Metabolomics Analysis of Litchi Leaves during Floral Induction Reveals Metabolic Improvement by Stem Girdling
Abstract
:1. Introduction
2. Results
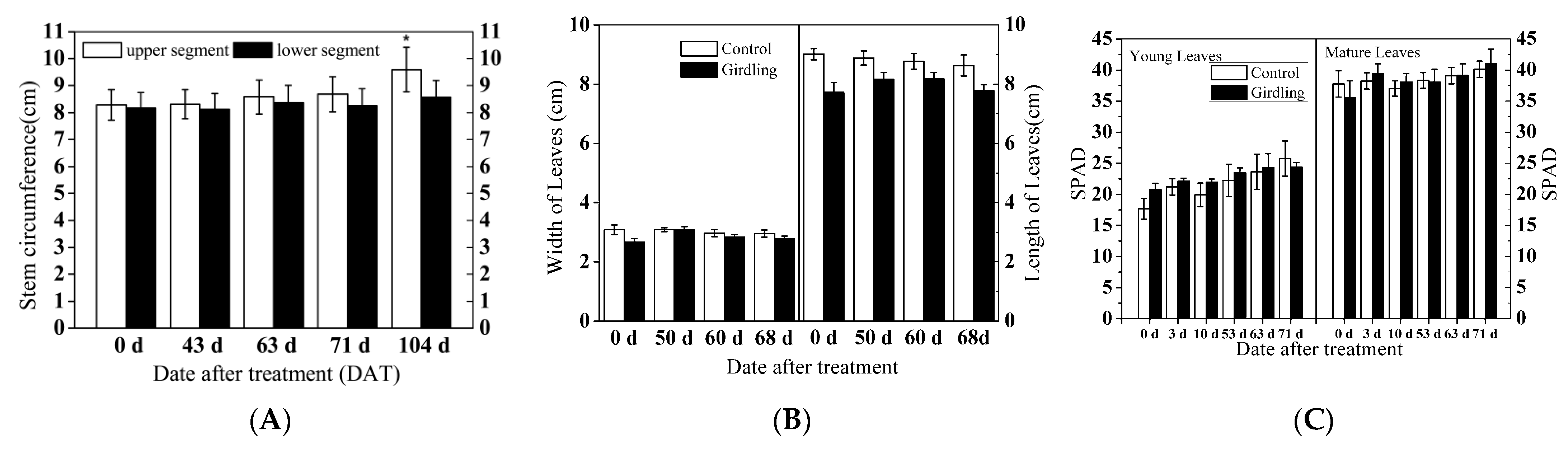
2.1. Biological Presentation of Litchi Trees under Experimental Conditions
2.2. Correlation Coefficient of Metabolite Profiles in Response to Stem Girdling and Low Temperatures
2.3. PCA of Metabolic Changes
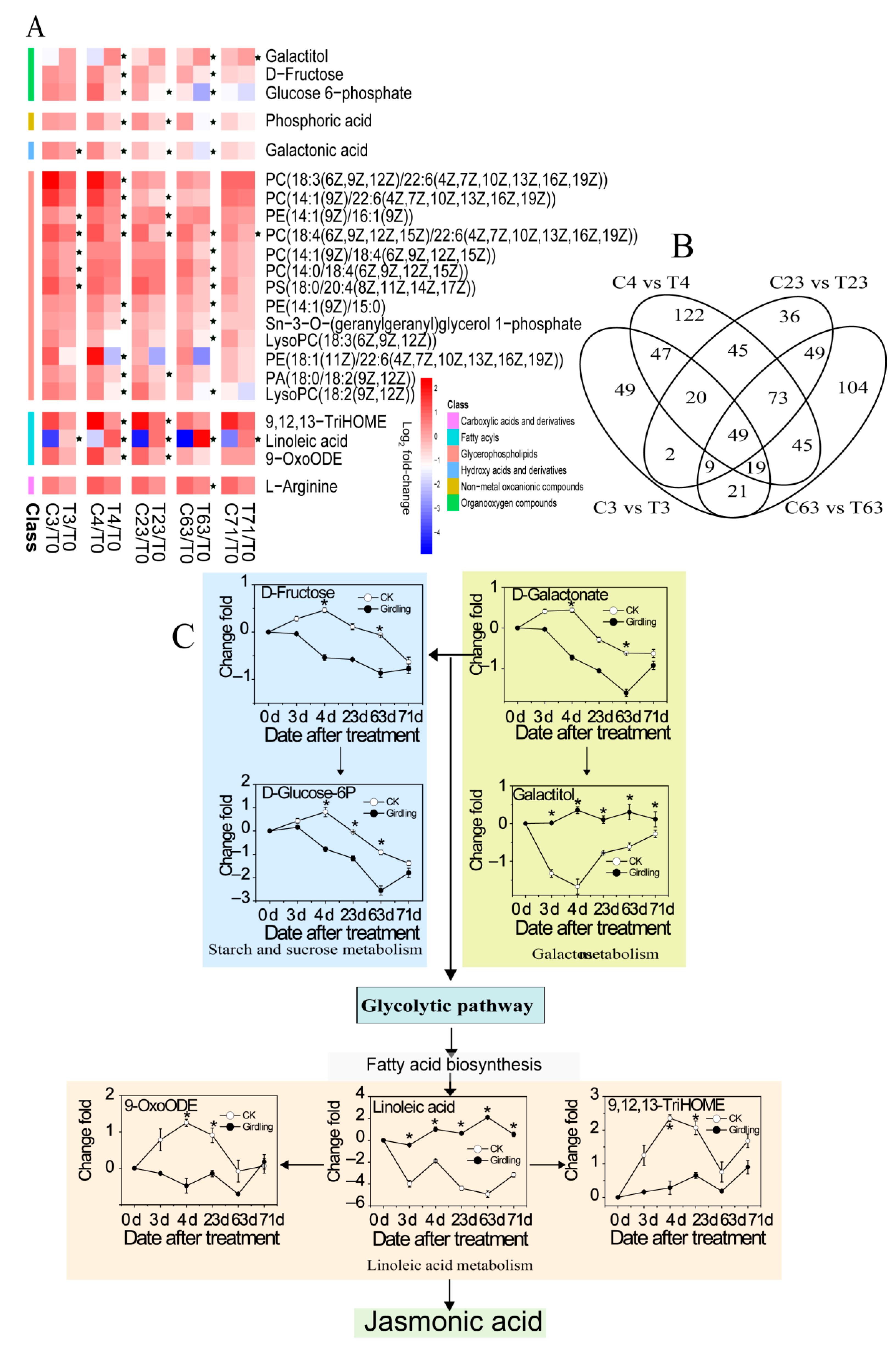
2.4. Global View of Metabolites Associated with Flowering
2.5. Metabolites Accounting for Different Flowering Rate
3. Discussion
3.1. Stem Girdling Promotes Litchi Flowering
3.2. Stem Girdling Alters the Carbohydrate Metabolites during Floral Induction
3.3. Stem Girdling Effect on the Phosphoenolpyruvate Metabolites during Floral Induction
3.4. Stem Girdling Effect on the Catecholamine Metabolites during Floral Induction
3.5. Stem Girdling Effect on the Acetyl-CoA Amount during Floral Induction
3.6. Stem Girdling Effect on the Fatty Acid Metabolites and Catabolism during Floral Induction
3.7. The Accumulation of Metabolites Accounting for Different Flowering Rate
4. Materials and Methods
4.1. Plant Materials and Experimental Scheme
4.2. Metabolite Extraction
4.3. Metabolite Profiling
4.4. Carbohydrates Determination
4.5. Indole-3-Acetic Acid (IAA) Determination
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| C | Control |
| DAT | Day after treatment |
| FDR | False discovery rate |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| OPLS-DA | Orthogonal projections to latent structures-discriminate analysis |
| PCA | Principal component analysis |
| SPAD | Soil and plant analyzer development |
| T | Girdling treatment |
| UPLCQ-TOF/MS | Ultra-high performance liquid chromatography-quadrupole time-of-Flight mass spectrometry |
| VIP | Variable important in projection |
References
- Menzel, C.M. The control of floral initiation in lychee: A review. Sci. Hortic. 1983, 21, 201–215. [Google Scholar] [CrossRef]
- Menzel, C.M.; Simpson, D.R. Effect of temperature on growth and flowering of litchi (Litchi chinensis Sonn.) cultivars. J. Hortic. Sci. 1988, 63, 349–360. [Google Scholar] [CrossRef]
- Menzel, C.M.; Simpson, D.R. Temperatures above 20 °C reduce flowering in lychee (Litchi chinensis Sonn.). J. Hortic. Sci. 1995, 70, 981–987. [Google Scholar] [CrossRef]
- Hu, J.; Kim, H.; Chen, H.; Zhou, B. Litchi Flowering is Regulated by Expression of Short Vegetative Phase Genes. J. Am. Soc. Hortic. Sci. 2018, 143, 101. [Google Scholar] [CrossRef]
- Malhotra, S.K.; Singh, S.K.; Nath, V. Physiology of flowering in litchi (Litchi chinensis): A review. Indian J. Agric. Sci. 2018, 88, 1319–1330. [Google Scholar]
