Antioxidant Serine-(NSAID) Hybrids with Anti-Inflammatory and Hypolipidemic Potency
Abstract
:1. Introduction
2. Results
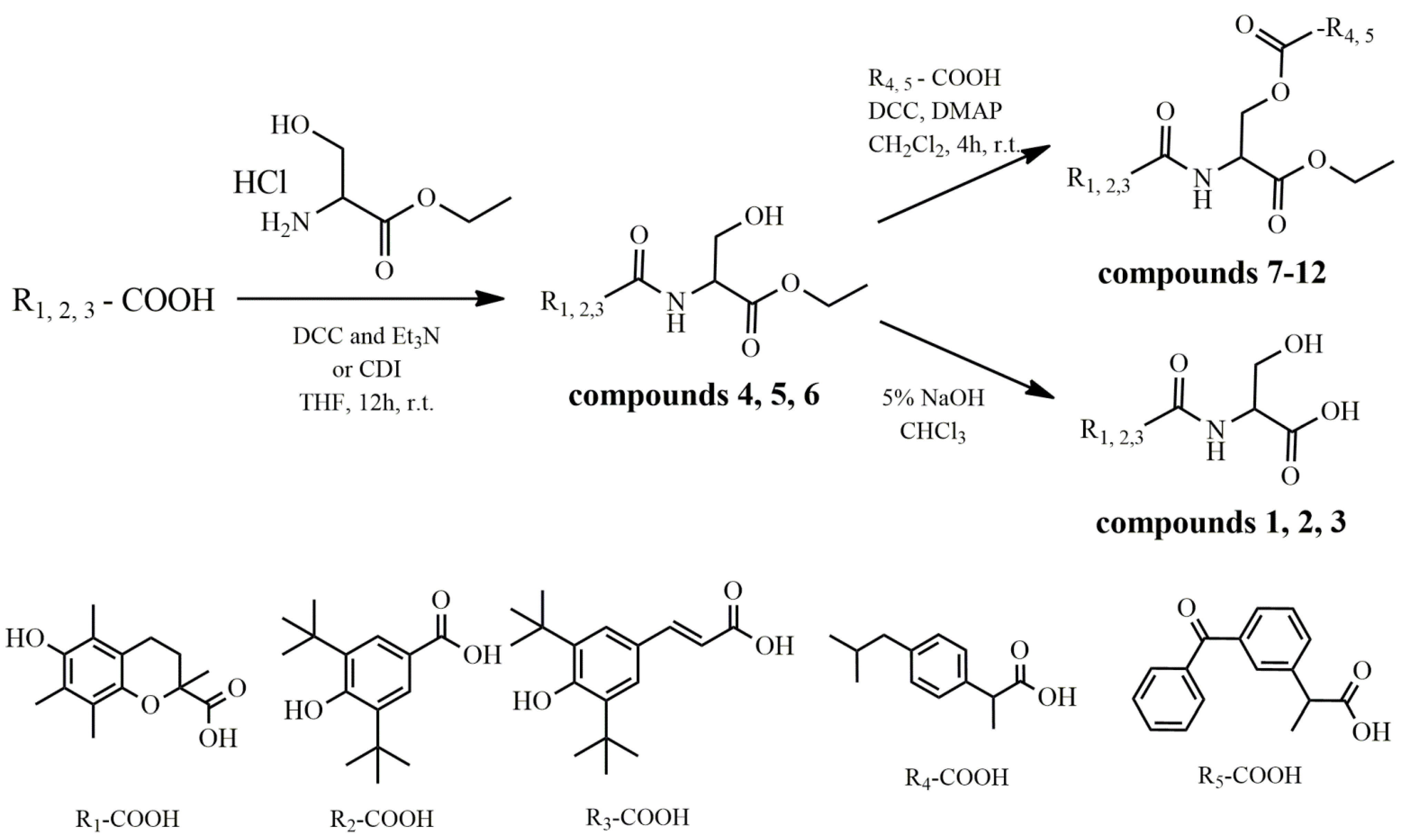
2.1. Synthesis
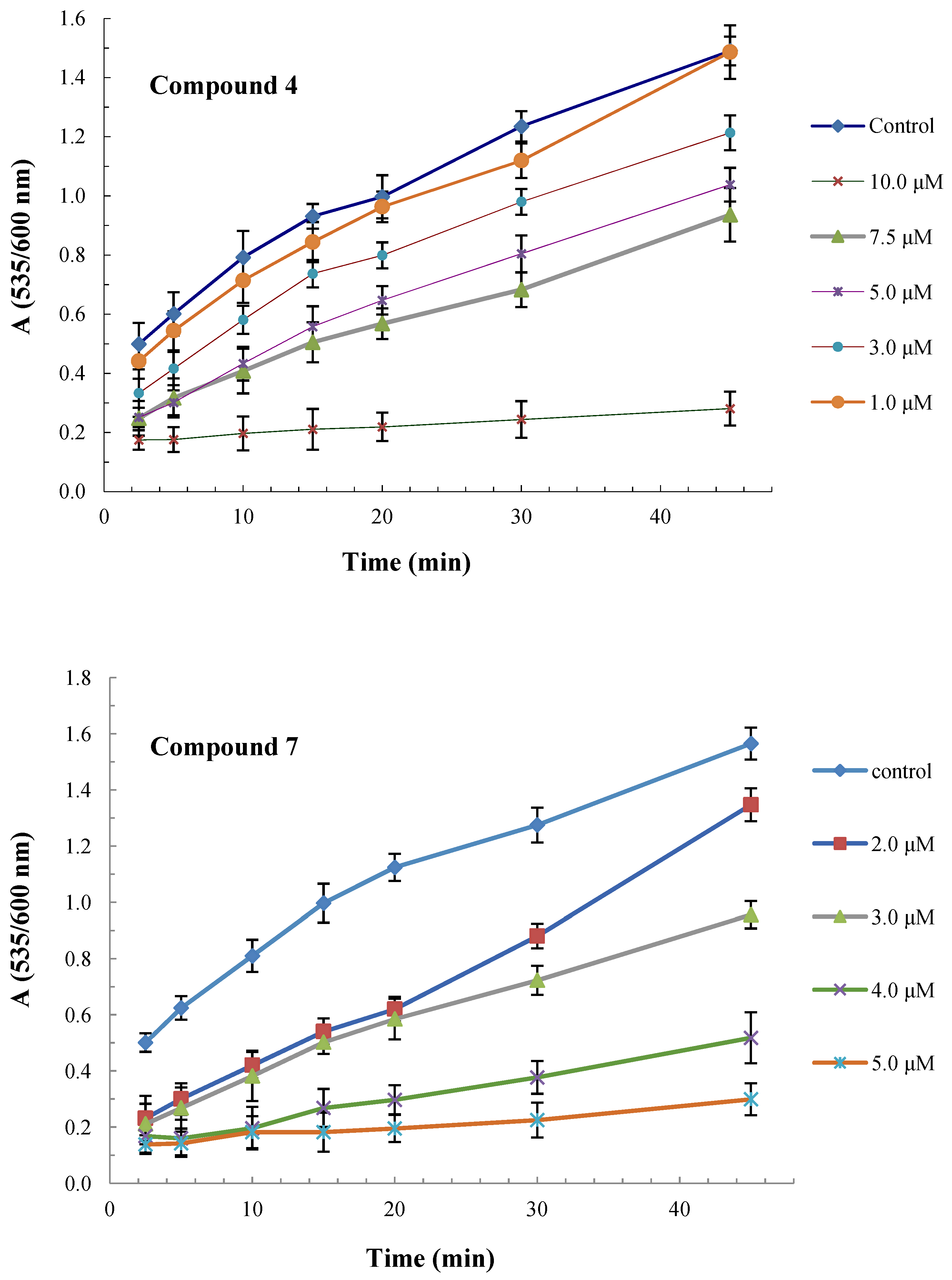
2.2. Antioxidant and Radical Scavenging Activity
2.3. In Vivo Anti-Inflammatory Activity
2.4. Lipoxygenase Inhibitory Activity
2.5. Hypolipidemic Effect
3. Discussion
3.1. Antioxidant and Radical Scavenging Activity
3.2. In Vivo Anti-Inflammatory Activity
3.3. Lipoxygenase Inhibitory Activity
3.4. Hypolipidemic Effect
4. Materials and Methods
4.1. General
4.2. Synthesis
4.2.1. General Methods for the Synthesis of Compounds 4–6
4.2.2. General Method for the Synthesis of Compounds 1–3
4.2.3. General Method for the Synthesis of Compounds 7–12
4.3. Biological Evaluation
4.3.1. In Vitro Lipid Peroxidation
4.3.2. In Vitro Interaction with the Stable Radical 1,1-Diphenyl-2-picrylhydrazyl (DPPH)
4.3.3. Carrageenan-Induced Paw Edema
4.3.4. In Vitro Evaluation of Lipoxygenase Activity
4.3.5. Effect on Plasma Cholesterol and Triglyceride Levels
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Baker, R.G.; Hayden, M.S.; Ghosh, S. Nf-κb, inflammation and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [Green Version]
- Nikolay, V.G.; Pavel, V.A.; Alexander, D.N.; Irina, L.Z.; Richard, O.J. Reactive oxygen species in pathogenesis of atherosclerosis. Curr. Pharm. Des. 2015, 21, 1134–1146. [Google Scholar]
- Li, Q.; Harraz, M.M.; Zhou, W.; Zhang, L.N.; Ding, W.; Zhang, Y.; Eggleston, T.; Yeaman, C.; Banfi, B.; Engelhardt, J.F. Nox2 and rac1 regulate H2O2-dependent recruitment of traf6 to endosomal interleukin-1 receptor complexes. Mol. Cell. Biol. 2006, 26, 140–154. [Google Scholar] [CrossRef] [Green Version]
- Park, H.S.; Jung, H.Y.; Park, E.Y.; Kim, J.; Lee, W.J.; Bae, Y.S. Cutting edge: Direct interaction of tlr4 with nad(p)h oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of nf-κb. J. Immunol. 2004, 173, 3589–3593. [Google Scholar] [CrossRef] [Green Version]
- Paneni, F.; Mocharla, P.; Akhmedov, A.; Costantino, S.; Osto, E.; Volpe, M.; Lüscher, T.F.; Cosentino, F. Gene silencing of the mitochondrial adaptor p66shc suppresses vascular hyperglycemic memory in diabetes. Circ. Res. 2012, 111, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Abu-Saleh, N.; Aviram, M.; Hayek, T. Aqueous or lipid components of atherosclerotic lesion increase macrophage oxidation and lipid accumulation. Life Sci. 2016, 154, 1–14. [Google Scholar] [CrossRef]
