Oregano and Thyme Essential Oils Encapsulated in Chitosan Nanoparticles as Effective Antimicrobial Agents against Foodborne Pathogens †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of the Degree of Deacetylation (DD) of Chitosan
2.2. Volatile Composition of Origanum vulgare ssp. hirtum and Thymus capitatus Oils
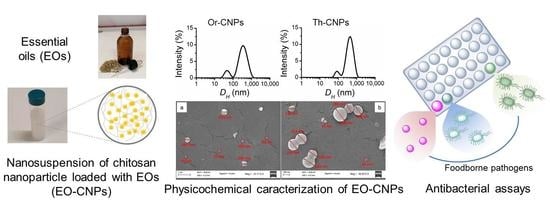
2.3. Preparation and Physicochemical Characterization of EO-CNPs
2.3.1. Encapsulation Efficiency (EE%) and Loading Capacity (LC%)
2.3.2. Physicochemical Stability of EO-CNPs
2.4. Antimicrobial Activity of Th-CNPs and Or-CNPs
3. Material and Methods
3.1. Materials
3.2. Determination of the Degree of Deacetylation (DD) of Chitosan
3.3. Origanum vulgare ssp. hirtum and Thymus capitatus Essential Oils
3.4. Preparation of Essential Oil-Loaded Chitosan Nanoparticles (EO-CNPs)
3.5. Characterization of EO-CNPs
3.5.1. Particle Size, Polydispersity Index, and Zeta Potential Measurements
3.5.2. Viscosity Analysis
3.5.3. Field Emission Scanning Electron Microscopy (FE-SEM)
3.5.4. Encapsulation Efficiency (EE) and Loading Capacity (LC) of EO-CNPs
3.5.5. Stability over Time of Essential Oil-Loaded Chitosan Nanoparticles (EO-CNPs)
3.5.6. Statistical Analysis
3.6. Microbiological Studies
3.6.1. Bacterial Strains and Growth Conditions
3.6.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeni, F.; Yavaş, S.; Alpas, H.A.M.I.; Soyer, Y.E.S.I.M. Most common foodborne pathogens and mycotoxins on fresh produce: A review of recent outbreaks. Crit. Rev. Food Sci. Nutr. 2016, 56, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.; McAuliffe, O. Listeria monocytogenes in Foods. Adv. Food Nutr. Res. 2018, 86, 181–213. [Google Scholar]
- Reineccius, G. Flavor Chemistry and Technology, 2nd ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Deligeorgis, S.G.; Bizelis, J.A.; Dardamani, A.; Theodosiou, I.; Fegeros, K. Effect of dietary oregano oil supplementation on lamb meat characteristics. Meat Sci. 2008, 79, 217–223. [Google Scholar] [CrossRef]
- Rai, M.; Kon, K. Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Chávez-González, M.L.; Rodrıguez-Herrera, R.; Aguilar, C.N. A natural alternative to combat antibiotics resistance. In Antibiotic Resistance-Mechanisms and New Antimicrobial Approaches; Kon, K., Rai, M., Eds.; Elsevier Inc.: London, UK, 2016; pp. 227–237. [Google Scholar]
- Napoli, E.; Ruberto, G.; Siracusa, L. New tricks for old guys: Recent developments in the chemistry, biochemistry, applications and exploitation of selected species from the Lamiaceae Family. Chem. Biodivers. 2020, 17, e1900677. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.R.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Cutter, C.N. Antimicrobial effect of herb extracts against Escherichia coli O157: H7, Listeria monocytogenes, and Salmonella typhimurium Associated with Beef. J. Food Prot. 2000, 63, 601–607. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [Green Version]
- Weiss, J.; Loeffler, M.; Terjung, N. The antimicrobial paradox: Why preservatives lose activity in foods. Curr. Opin. Food Sci. 2015, 4, 69–75. [Google Scholar] [CrossRef]
- Skandamis, P.; Tsigarida, E.; Nychas, G.J. Ecophysiological attributes of Salmonella typhimurium in liquid culture and within a gelatin gel with or without the addition of oregano essential oil. World J. Microbiol. Biotechnol. 2000, 16, 31–35. [Google Scholar] [CrossRef]
- Weiss, J.; Gaysinsky, S.; Davidson, M.; McClements, J. Nanostructured encapsulation systems: Food antimicrobials. In Global Issues in Food Science and Technology; Academic Press: Cambridge, MA, USA, 2009; pp. 425–479. [Google Scholar]
- FDA. GRAS Notices: Shrimp-Derived Chitosan. 2012. Available online: http://www.accessdata.fda.gov/scripts/fdcc/?set=GRASNotices&id=443&sort=GRN_No&order=DESC&startrow=1&type=basic&search=chitosan (accessed on 6 June 2021).