- Xiao, Q.; Su, Z.; Chen, H.; Shen, J. Genome-wide identification and involvement of litchi SPL genes in flowering in response to cold and leaf maturity. J. Hortic. Sci. Biotechnol. 2019, 94, 428–440. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, J.; Wei, Y.; Chen, H. Transcriptome profiling of litchi leaves in response to low temperature reveals candidate regulatory genes and key metabolic events during floral induction. BMC Genom. 2017, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- O’Hare, T.J. Interaction of temperature and vegetative flush maturity influences shoot structure and development of lychee (Litchi chinensis Sonn.). Sci. Hortic. 2002, 95, 203–211. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, S.K.; Mahato, A.K.; Ravishankar, H.; Dubey, A.K.; Singh, N.K. Physiological and molecular basis of alternate bearing in perennial fruit crops. Sci. Hortic. 2019, 243, 214–225. [Google Scholar] [CrossRef]
- Menzel, C.M.; Rasmussen, T.S.; Simpson, D.R. Effects of temperature and leaf water stress on growth and flowering of litchi (Litchi chinensis Sonn.). J. Hortic. Sci. 1989, 58, 75–82. [Google Scholar]
- Chen, H.B.; Huang, H.B. Low temperature requirements for floral induction in lychee. Acta. Hortic. 2005, 665, 195–202. [Google Scholar] [CrossRef]
- Groff, G. Some ecological factors involved in successful lychee culture. Proc. Fla. State Hortic. Soc. 1943, 56, 134–155. [Google Scholar]
- Chen, P.; Roan, S.; Lee, C.; Chen, I. The effect of temperature during inflorescence development to flowering and inflorescence length on yield of ‘Yu Her Pau’ litchi. Sci. Hortic. 2013, 159, 186–189. [Google Scholar] [CrossRef]
- Shen, J.; Xiao, Q.; Qiu, H.; Chen, C.; Chen, H. Integrative effect of drought and low temperature on litchi (Litchi chinensis Sonn.) floral initiation revealed by dynamic genome-wide transcriptome analysis. Sci. Rep. 2016, 6, 32005. [Google Scholar] [CrossRef]
- Carr, M.; Menzel, C.M. The water relations and irrigation requirements of lychee (Litchi chinensis Sonn.): A review. Exp. Agric. 2014, 50, 481–497. [Google Scholar] [CrossRef] [Green Version]
- Khali, F.; Qureshi, K.; Khan, A.; Nabila, B. Effect of girdling and plant growth regulators on productivity in olive (Olea europaea). Pak. J. Agric. Res. 2012, 25, 120–128. [Google Scholar]
- Li, C.; Xiao, Y. Girdling increases yield of ‘Nuomici’ litchi. Acta. Hortic. 2001, 558, 233–235. [Google Scholar] [CrossRef]
- Moreira, R.; Fernandes, D.; da Cruz, M.D.C.M.; Lima, J.; de Oliveira, A. Water restriction, girdling and paclobutrazol on flowering and production of olive cultivars. Sci. Hortic. 2016, 200, 197–204. [Google Scholar] [CrossRef]