- Csonka, C.; Sárközy, M.; Pipicz, M.; Dux, L.; Csont, T. Modulation of Hypercholesterolemia-Induced Oxidative/Nitrative Stress in the Heart. Oxid. Med. Cell. Longev. 2016, 2016, 3863726. [Google Scholar] [CrossRef]
- Hegazy, G.H.; Ali, H.I. Design, synthesis, biological evaluation, and comparative Cox1 and Cox2 docking of p-substituted benzylidenamino phenyl esters of ibuprofenic and mefenamic acids. Bioorg. Med. Chem. 2012, 20, 1259–1270. [Google Scholar] [CrossRef]
- Kourounakis, P.N.; Tsiakitzis, K.; Kourounakis, A.P.; Galanakis, D. Reduction of gastrointestinal toxicity of NSAIDs via molecular modifications leading to antioxidant anti-inflammatory drugs. Toxicology 2000, 144, 205–210. [Google Scholar] [CrossRef]
- Funk, C.D.; FitzGerald, G.A. COX-2 inhibitors and cardiovascular risk. J. Cardiovasc. Pharmacol. 2007, 50, 470–479. [Google Scholar] [CrossRef]
- Tziona, P.; Theodosis-Nobelos, P.; Rekka, E.A. Medicinal Chemistry approaches of controlling gastrointestinal side effects of non-steroidal anti-inflammatory drugs. Endogenous protective mechanisms and drug design. Med. Chem. 2017, 13, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Maralani, M.N.; Movahedian, A.; Javanmard, S.H. Antioxidant and cytoprotective effects of L-Serine on human endothelial cells. Res. Pharm. Sci. 2012, 7, 209–215. [Google Scholar]
- Movahedian, A.; Naderi, G.A.; Dashti, G.R.; Asgary, S.; Zadhoosh, F. Antioxidant effects of L-Serine against fatty streak formation in hypercholesterolemic animals. ARYA Atheroscler. 2010, 2, 126–129. [Google Scholar]
- Garofalo, K.; Penno, A.; Schmidt, B.P.; Lee, H.J.; Frosch, M.P.; von Eckardstein, A.; Brown, R.H.; Hornemann, T.; Eichler, F.S. Oral L-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J. Clin. Investig. 2011, 121, 4735–4745. [Google Scholar] [CrossRef] [Green Version]
- Mothet, J.P.; Parent, A.T.; Wolosker, H.; Brady, R.O., Jr.; Linden, D.J.; Ferris, C.D.; Rogawski, M.A.; Snyder, S.H. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. USA 2000, 25, 4926–4931. [Google Scholar] [CrossRef] [Green Version]
- de Koning, T.J.; Duran, M.; Dorland, L.; Gooskens, R.; Van Schaftingen, E.; Jaeken, J.; Blau, N.; Berger, R.; Poll-The, B.T. Beneficial effects of L-serine and glycine in the management of seizures in 3-phosphoglycerate dehydrogenase deficiency. Ann. Neurol. 1998, 44, 261–265. [Google Scholar] [CrossRef]
