.
The overall changes in the distribution curves were also reflected in the average particle size of each treatment. In this case, the factor that caused the highest variability in the average particle size was the esterification type: triglycerides displayed a larger average size. In a previous study [
14], we showed that during emulsification, the fish oil triglycerides have more affinity for the granule fraction of the egg yolk (micro-size region), thus increasing its volume percentage, which results in a larger average particle size. On the other hand, a high-pressure homogenization process was shown to form emulsions with smaller particle size, usually in the nano-size range, regardless of the biopolymer used as the coating material. For instance, high-pressure homogenization of sodium caseinate-coated fish oil emulsions at 12 kpsi showed an average particle diameter of 168 nm [
7]; this trend was also observed in samples A22TH and A44TH. In comparison, the larger particle size obtained for their counterparts A22TS and A44TS could be the result of larger particles formed by a bridging flocculation effect [
6,
11]. These flocculated particles could be responsible for the peak observed in the micro-size region of the distribution curves (
Figure 1a). Xu et al. [
6] observed the same effect in emulsions using rice glutelin as a primary coating and 0.01% of anionic polysaccharides as the secondary layer.
Polysaccharides can form thick interfacial layers, which can prevent droplet flocculation and coalescence [
10,
20]. Nevertheless, the bridging flocculation effect is observed if the concentration of polysaccharide molecules is not enough to cover the entire oil droplet in the case of single-layer emulsions or to bond with protein-coated droplets in double-layer emulsions, causing one molecule to adsorb to the surface of several droplets at the same time [
4]. Bouyer et al. [
11] suggested that this effect can also be related to the emulsification process in turbulent flow and storage. Compared to other surface-active biopolymers, gum arabic displays a lower affinity for oil-in-water interfaces; thus, a ratio as high as 1:1 gum arabic:oil phase ratio is needed to form emulsions that are more resistant to flocculation [
12]. The observations from our study suggest that the concentration of gum arabic as a secondary layer was not high enough to prevent this effect; however, emulsions using gum guar at the same concentration did not show bridging flocculation. The effectiveness of gum guar, a nonionic polysaccharide, to prevent aggregation was the result of the hydration of its polar head groups. However, the accelerated shelf-life conditions tested in this study caused dehydration of these head groups that eventually resulted in aggregation [
12], as observed by the increase in the average particle size. Hence, a significant effect of storage on the particle size of emulsions stabilized by gum guar was observed only on day 10. Shi et al. [
18] also observed a significant increase of the particle size over the storage of fish oil-in-water emulsions stabilized by lecithin, gum arabic, or Tween 80. On the other hand, the resulting smaller particle size obtained at lower concentrations of EPA + DHA could be explained by the higher availability and faster adsorption of the biopolymer molecules used as emulsifiers to the oil phase surface due to the higher wall material:oil phase ratio [
2], resulting in greater efficiency when coating the lipid droplets and forming smaller particles.
Another important characteristic to consider during the study of emulsions is their efficiency at preventing the release of the core material during processing and storage. Polysaccharides have shown potential to enhance the stability of oil-in-water emulsions using proteins as primary emulsifiers [
6,
13]. In these double-layer emulsions, proteins and polysaccharides can interact via covalent or noncovalent bonds, which influences the stability of the emulsions [
9]. Therefore, this study assessed the differences between adding a neutral (gum guar) or an anionic (gum arabic) polysaccharide on the encapsulation efficiency and stability given by the total EPA+DHA that remained encapsulated during storage under accelerated shelf-life conditions (up to 10 days at 45 °C).
The lack of significant differences in the encapsulation efficiencies between the two main plots in our experimental design (gum arabic versus gum guar) indicates that despite the bridging flocculation effect observed in treatments with gum arabic, the core material remained encapsulated within the continuous phase of the emulsion. Nevertheless, the clear differences between the efficiencies of the two polysaccharides to keep the EPA + DHA encapsulated after 10 days of storage could be partially attributed to the behavior of gum arabic under the pH conditions used during the processing of the emulsions. The initial egg yolk solutions were adjusted to pH 6.0 before proceeding with the primary homogenization. Under these conditions, the zeta potential of egg yolk is slightly negative [
21], which could have caused repulsion between the gum arabic molecules and the proteins adsorbed at the surface of the lipid droplets, resulting in poor efficiency when forming a secondary layer [
7]. Furthermore, as previously discussed, gum arabic requires a ratio of 1:1 (
w/
w) with the oil phase to reach its stable effect. Results from the encapsulation efficiency over time from this study seemed to be related to the overall particle stability found for the same treatments. The lack of significant differences between processing types on emulsions containing gum guar suggests that the stability of the emulsions at this stage was given mostly by the ability of the polysaccharide to act as an extra barrier to entrap the lipids in the core material and not due to the particle size of the emulsion.