- Maier, C.; Johnsen, K.; Clinton, B.; Ludovici, K. Relationships between stem CO2 efflux, substrate supply, and growth in young loblolly pine trees. New. Phytol. 2010, 185, 502–513. [Google Scholar] [CrossRef]
- Yuan, R.; Huang, H. Regulation of roots and shoots growth and fruit-drop of young litchi trees by trunk girdling in view of source-sink relationships. J. Fruit Sci. 1993, 10, 195–198. [Google Scholar]
- Zhou, X.; Wu, D.; Huang, H.; Lai, J.; Huang, D. Manipulating Growth and Fruiting of Young Litchi Trees by Spiral Girdling. Acta. Hortic. 1996, 23, 13–18. [Google Scholar]
- Hogberg, P.; Nordgren, A.; Buchmann, N.; Taylor, A.F.; Ekblad, A.; Hogberg, M.; Nyberg, G.; Ottosson-Lofvenius, M.; Read, D. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 2001, 411, 789–792. [Google Scholar] [CrossRef]
- Parrott, D.; McInnerney, K.; Feller, U.; Fischer, A. Steam-girdling of barley (Hordeum vulgare) leaves leads to carbohydrate accumulation and accelerated leaf senescence, facilitating transcriptomic analysis of senescence-associated genes. New. Phytol. 2007, 176, 56–69. [Google Scholar] [CrossRef]
- Lopez, R.; Brossa, R.; Gil, L.; Pita, P. Stem girdling evidences a trade-off between cambial activity and sprouting and dramatically reduces plant transpiration due to feedback inhibition of photosynthesis and hormone signaling. Front. Plant Sci. 2015, 6, 285. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, D.; Huang, H.; Wu, D. Carbohydrate and Endohormone Status in Relation to Fruit Set as Influenced by Trunk Spiral Girdling of Young Litchi Trees. Acta. Hortic. 1999, 26, 77–80. [Google Scholar]
- Van de Wal, B.; Windt, C.; Leroux, O.; Steppe, K. Heat girdling does not affect xylem integrity: An in vivo magnetic resonance imaging study in the tomato peduncle. New. Phytol. 2017, 215, 558–568. [Google Scholar] [CrossRef] [Green Version]
- Bloemen, J.; Agneessens, L.; Van Meulebroek, L.; Aubrey, D.; McGuire, M.; Teskey, R.; Steppe, K. Stem girdling affects the quantity of CO2 transported in xylem as well as CO2 efflux from soil. New. Phytol. 2014, 201, 897–907. [Google Scholar] [CrossRef]
- Tang, G.; Li, X.; Lin, L.; Guo, H.; Li, L. Combined effects of girdling and leaf removal on fluorescence characteristic of Alhagi sparsifolia leaf senescence. Plant Biol. 2015, 17, 980–989. [Google Scholar] [CrossRef]
- Tang, G.; Li, X.; Lin, L.; Zeng, F. Impact of girdling and leaf removal on Alhagi sparsifolia leaf senescence. Plant Growth Regul. 2016, 78, 205–216. [Google Scholar] [CrossRef]