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J. Chitosan applications for the food industry. In Chitosan: Derivatives, Composites and Applications; Scrivener Publishing, LLC/Wiley: Salem, MA, USA, 2017; pp. 185–232. [Google Scholar]
- Hu, J.; Wang, X.; Xiao, Z.; Bi, W. Effect of chitosan nanoparticles loaded with cinnamon essential oil on the quality of chilled pork. LWT Food Sci. Technol. 2015, 63, 519–526. [Google Scholar] [CrossRef]
- Karimirad, R.; Behnamian, M.; Dezhsetan, S.; Sonnenberg, A. Chitosan nanoparticles-loaded Citrus aurantium essential oil: A novel delivery system for preserving the postharvest quality of Agaricus bisporus. J. Sci. Food Agric. 2018, 98, 5112–5119. [Google Scholar] [CrossRef] [PubMed]
- Sotelo-Boyás, M.E.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Corona-Rangel, M.L. Physicochemical characterization of chitosan nanoparticles and nanocapsules incorporated with lime essential oil and their antibacterial activity against food-borne pathogens. LWT Food Sci. Technol. 2017, 77, 15–20. [Google Scholar] [CrossRef]
- Grande-Tovar, C.D.; Chaves-Lopez, C.; Serio, A.; Rossi, C.; Paparella, A. Chitosan coatings enriched with essential oils: Effects on fungi involved in fruit decay and mechanisms of action. Trends Food Sci. Technol. 2018, 78, 61–71. [Google Scholar] [CrossRef]
- Barrera-Ruiz, D.G.; Cuestas-Rosas, G.C.; Sánchez-Mariñez, R.I.; Álvarez-Ainza, M.L.; Moreno-Ibarra, G.M.; López-Meneses, A.K.; Plascencia-Jatomea, M.; Cortez-Rocha, M.O. Antibacterial activity of essential oils encapsulated in chitosan nanoparticles. Food Sci. Technol. Campinas 2020, 40, 568–573. [Google Scholar] [CrossRef]
- Barzegar, M.; Ghaderi Ghahfarokhi, M.; Sahari, M.A.; Azizi, M.H. Enhancement of thermal stability and antioxidant activity of thyme essential oil by encapsulation in chitosan nanoparticles. J. Agric. Sci. Technol. 2016, 18, 1781–1792. [Google Scholar]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef]
- Electronic Code of Federal Regulations (e-CFR). 2020. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=42bb02884fc7049a74766606865e2a61&mc=true&node=pt21.6.582&rgn=div5 (accessed on 6 June 2021).
- Kasaai, M.R. A review of several reported procedures to determine the degree of N-acetylation for chitin and chitosan using infrared spectroscopy. Carbohydr. Polym. 2008, 71, 497–508. [Google Scholar] [CrossRef]
- Foster, L.J.R.; Ho, S.; Hook, J.; Basuki, M.; Marcal, H. Chitosan as a biomaterial: Influence of degree of deacetylation on its physiochemical, material and biological properties. PLoS ONE 2015, 10, e0135153. [Google Scholar] [CrossRef] [Green Version]
- American Standard Test Method, ASTM International. F2260-03. 2003. Available online: http://www.astm.org (accessed on 6 June 2021).
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Consoli, G.M.L.; Cafiso, V.; Geraci, C. Essential oils encapsulated in polymer-based nanocapsules as potential candidates for application in food preservation. Food Chem. 2018, 269, 286–292. [Google Scholar] [CrossRef]
- Poulose, A.J.; Croteau, R. Biosynthesis of aromatic monoterpenes: Conversion of γ-terpinene to p-cymene and thymol in Thymus vulgaris L. Arch. Biochem. Biophys. 1978, 187, 2, 307–314. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Dugo, G.; Ruberto, G.; Leto, C.; Napoli, E.M.; Rando, R.; Fede, M.R.; La Bella, S.; Licata, M.; Virga, G.; et al. Study of quantitative and qualitative variations in essential oils of sicilian oregano biotypes. J. Essent. Oil Res. 2015, 27, 4, 293–306. [Google Scholar]
- Tuttolomondo, T.; leto, C.; Leone, R.; Licata, M.; Virga, G.; Ruberto, G.; Napoli, E.M.; La Bella, S. Essential oil characteristics of wild Sicilian oregano population in relation to environmental conditions. J. Essent. Oil Res. 2014, 26, 210–220. [Google Scholar] [CrossRef]
- Napoli, E.M.; Curcuruto, G.; Ruberto, G. Screening of the essential oil composition of wild Sicilian thyme. Biochem. Syst. Ecol. 2010, 38, 816–822. [Google Scholar] [CrossRef]