- de Koning, T.J.; Snell, K.; Duran, M.; Berger, R.; Poll-The, B.T.; Surtees, R. L-serine in disease and development. Biochem. J. 2003, 371, 653–661. [Google Scholar] [CrossRef]
- Song, F.; Li, H.; Sun, J.; Wang, S. Protective effects of cinnamic acid and cinnamic aldehyde on isoproterenol-induced acute myocardial ischemia in rats. J. Ethnopharmacol. 2013, 150, 125–130. [Google Scholar] [CrossRef]
- Liao, J.C.; Deng, J.S.; Chiu, C.S.; Hou, W.C.; Huang, S.S.; Shie, P.H.; Huang, G.J. Anti-Inflammatory Activities of Cinnamomum cassia Constituents In Vitro and In Vivo. Evid. Based Complement. Altern. Med. 2012, 2012, 429320. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Ge, S.; Li, S.; Lin, H.; Lin, S. Anti-obesity effect of trans-cinnamic acid on HepG2 cells and HFD-fed mice. Food Chem. Toxicol. 2020, 137, 111148. [Google Scholar] [CrossRef]
- Ziakas, G.N.; Rekka, E.A.; Gavalas, A.M.; Eleftheriou, P.T.; Kourounakis, P.N. New analogues of butylated hydroxytoluene as anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. 2006, 14, 5616–5624. [Google Scholar] [CrossRef]
- Galvão, G.M.; Florentino, I.F.; Sanz, G.; Vaz, B.G.; Lião, L.M.; Sabino, J.R.; Cardoso, C.S.; da Silva, D.P.B.; Costa, E.A.; Silva, A.L.P.; et al. Anti-inflammatory and antinociceptive activity profile of a new lead compound—LQFM219. Int. Immunopharmacol. 2020, 88, 106893. [Google Scholar] [CrossRef]
- Τheodosis-Nobelos, P.; Papagiouvannis, G.; Rekka, E.A. A Review on Vitamin E Natural Analogues and on the Design of Synthetic Vitamin E Derivatives as Cytoprotective Agents. Mini Rev. Med. Chem. 2021, 21, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Theodosis-Nobelos, P.; Kourounakis, P.Ν.; Rekka, E.A. Anti-inflammatory and Hypolipidemic Effect of Novel Conjugates with Trolox and Other Antioxidant Acids. Med. Chem. 2017, 13, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Theodosis-Nobelos, P.; Athanasekou, C.; Rekka, E.A. Dual antioxidant structures with potent anti-inflammatory, hypolipidemic and cytoprotective properties. Bioorg. Med. Chem. Lett. 2017, 27, 4800–4804. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Hemmati, A.A.; Naghizadeh, B.; Mard, S.A.; Rezaie, A.; Ghorbanzadeh, B. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J. Pharmacol. 2015, 47, 292–298. [Google Scholar] [PubMed] [Green Version]