- Cutting, J.; Lyne, M. Girdling and the reduction in shoot xylem sap concentrations of cytokinins and gibberellins in peach. J. Hortic. Sci. 1993, 68, 619–626. [Google Scholar] [CrossRef]
- Schaffer, A.; Liu, K.; Goldschmidt, E.; Boyer, C.; Goren, R. Citrus Leaf Chlorosis Induced by Sink Removal: Starch, Nitrogen, and Chloroplast Ultrastructure. J. Plant Physiol. 1986, 124, 111–121. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef]
- Holland, V.; Fragner, L.; Jungcurt, T.; Weckwerth, W.; Brueggemann, W. Girdling interruption between source and sink in Quercus pubescens does not trigger leaf senescence. Photosynthetica 2016, 54, 589–597. [Google Scholar] [CrossRef]
- Weichert, N.; Saalbach, I.; Weichert, H.; Kohl, S.; Erban, A.; Kopka, J.; Hause, B.; Varshney, A.; Sreenivasulu, N.; Strickert, M. Increasing sucrose uptake capacity of wheat grains stimulates storage protein synthesis. Plant Physiol. 2010, 152, 698–710. [Google Scholar] [CrossRef] [Green Version]
- Bolouri, M.; Van den Ende, W. Sugars, the clock and transition to flowering. Front. Plant Sci. 2013, 4, 22. [Google Scholar]
- Turnbull, C. Long-distance regulation of flowering time. J. Exp. Bot. 2011, 62, 4399–4413. [Google Scholar] [CrossRef] [Green Version]
- Hoekstra, F.; Golovina, E.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef]
- Hanson, J.; Smeekens, S. Sugar perception and signaling--an update. Curr. Opin. Plant Biol. 2009, 12, 562–567. [Google Scholar] [CrossRef]
- Lebon, G.; Wojnarowiez, G.; Holzapfel, B.; Fontaine, F.; Vaillant-Gaveau, N.; Clement, C. Sugars and flowering in the grapevine (Vitis vinifera L.). J. Exp. Bot. 2008, 59, 2565–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz-Marchena, M.; Albi, T.; Lucas-Reina, E.; Said, F.; Romero-Campero, F.; Cano, B.; Ruiz, M.; Romero, J.; Valverde, F. Photoperiodic control of carbon distribution during the floral transition in Arabidopsis. Plant Cell 2014, 26, 565–584. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Jin, S.; Hu, H.; Sun, Y.; Wang, Y.; Han, P.; Hou, B. UGT87A2, an Arabidopsis glycosyltransferase, regulates flowering time via FLOWERING LOCUS C. New. Phytol. 2012, 194, 666–675. [Google Scholar] [CrossRef]
- Bernier, G. Physiological Signals That Induce Flowering. Plant Cell 1993, 5, 1147–1155. [Google Scholar] [CrossRef] [Green Version]
- Lejeune, P. Sucrose levels in leaf exudate as a function of floral induction in the long day plant Sinapis alba L. Plant Physiol. Biochem. 1991, 29, 153–157. [Google Scholar]