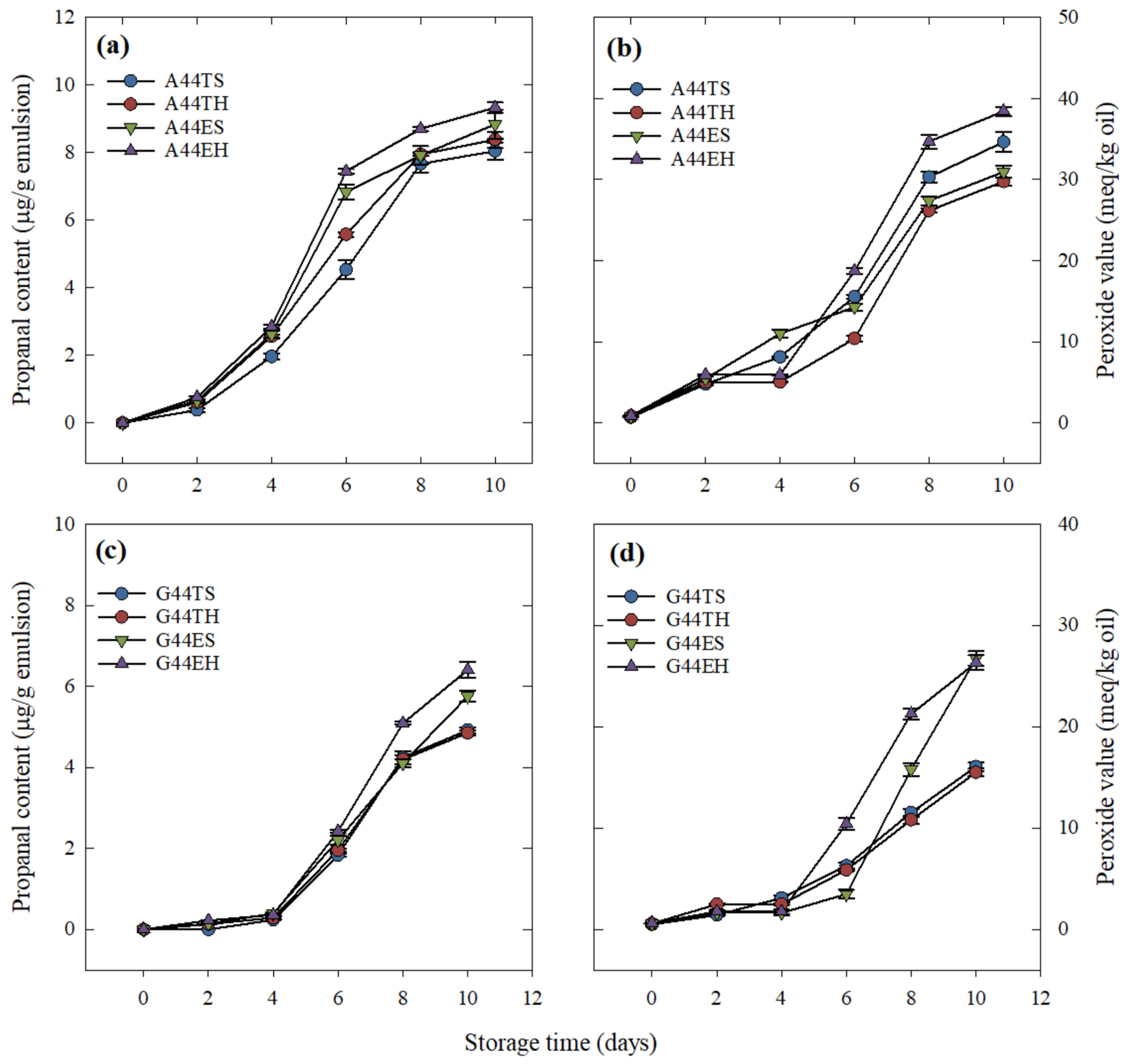
The encapsulation efficiency of an emulsion and its ability to prevent the core material release during processing and storage will impact its oxidative stability, as it is known that the nonencapsulated material will be more prone to oxidation in the presence of oxygen and high temperatures [
18,
22,
23]. Therefore, a higher concentration of surface oil results in higher oxidation. This oxidation will adversely impact the nutritional value and sensory quality of the product [
18]. Moreover, oxidation of long-chain polyunsaturated fatty acids, such as EPA and DHA, produces toxic compounds that are related to aging, mutagenesis, and carcinogenesis [
24,
25]. Thus, the prevention of lipid oxidation is of crucial importance in an oil-in-water emulsion system. The lack of significant effects of processing type on the oxidative stability of emulsions containing gum guar suggests that their stability was mostly the result of an efficient interaction between the egg yolk and the polysaccharide when acting as a barrier between the environment and the core material.