- Theodosis-Nobelos, P.; Tziona, P.; Poptsis, A.; Athanasekou, C.; Kourounakis, P.N.; Rekka, E.A. Novel polyfunctional esters of ibuprofen and ketoprofen with hypolipidemic, lipoxygenase inhibitory and enhanced anti-inflammatory activity. Med. Chem. Res. 2017, 26, 461–472. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Kourti, M.; Gavalas, A.; Rekka, E.A. Amides of non-steroidal anti-inflammatory drugs with thiomorpholine can yield hypolipidemic agents with improved anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2016, 26, 910–913. [Google Scholar] [CrossRef]
- Tsolaki, E.; Nobelos, P.; Geronikaki, A.; Rekka, E.A. Selected heterocyclic compounds as antioxidants. Synthesis and biological evaluation. Curr. Top. Med. Chem. 2014, 14, 2462–2477. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.L.; Luft, C.; Lunardelli, A.; Amaral, R.H.; Melo, D.A.; Donadio, M.V.; Nunes, F.B.; de Azambuja, M.S.; Santana, J.C.; Moraes, C.M.; et al. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. An. Acad. Bras. Cienc. 2015, 87, 1397–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witaicenis, A.; Seito, L.N.; da Silveira Chagas, A.; de Almeida, L.D., Jr.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Di Stasi, L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar] [CrossRef]
- Steinhilber, D.; Hofmann, B. Recent advances in the search for novel 5-lipoxygenase inhibitors. Basic Clin. Pharmacol. Toxicol. 2014, 114, 70–77. [Google Scholar] [CrossRef]
- Somvanshi, R.K.; Singh, A.K.; Saxena, M.; Mishra, B.; Dey, S. Development of novel peptide inhibitor of Lipoxygenase based on biochemical and BIAcore evidences. Biochim. Biophys. Acta 2008, 1784, 1812–1817. [Google Scholar] [CrossRef] [PubMed]
- Manju, S.L.; Ethiraj, K.R.; Elias, G. Safer anti-inflammatory therapy through dual COX-2/5-LOX inhibitors: A structure-based approach. Eur. J. Pharm. Sci. 2018, 121, 356–381. [Google Scholar]
- Theodosis-Nobelos, P.; Papagiouvanis, G.; Pantelidou, M.; Kourounakis, P.N.; Athanasekou, C.; Rekka, E.A. Design, synthesis and study of nitrogen monoxide donors as potent hypolipidaemic and anti-inflammatory agents. Molecules 2020, 25, 19. [Google Scholar] [CrossRef] [Green Version]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Kourounakis, P.N.; Rekka, E.A. Active Anti-Inflammatory and Hypolipidemic Derivatives of Lorazepam. Molecules 2019, 24, 3277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landberg, R.; Sunnerheim, K.; Dimberg, L.H. Avenanthramides as lipoxygenase inhibitors. Heliyon 2020, 6, e04304. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Kourti, M.; Tziona, P.; Rekka, E.A.; Kourounakis, P.Ν. Esters of some non-steroidal anti-inflammatory drugs with cinnamyl alcohol are potent lipoxygenase inhibitors with enhanced anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2015, 25, 5028–5031. [Google Scholar] [CrossRef]
- Korolenko, T.A.; Tuzikov, F.V.; Vasil’eva, E.D.; Cherkanova, M.S.; Tuzikova, N.A. Fractional composition of blood serum lipoproteins in mice and rats with Triton WR 1339-induced lipemia. Bull. Exp. Biol. Med. 2010, 149, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Anter, E.; Keaney, J.F., Jr. Oxidative stress, antioxidants, and endothelial function. Curr. Med. Chem. 2004, 11, 1093–1104. [Google Scholar] [CrossRef]
- Gamba, P.; Testa, G.; Gargiulo, S.; Staurenghi, E.; Poli, G.; Leonarduzzi, G. Oxidized cholesterol as the driving force behind the development of Alzheimer’s disease. Front. Aging Neurosci. 2015, 7, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzon Dos Santos, J.; Quadros, A.S.; Weschenfelder, C.; Garofallo, S.B.; Marcadenti, A. Oxidative Stress Biomarkers, Nut-Related Antioxidants, and Cardiovascular Disease. Nutrients 2020, 12, 682. [Google Scholar] [CrossRef] [Green Version]
- Tsiakitzis, K.C.; Papagiouvannis, G.; Theodosis-Nobelos, P.; Tziona, P.; Kourounakis, P.N.; Rekka, E.A. Synthesis, antioxidant and anti-inflammatory effects of antioxidant acid amides with GABA and n-acyl-pyrrolidin-2-ones. Curr. Chem. Biol. 2017, 11, 127–139. [Google Scholar] [CrossRef]
- Tooulia, K.K.; Theodosis-Nobelos, P.; Rekka, E.A. Thiomorpholine derivatives with hypolipidemic and antioxidant activity. Arch. Pharm. 2015, 348, 629–634. [Google Scholar] [CrossRef]

| Compound | Inhibition of Lipid Peroxidation IC50 (μΜ) a | clogP b | TPSA b, Å2 | logD b |
|---|---|---|---|---|
| 1 | 150 | 2.06 | 116.09 | −2.320 |
| 2 | 40%/1 mM | 2.90 | 106.85 | −0.563 |
| 3 | 80 | 3.40 | 106.85 | 0.161 |
| 4 | 7.3 | 2.75 | 105.09 | |
| 5 | 163 | 3.69 | 95.86 | |
| 6 | 22 | 4.21 | 95.86 | |
| 7 | 3.4 | 7.54 | 111.17 | |
| 8 | 30%/500 μM | 8.47 | 101.94 | |
| 9 | 20%/50 μM | 8.99 | 101.94 | |
| 10 | 2.8 | 6.62 | 128.24 | |
| 11 | 30%/1 mM | 7.55 | 119.01 | |
| 12 | 20%/100 μM | 8.07 | 119.01 | |
| Trolox | 25 | 3.09 |