- Lejeune, P.; Bernier, G.; Requier, M.; Kinet, J. Sucrose increase during floral induction in the phloem sap collected at the apical part of the shoot of the long-day plant Sinapis alba L. Planta 1993, 190, 71–74. [Google Scholar] [CrossRef]
- Hisamatsu, T.; King, R. The nature of floral signals in Arabidopsis. II. Roles for Flowering Locus T (FT) and gibberellin. J. Exp. Bot. 2008, 59, 3821–3829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsoukas, I.; Massiah, A.; Thomas, B. Florigenic and antiflorigenic signaling in plants. Plant Cell Physiol. 2012, 53, 1827–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, S.; Giraldo, J.; Lew, M.; Liu, P.; Yang, Y.; Koman, V.; McGee, M.; Olsen, B.; Strano, M. Polymethacrylamide and Carbon Composites that Grow, Strengthen, and Self-Repair using Ambient Carbon Dioxide Fixation. Adv. Mater. 2018, 30, 1804037. [Google Scholar] [CrossRef]
- Kostal, V.; Tollarova, M.; Sula, J. Adjustments of the enzymatic complement for polyol biosynthesis and accumulation in diapausing cold-acclimated adults of Pyrrhocoris apterus. J. Insect Physiol. 2004, 50, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Fleischmacher, O.; Vattuone, M.; Prado, F.; Sampietro, A. Specificity of sugar cane trehalase. Phytochemistry 1980, 19, 37–41. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino Acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Rohr, J. Shikimic Acid. Metabolism and Metabolites. Angew. Chem. 1995, 107, 653. [Google Scholar] [CrossRef]
- Razal, R.; Ellis, S.; Singh, S.; Lewis, N.; Towers, G. Nitrogen recycling in phenylpropanoid metabolism. Phytochemistry 1996, 41, 31–35. [Google Scholar] [CrossRef]
- Bonawitz, N.; Chapple, C. The genetics of lignin biosynthesis: Connecting genotype to phenotype. Annu. Rev. Genet. 2010, 44, 337–363. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New. Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlot, A.; Dempsey, D.; Klessig, D. Salicylic Acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [Green Version]
- Dudareva, N.; Negre, F.; Nagegowda, D.; Orlova, I. Plant Volatiles: Recent Advances and Future Perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Vickers, C.; Gershenzon, J.; Lerdau, M.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Murfitt, L.; Mann, C.; Gorenstein, N.; Kolosova, N.; Kish, C.; Bonham, C.; Wood, K. Developmental Regulation of Methyl Benzoate Biosynthesis and Emission in Snapdragon Flowers. Plant Cell 2000, 12, 949–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassao, D.; Gang, D.; Koeduka, T.; Jackson, B.; Pichersky, E.; Davin, L.; Lewis, N. Chavicol formation in sweet basil (Ocimum basilicum): Cleavage of an esterified C9 hydroxyl group with NAD(P)H-dependent reduction. Org. Biomol. Chem. 2006, 4, 2733–2744. [Google Scholar] [CrossRef]