The development of cytotoxic oxidation products from fish oil was studied to complete the assessment of the developed egg yolk/polysaccharide emulsions. For this purpose, the relative viability of Caco-2 cells was measured after incubation with fish oil from emulsions throughout storage and compared to that of cells incubated with nonencapsulated fish oil. Some compounds can display dose-dependent cytotoxicity on Caco-2 cells [
26]; however, our emulsions did not show this effect at concentrations ranging from 50–100 μg fish oil/mL of incubation medium. Additionally, the lack of significant cell viability reduction of Caco-2 cells showed that our developed egg yolk/polysaccharide emulsions can prevent the formation of cytotoxic components from fish oil. Further studies are needed to evaluate the mechanisms with which these polysaccharides exert these beneficial properties.
4. Materials and Methods
4.1. Materials
White-shelled eggs were collected from the Alberta Poultry Research Centre (Edmonton, AB, Canada). Egg yolks were manually separated from the whites and rolled on Whatman no. 1 paper to eliminate the albumen residues. After puncturing the vitelline membrane, the egg yolk content was collected in a container placed in an ice bath and used to form the monolayer emulsions.
Fish oil from Alaska Pollock (Gadus chalcogrammus) with a high content of eicosapentaenoic (EPA) and docosahexaenoic (DHA) fatty acids as triglycerides or ethyl esters was obtained from AlaskOmega Products (Organic Technologies, Coshocton, OH, USA). Caco-2 cells were obtained from the American Type Culture Collection and used from passages 19–32. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), phosphate buffer saline (PBS), Hank’s balanced salt solution (HBSS), 0.25% (w/v) trypsin–0.53 mM EDTA, 1% non-essential amino acids, 1% (w/v) penicillin–streptomycin, and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) were obtained from Gibco Invitrogen (Burlington, ON, Canada). Gum guar, gum arabic, an in vitro toxicology assay, and all reagents used for chemical analyses were obtained from Sigma-Aldrich (Oakville, ON, Canada).
4.2. Emulsion Formation
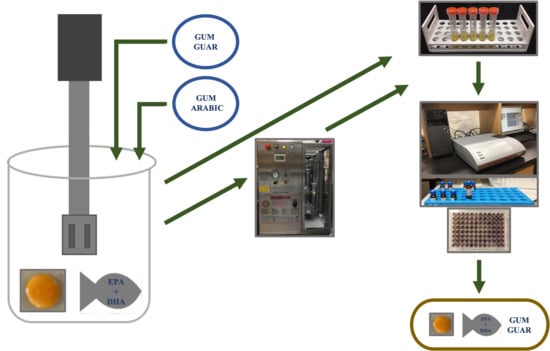
A graphical overview of the process followed to produce the emulsions is shown in
Figure 7.
The moisture content of the freshly extracted egg yolk was determined using a convection oven set a 105–110 °C for 5 h. The 1%
w/
w polysaccharide solutions were prepared by stirring gum guar or gum arabic in cool Milli-Q water at 400 rpm until complete dissolution. The concentration and type of fish oil added followed the experimental design further described in
Section 4.4. Egg yolk/polysaccharide emulsions were formed using two processing approaches: simple and high-pressure homogenization, as described below.
4.2.1. Simple (Primary) Homogenization
Fish oil was added to the egg yolk and mixed manually to facilitate its dispersion, then homogenized at 24,000 rpm for 2 min using a T25 Ultra Turrax (IKA Works Inc., Wilmington, DE, USA). The polysaccharide solution was then added to reach a final concentration of 0.25 g polysaccharide per 100 g of emulsion. Based on the egg yolk moisture content, the necessary amount of Milli-Q water was added to obtained 30% egg yolk solids in the final emulsion. This mixture was homogenized again at 24,000 rpm for 2 min. Thus, this ratio of 1:0.0083 egg yolk solids:polysaccharide was kept constant in all treatments, varying only the proportion of fish oil.