| Compound | Percent Interaction with DPPH | |||
|---|---|---|---|---|
| 200 μΜ | 100 μΜ | 50 μΜ | 25 μΜ | |
| 1 | 90.4 | 87.0 | 48.0 | 19.6 |
| 3 | 82.3 | 35.9 | 17.4 | 12.9 |
| 4 | 92.4 | 88.2 | 53.3 | 24.5 |
| 6 | 88.7 | 48.6 | 21.2 | 17.9 |
| 7 | 91.6 | 89.0 | 54.2 | 22.6 |
| 8 | 18.7 | 4.0 | 3. 6 | - |
| 9 | 90.9 | 58.0 | 31.8 | 15.5 |
| 10 | 87.0 | 64.6 | 30.1 | 12.4 |
| 12 | 94.0 | 53.8 | 24.7 | 20.0 |
| Trolox | 92.0 | 90.0 | 38.0 | 22.0 |
| Compound | % Edema Reduction |
|---|---|
| 1 | 43 ** |
| 2 | 44 ** |
| 3 | 27 * |
| 4 | 37 * |
| 5 | 47 * |
| 6 | 44 * |
| 7 | 45 ** |
| 8 | 48 ** |
| 9 | 52 *** |
| 10 | 42 * |
| 11 | 67 *** |
| 12 | 59 *** |
| Ibuprofen | 36 * |
| Ketoprofen | 47 ** |
| Compound | IC50 (μM) or %/100 μM |
|---|---|
| 1 | 30% |
| 2 | 15% |
| 3 | 20% |
| 4 | 15% |
| 5 | 200 |
| 6 | 156 |
| 7 | - |
| 8 | 13 |
| 9 | 80 |
| 10 | 23% |
| 11 | 86 |
| 12 | 90 |
| Ibuprofen | 200 |
| Ketoprofen | 220 |
| BHT | 192 |
| NDGA | 1.3 |
| Compound | % Reduction | |||
|---|---|---|---|---|
| Dose (i.p.) (μmol/kg) | TC a | TG b | LDL-C c | |
| 4 | 150 | 46.2 ** | 53.4 ** | 60.2 * |
| 5 | 150 | 52.3 ** | 62.0 ** | 66.0 ** |
| 9 | 150 | 61.2 ** | 52.1 ** | 70.1 * |
| 11 | 150 | 57.6 ** | 44.5 * | 51.2 ** |
| 12 | 150 | 53.4 ** | 33.2 * | 56.2 * |
| Simvastatin | 150 | 73.0 ** | - | 70.0 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodosis-Nobelos, P.; Papagiouvannis, G.; Tziona, P.; Kourounakis, P.N.; Rekka, E.A. Antioxidant Serine-(NSAID) Hybrids with Anti-Inflammatory and Hypolipidemic Potency. Molecules 2021, 26, 4060. https://doi.org/10.3390/molecules26134060
Theodosis-Nobelos P, Papagiouvannis G, Tziona P, Kourounakis PN, Rekka EA. Antioxidant Serine-(NSAID) Hybrids with Anti-Inflammatory and Hypolipidemic Potency. Molecules. 2021; 26(13):4060. https://doi.org/10.3390/molecules26134060
Chicago/Turabian StyleTheodosis-Nobelos, Panagiotis, Georgios Papagiouvannis, Paraskevi Tziona, Panos N. Kourounakis, and Eleni A. Rekka. 2021. "Antioxidant Serine-(NSAID) Hybrids with Anti-Inflammatory and Hypolipidemic Potency" Molecules 26, no. 13: 4060. https://doi.org/10.3390/molecules26134060
APA StyleTheodosis-Nobelos, P., Papagiouvannis, G., Tziona, P., Kourounakis, P. N., & Rekka, E. A. (2021). Antioxidant Serine-(NSAID) Hybrids with Anti-Inflammatory and Hypolipidemic Potency. Molecules, 26(13), 4060. https://doi.org/10.3390/molecules26134060