- Hegele, M.; Sritontip, C.; Chattrakul, A.; Tiyayon, P.; Naphrom, D.; Sringarm, K.; Sruamsiri, P.; Manochai, P.; Wuensche, J. Hormonal Control of Flower Induction in Litchi and Longan. Acta Hortic. 2010, 863, 305–313. [Google Scholar] [CrossRef]
- Murata, A.; Kai, K.; Tsutsui, K.; Takeuchi, J.; Todoroki, Y.; Furihata, K.; Yokoyama, M.; Baldermann, S.; Watanabe, N. Enantio-selective reduction of the flowering related compound KODA and its analogues in Pharbitis nil cv. Violet. Tetrahedron 2012, 68, 5583–5589. [Google Scholar] [CrossRef] [Green Version]
- Ives, J.; Posner, H. Epinephrine, Propranolol, and the Sucrose-Ammonium Inhibition of Flowering in Lemna paucicostata 6746. Plant Physiol. 1982, 70, 311–312. [Google Scholar] [CrossRef] [Green Version]
- Ueno, E.; Shinozaki, M. Effects of the inhibitors of biosynthesis and degradation of catecholamines on photoperiodic induction of flowering in Pharbitis Nil. J. Plant Physiol. 1999, 155, 332–337. [Google Scholar] [CrossRef]
- Kai, K.; Oba, Y.; Akaike, R.; Murata, A.; Nishikawa, T.; Watanabe, N. Insight into the chemistry of cycloaddition between alpha-ketol oxylipin and epinephrine: Isolation and structure elucidation of a new reaction product. Tetrahedron Lett. 2013, 54, 2247–2250. [Google Scholar] [CrossRef]
- Yokoyama, M.; Yamaguchi, S.; Inomata, S.; Komatsu, K.; Yoshida, S.; Iida, T.; Yokokawa, Y.; Yamaguchi, M.; Kaihara, S.; Takimoto, A. Stress-induced factor involved in flower formation of Lemna is an α-ketol derivative of linolenic acid. Plant Cell Physiol. 2000, 41, 110–113. [Google Scholar] [CrossRef] [Green Version]
- Khurana, J.; Tamot, B.; Maheshwari, N.; Maheshwari, S. Role of Catecholamines in Promotion of Flowering in a Short-Day Duckweed, Lemna paucicostata 6746. Plant Physiol. 1987, 85, 10–12. [Google Scholar] [CrossRef] [Green Version]
- Kai, K.; Takeuchi, J.; Kataoka, T.; Yokoyama, M.; Watanabe, N. Structure-activity relationship study of flowering-inducer FN against Lemna paucicostata. Tetrahedron 2008, 64, 6760–6769. [Google Scholar] [CrossRef] [Green Version]
- Baud, S.; Guyon, V.; Kronenberger, J.; Wuilleme, S.; Miquel, M.; Caboche, M.; Lepiniec, L.; Rochat, C. Multifunctional acetyl-CoA carboxylase 1 is essential for very long chain fatty acid elongation and embryo development in Arabidopsis. Plant J. 2003, 33, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Footitt, S.; Cornah, J.; Pracharoenwattana, I.; Bryce, J.; Smith, S. The Arabidopsis 3-ketoacyl-CoA thiolase-2 (kat2-1) mutant exhibits increased flowering but reduced reproductive success. J. Exp. Bot. 2007, 58, 2959–2968. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Song, Z.; Nikolau, B. Reverse genetic characterization of two paralogous acetoacetyl CoA thiolase genes in Arabidopsis reveals their importance in plant growth and development. Plant J. 2012, 70, 1015–1032. [Google Scholar] [CrossRef]