4.2.2. Secondary (High-Pressure) Homogenization
Egg yolk/fish oil mixtures were homogenized at 24,000 rpm for 2 min using a T25 Ultra Turrax. The corresponding polysaccharide solution was then added and mixed manually until homogeneous, keeping the 1:0083 egg yolk solids:polysaccharide ratio in the final emulsion. This mixture was subjected to secondary homogenization in a Pressure Cell Homogenizer FPG 12,800 (Stansted Fluid Power LTD, London, UK) at 200 MPa and a hydraulic relief pressure of 240 MPa.
4.2.3. Accelerated Shelf-Life
To simulate accelerated shelf-life conditions, freshly prepared emulsions (simple and high-pressure) were transferred to air-tight sterile plastic tubes and stored at 45 °C for up to 10 days. Samples from the same experimental unit were analyzed right after preparation (day 0) and at days 2, 4, 6, 8, and 10. Based on preliminary results, all treatments were characterized according to their particle size and distribution over time, but only treatments displaying significant differences with the controls (mainly those containing 50% fish oil) were analyzed for viscosity, encapsulation efficiency, oxidative stability, and cytotoxicity.
4.3. Characterization of the Emulsions
4.3.1. Viscosity
The apparent viscosity at 25 °C was determined using steady-state shear measurements (0.1–100 s−1) in a Modular Compact Rheometer 302 (Anton Paar, Graz, Austria). A concentric cylinder measuring tool with an active length of 120.2 mm and a positioning length of 72.5 mm was used to determine the viscosity curves of emulsions formed using primary homogenization. A 50 mm diameter parallel plate measuring tool and a 1 mm gap setting were used for the emulsions resulting from the secondary homogenization.
4.3.2. Particle Size and Distribution (PSD)
Emulsions were diluted 1:4 (w/w) with 1% (w/w) sodium dodecyl sulfate solution, then vortexed for 30 s prior to analysis to avoid multiple scattering. The 2 mL samples were placed in a disposable polystyrene cuvette for measurement. The particle size (as given by the Sauter mean diameter, d32) and distribution (given by the volume percentage) of the emulsions were measured via dynamic light scattering (DLS) in a LitesizerTM 500 (Anton Paar, Graz, Austria) using backscattered angle (175 degrees) at 25 °C and 2 min of equilibration time.
4.3.3. Encapsulation Efficiency (EEf)
The encapsulation efficiency was calculated based on the concentration of EPA + DHA in the total and surface oil of the emulsions using the following formula:
Total oil was extracted following the chloroform:methanol method developed by Bligh and Dyer [
27], whereas the surface oil extraction was conducted by washing with hexanes [
22]. Both methods were modified to adapt to the matrix of the emulsions to obtain dependable yields according to previous observations [
14].
Furthermore, the methylation and quantification of EPA + DHA were conducted following the method developed by Joseph and Ackman [
28] using internal standard 23:0 methyl esters for triglycerides samples, and 23:0 ethyl esters for ethyl ester samples. The results are expressed as mg EPA + DHA/g oil.
The amount of surface oil obtained from most emulsions was below 25 mg; therefore, the weight of the oil was recorded for considerations in the final calculation. The appropriate internal standard was directly added into the tube containing the surface oil for further methylation.
The EPA/DHA content was quantified after methylation by injecting 1 μL samples into an Agilent 7890 Gas Chromatograph with 5975 Mass Selective Detector using MSD Chemstation software. The carrier gas was Helium 5.0 and the column was an Agilent HP-5 MS with 30 m × 250 μm × 0.25 μm dimensions. The inlet temperature was set at 200 °C and operated in splitless mode. The oven was set to 50 °C for 5 min, then increased at 5 °C/min until 320 °C for 5 min. Total run time was 64 min. The mass spectrum was scanned from 40–500 m/z.
4.3.4. Propanal Content
The propanal formation in the emulsions, as assessed using headspace chromatography, was used as a marker for the oxidation of omega-3 fatty acids. About 2 g of the corresponding emulsion was placed into a 20 mL headspace vial, sealed, and heated at 60 °C for 30 min, followed by 30 min of equilibration time at room temperature. The headspace was then sampled manually using a Hamilton® GASTIGHT syringe, 1725 N (Sigma-Aldrich, Oakville, ON, Canada). A total of 100 μL was manually injected in a capillary gas chromatograph equipped with a Restek Stabilwax (Crossbond Carbowax, Restek, Bellefonte, PA, USA) column measuring 20 m in length with a 0.53 mm internal diameter and 0.5 μm df while using helium as the carrier gas. The oven was set to 45 °C and the total run time was 2 min. The temperature of the detector and injector was 250 °C. The propanal content was calculated using a standard curve (r2 = 0.99) built with 0, 3, 6, 9, 12, and 15 μg propanal (98% purity) in Milli-Q water and expressed as μg propanal/g sample.