- Koukaras, E.N.; Papadimitriou, S.A.; Bikiaris, D.N.; Froudakis, G.E. Insight on the formation of chitosan nanoparticles through ionotropic gelation with tripolyphosphate. Mol. Pharm. 2012, 9, 2856–2862. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf. B 2005, 44, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Sonia, T.A.; Sharma, C.P. Chitosan and its derivatives for drug delivery perspective. Adv. Polym. Sci. 2011, 243, 23–53. [Google Scholar]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ergin, A.D.; Bayindir, Z.S.; Yüksel, N. Characterization and optimization of colon targeted S-adenosyl-L-methionine loaded chitosan nanoparticles. J. Pharm. Res. 2019, 23, 914–926. [Google Scholar] [CrossRef] [Green Version]
- Patravale, V.B.; Date, A.A.; Kulkarni, R.M. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, A.M.M.; Dorkoosh, F.A.; Avadi, M.R.; Saadat, P.; Rafiee-Tehrani, M.; Junginger, H.E. Preparation, characterization and antibacterial activities of chitosan, N-trimethyl chitosan (TMC) and N-diethylmethyl chitosan (DEMC) nanoparticles loaded with insulin using both the ionotropic gelation and polyelectrolyte complexation methods. Int. J. Pharm. 2008, 355, 299–306. [Google Scholar] [CrossRef]
- Rehman, A.; Tong, Q.; Shezad, Q.; Aadil, R.M.; Khan, I.M.; Riaz, T.; Jafari, S.M. Rheological Analysis of Solid-Like Nanoencapsulated Food Ingredients by Rheometers. Characterization of Nanoencapsulated Food Ingredients in Nanoencapsulation in the Food Industry; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 547–583. [Google Scholar]
- Sotelo-Boyás, M.; Correa-Pacheco, Z.; Bautista-Baños, S.; Gómez y Gómez, Y. Release study and inhibitory activity of thyme essential oil-loaded chitosan nanoparticles and nanocapsules against foodborne bacteria. Int. J. Biol. Macromol. 2017, 103, 409–414. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef]
- Granata, G.; Consoli, G.M.L.; Lo Nigro, R.; Geraci, C. Hydroxycinnamic acids loaded in lipid-core nanocapsules. Food Chem. 2018, 245, 551–556. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.E.; Valverde-Aguilar, G.; Plascencia-Jatomea, M.; Correa-Pacheco, Z.N.; Jiménez-Aparicio, A.; Solorza-Feria, J.; Barrera-Necha, L.; Bautista-Baños, S. Characterization of chitosan nanoparticles added with essential oils. In vitro effect on Pectobacterium carotovorum. Rev. Mex. Ing. Quím. 2015, 14, 589–599. [Google Scholar]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Czechowska-Biskup, R.; Jarosińska, D.; Rokita, B.; Ulański, P.; Rosiak, J.M. Determination of degree of deacetylation of chitosan-comparision of methods. Prog. Chem. Appl. Chitin Deriv. 2012, 17, 5–20. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatographic/Quadrupole Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2012. [Google Scholar]
- Wayne, P.A. M100–S23: Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Third Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013. [Google Scholar]





| S. aureus ATCC 29213 | E. coli ATCC 25922 | L. monocytogenes ATCC 19118 | ||||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| Th-CNPs | 0.06 | 0.06 | 0.12 | 0.12 | 0.03 | 0.03 |
| Th-EO | 2 | 2 | 2 | 2 | 1 | 2 |
| Or-CNPs | 0.03 | 0.06 | 0.06 | 0.06 | 0.03 | 0.03 |
| Or-EO | 4 | 4 | 4 | 4 | 2 | 4 |
| CNPs | 0.03 | >0.50 | 0.06 | 0.50 | 0.03 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Malandrino, G.; Cafiso, V.; Stefani, S.; Geraci, C. Oregano and Thyme Essential Oils Encapsulated in Chitosan Nanoparticles as Effective Antimicrobial Agents against Foodborne Pathogens. Molecules 2021, 26, 4055. https://doi.org/10.3390/molecules26134055
Granata G, Stracquadanio S, Leonardi M, Napoli E, Malandrino G, Cafiso V, Stefani S, Geraci C. Oregano and Thyme Essential Oils Encapsulated in Chitosan Nanoparticles as Effective Antimicrobial Agents against Foodborne Pathogens. Molecules. 2021; 26(13):4055. https://doi.org/10.3390/molecules26134055
Chicago/Turabian StyleGranata, Giuseppe, Stefano Stracquadanio, Marco Leonardi, Edoardo Napoli, Graziella Malandrino, Viviana Cafiso, Stefania Stefani, and Corrada Geraci. 2021. "Oregano and Thyme Essential Oils Encapsulated in Chitosan Nanoparticles as Effective Antimicrobial Agents against Foodborne Pathogens" Molecules 26, no. 13: 4055. https://doi.org/10.3390/molecules26134055
APA StyleGranata, G., Stracquadanio, S., Leonardi, M., Napoli, E., Malandrino, G., Cafiso, V., Stefani, S., & Geraci, C. (2021). Oregano and Thyme Essential Oils Encapsulated in Chitosan Nanoparticles as Effective Antimicrobial Agents against Foodborne Pathogens. Molecules, 26(13), 4055. https://doi.org/10.3390/molecules26134055