- Blée, E. Impact of phyto-oxylipins in plant defense. Trends Plant Sci. 2002, 7, 315–322. [Google Scholar] [CrossRef]
- Hamberg, M.; León, I.; Sanz, A.; Castresana, C. Fatty acid α-dioxygenases. Prostag. Oth. Lipid Mediat. 2002, 68, 363–374. [Google Scholar] [CrossRef]
- Andreou, A.; Brodhun, F.; Feussner, I. Biosynthesis of oxylipins in non-mammals. Prog. Lipid Res. 2009, 48, 148–170. [Google Scholar] [CrossRef]
- Mosblech, A.; Feussner, I.; Heilmann, I. Oxylipins: Structurally diverse metabolites from fatty acid oxidation. Plant Physiol. Bioch. 2009, 47, 511–517. [Google Scholar] [CrossRef]
- Vollenweider, S.; Weber, H.; Stolz, S.; Chételat, A.; Farmer, E. Fatty acid ketodienes and fatty acid ketotrienes: Michael addition acceptors that accumulate in wounded and diseased Arabidopsis leaves. Plant J. 2000, 24, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Stintzi, A.; Weber, H.; Reymond, P.; Browse, J.; Farmer, E. Plant Defense in the Absence of Jasmonic Acid: The Role of Cyclopentenones. Proc. Natl. Acad. Sci. USA 2001, 98, 12837–12842. [Google Scholar] [CrossRef] [Green Version]
- Montillet, J.; Chamnongpol, S.; Rustérucci, C.; Dat, J.; van de Cotte, B.; Agnel, J.; Battesti, C.; Inzé, D.; Van Breusegem, F.; Triantaphylidès, C. Fatty Acid Hydroperoxides and H2O2 in the Execution of Hypersensitive Cell Death in Tobacco Leaves. Plant Physiol. 2005, 138, 1516–1526. [Google Scholar] [CrossRef] [Green Version]
- Obregón, P.; Martín, R.; Sanz, A.; Castresana, C. Activation of defence-related genes during senescence: A correlation between gene expression and cellular damage. Plant Mol. Biol. 2001, 46, 67–77. [Google Scholar] [CrossRef]
- Royo, J.; Vancanneyt, G.; Pà Rez, A.; Sanz, C.; Stà Rmann, K.; Rosahl, S.; Sà Nchez-Serrano, J. Characterization of Three Potato Lipoxygenases with Distinct Enzymatic Activities and Different Organ-specific and Wound-regulated Expression Patterns. J. Biol. Chem. 1996, 271, 21012–21019. [Google Scholar] [CrossRef] [Green Version]