4.3.5. Peroxide Value
The peroxide value (POV) was measured following the AOAC official method 965.33 [
29]. The POV was expressed as meq peroxide per kg oil and calculated using the following formula:
4.3.6. Cytotoxicity Analysis
Caco-2 cells were routinely grown in supplemented DMEM, refreshing the medium every other day, and used for seeding at 80% confluence. The toxicity of emulsions was evaluated on days 0, 2, 4, 6, 8, and 10 of storage at 45 °C. The egg yolk/polysaccharide solutions without fish oil (controls) were tested under the same conditions to eliminate the effect of the carrier components. Non-encapsulated fish oil ethyl esters (FOE) were used as a negative control.
The toxicity of the samples was assessed by measuring the relative viability of Caco-2 cells using the In Vitro Toxicology Assay Kit from Sigma-Aldrich, which is based on the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) method developed by Mosmann [
30].
Caco-2 cells were harvested with PBS containing 0.25% trypsin and 0.53 mM EDTA, then seeded in high-glucose and L-glutamine DMEM medium supplemented with 10% FBS, 1% non-essential amino acids, 1% penicillin and streptomycin, and 2.5% (v/v) HEPES to a concentration of 50,000 cells/mL. Then, 10,000 cells/well (200 μL) were placed on a 96-well plate and incubated at 37 °C in a humidified incubator with 5% CO2 for 24 h.
After incubation, the original medium was replaced with the corresponding treatment suspended in the supplemented DMEM at final concentrations of 50, 75, and 100 μg EPA + DHA/mL medium and incubated for 24 h. The same concentration of EPA + DHA from FOE was used for comparisons with the negative control. Supplemented DMEM without emulsions was used as a blank reference for 100% viability. After the second incubation, the wells were gently washed 3 times with PBS. The cells were then incubated for 4 h with DMEM medium containing 10% MTT solution (5 mg/mL PBS). The medium was then removed and the formazan crystals formed on each well were observed at 40× in a Zeiss Axio Scope A1 light microscope (Zeiss, Oberkochen, Germany). The crystals were then dissolved by pipetting up and down with 200 μL of acidified isopropanol. The absorbance of each well was measured spectrophotometrically at a wavelength of 570 nm. The background absorbance of the plate was measured at 690 nm and subtracted from the value at 570 nm. This result was used to calculate the relative cell viability (%) using the following formula:
4.4. Sample Identification and Statistical Analysis
As an example, sample IDs were structured as follows: G44TS. The first letter refers to the polysaccharide type: G for gum guar or A for gum arabic. The following two digits refer to the percentage of total EPA + DHA from fish oil added, which was calculated based on the egg yolk’s dry matter. The third character indicates the esterification type: T for triglycerides and E for ethyl esters. Finally, the last letter refers to the process used for the emulsion formation: S means simple homogenization and H means high-pressure homogenization. Each group of treatments was paired with its control without fish oil, which were identified as Control-AS, Control-AH, Control-GS, and Control-GH, where the last two letters mean the same as specified before. Non-encapsulated fish oil ethyl esters (FOE) were used as negative controls for toxicity assays and oxidative stability.
Emulsions were formed using a combination of polysaccharide type, processing method, concentration, and type of esterification of EPA + DHA. The experimental design was a split-split-plot in time. The main plots were the egg yolk:polysaccharide solutions. The fixed factor for the split-plot was the processing type at two levels: simple or high-pressure homogenization. Finally, the fixed factor for the split-split-plot was the type and concentration of fish oil at 4 levels: 22 and 44% EPA + DHA triglycerides and 22 and 44% EPA + DHA ethyl esters. Time (at 6 levels) was considered a fixed effect since its effect on the output variables was of interest. All chemical analyses were carried out in triplicates. Four replications were conducted for the toxicity analysis. Results were expressed as the mean and the standard error. A linear mixed-effects model was used to assess the significance of time as a factor and the fixed factors over time; a one-way analysis of variance (ANOVA) was used to find significant factors among treatments at a given time. Significant differences in the mean values were considered at
p < 0.05 using Tukey’s test. All the statistical analyses were conducted using the statistical package R version 3.3.2 in R studio version 1.0.136 [
13].