- León, J.; Rojo, E.; Sánchez Serrano, J. Wound signalling in plants. J. Exp. Bot. 2001, 52, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, A.; Akopian, A.; Ruparel, N.; Diogenes, A.; Weintraub, S.; Uhlson, C.; Murphy, R.; Hargreaves, K. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J. Clin. Investig. 2010, 120, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium Oligomannate Therapeutically Remodels Gut Microbiota and Suppresses Gut Bacterial Amino Acids-Shaped Neuroinflammation to Inhibit Alzheimer’s Disease Progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Huang, Y.; Liu, S.; Zhang, S.; Zou, H.; Wang, Z.; Zhu, W.; Geng, J. Toxicological Effects of Nano- and Micro-Polystyrene Plastics on Red Tilapia: Are Larger Plastic Particles More Harmless? J. Hazard. Mater. 2020, 396, 122693. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, T.; Wang, H.; Huang, X.; Qin, Y.; Hu, G. Patterns of enzyme activities and gene expressions in sucrose metabolism in relation to sugar accumulation and composition in the aril of Litchi chinensis Sonn. J. Plant Physiol. 2013, 170, 731–740. [Google Scholar] [CrossRef]
- Xu, C.; Chen, W.; Chen, K.; Zhang, S. A Simple Method for Determining the Content of Starch-Iodine Colorimety. Biotechnology 1998, 8, 41–43. [Google Scholar]
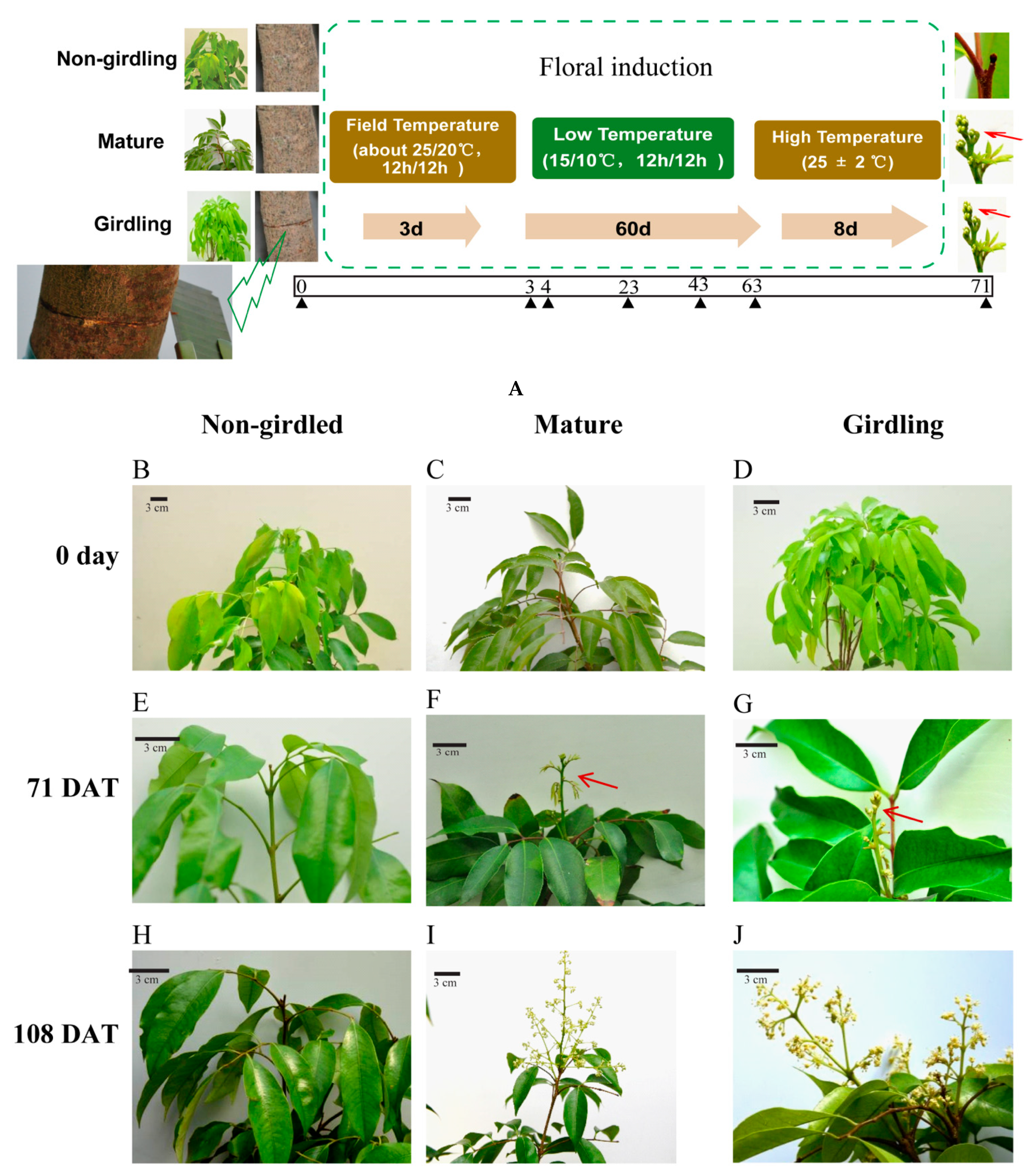




| Treatment | Non-Girdled | Mature | Girdled |
|---|---|---|---|
| Percentage of flowering trees (%) | 16.67 | 100 | 100 |
| Percentage of flowering shoots (%) | 11.08 ± 9.14 b | 74.23 ± 17.93 a | 71.97 ± 10.06 a |
| Days to floral initiation | 81.50 ± 0.50 a | 72.50 ± 2.47 a | 78.00 ± 2.07 a |
| Days to floral differentiation (Male flowers) | 29.00 ± 6.00 a | 35.00 ± 1.41 a | 34.33 ± 2.56 a |
| Days to floral differentiation (Female flowers) | 29.00 ± 4.00 a | 33.25 ± 1.03 a | 30.00 ± 1.83 a |
| Panicle length (cm) | 10.67 ± 0.16 b | 15.52 ± 0.30 a | 8.59 ± 0.97 b |
| Panicle width (cm) | 5.04 ± 0.04 b | 9.1 ± 0.86 a | 6.01 ± 0.84 ab |
| Panicle length/Panicle width (cm) | 2.11 ± 0.01 a | 1.86 ± 0.20 ab | 1.53 ± 0.09 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Z.; Xiao, Q.; Shen, J.; Chen, H.; Yan, S.; Huang, W. Metabolomics Analysis of Litchi Leaves during Floral Induction Reveals Metabolic Improvement by Stem Girdling. Molecules 2021, 26, 4048. https://doi.org/10.3390/molecules26134048
Su Z, Xiao Q, Shen J, Chen H, Yan S, Huang W. Metabolomics Analysis of Litchi Leaves during Floral Induction Reveals Metabolic Improvement by Stem Girdling. Molecules. 2021; 26(13):4048. https://doi.org/10.3390/molecules26134048
Chicago/Turabian StyleSu, Zuanxian, Qiushen Xiao, Jiyuan Shen, Houbin Chen, Shijuan Yan, and Wenjie Huang. 2021. "Metabolomics Analysis of Litchi Leaves during Floral Induction Reveals Metabolic Improvement by Stem Girdling" Molecules 26, no. 13: 4048. https://doi.org/10.3390/molecules26134048





