Pristine and Modified Porous Membranes for Zinc Slurry–Air Flow Battery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Commercial Membranes
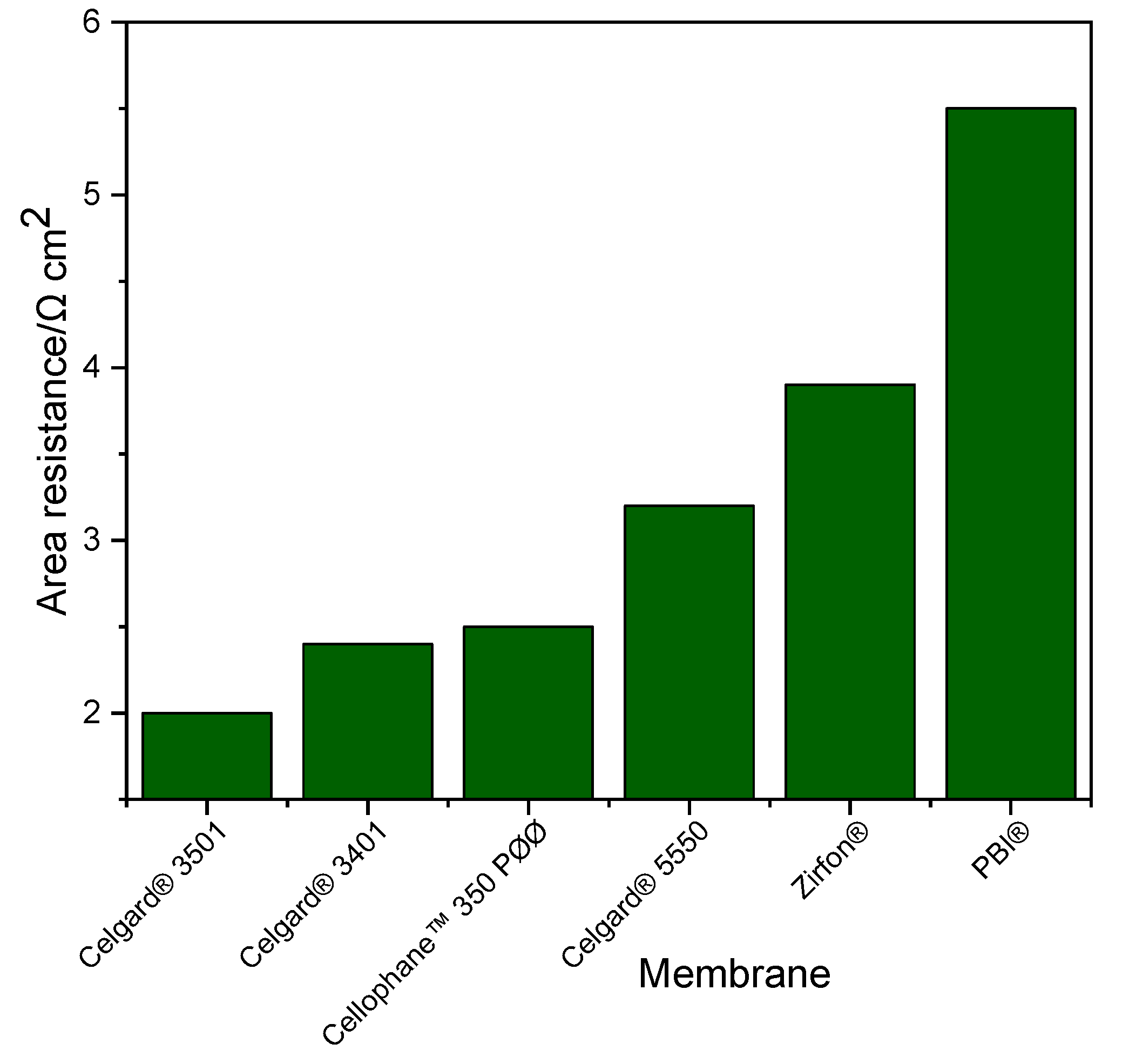
2.1.1. Electrolyte Uptake and Ion Conductivity
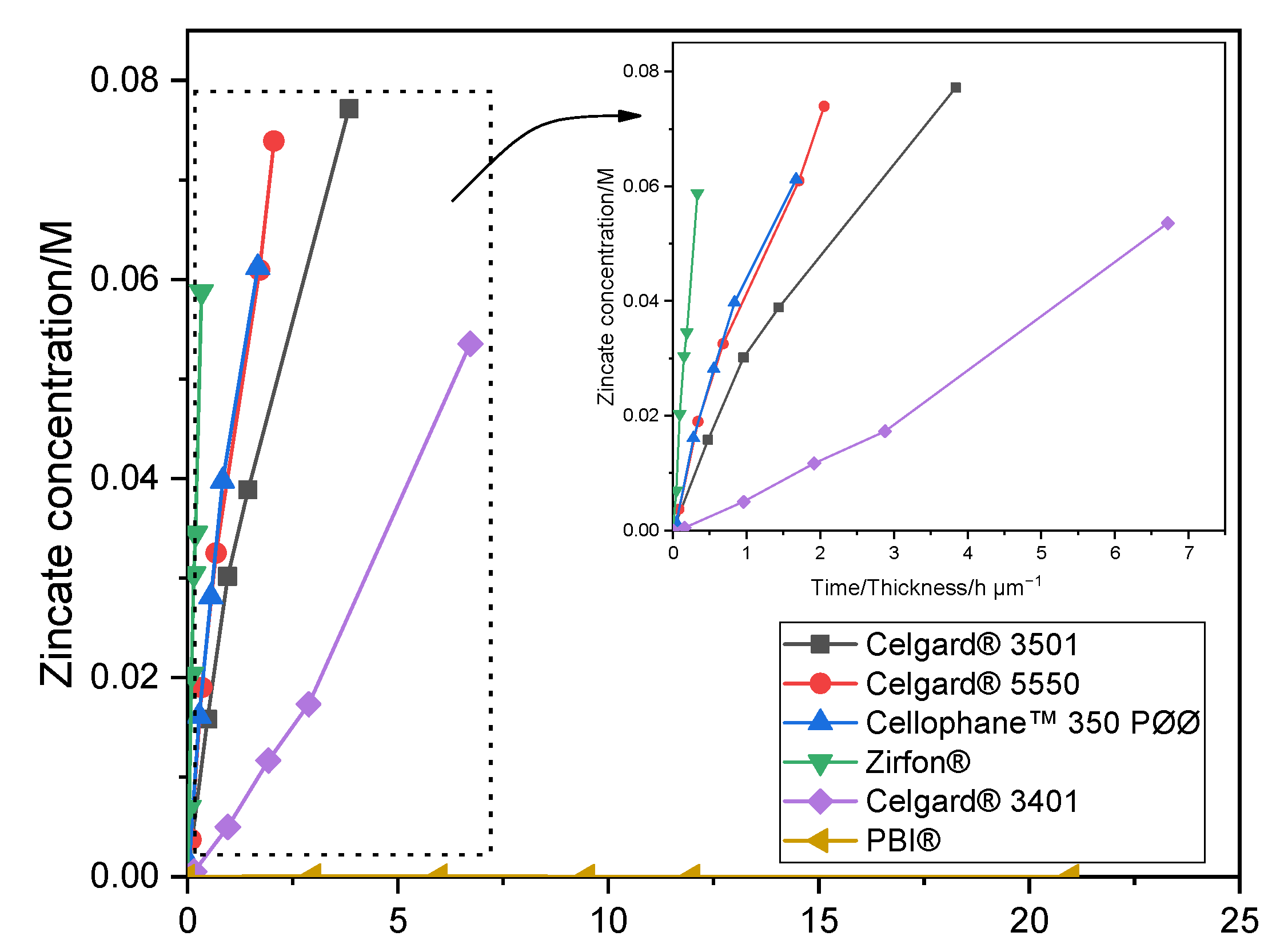
2.1.2. Zincate Ions Crossover
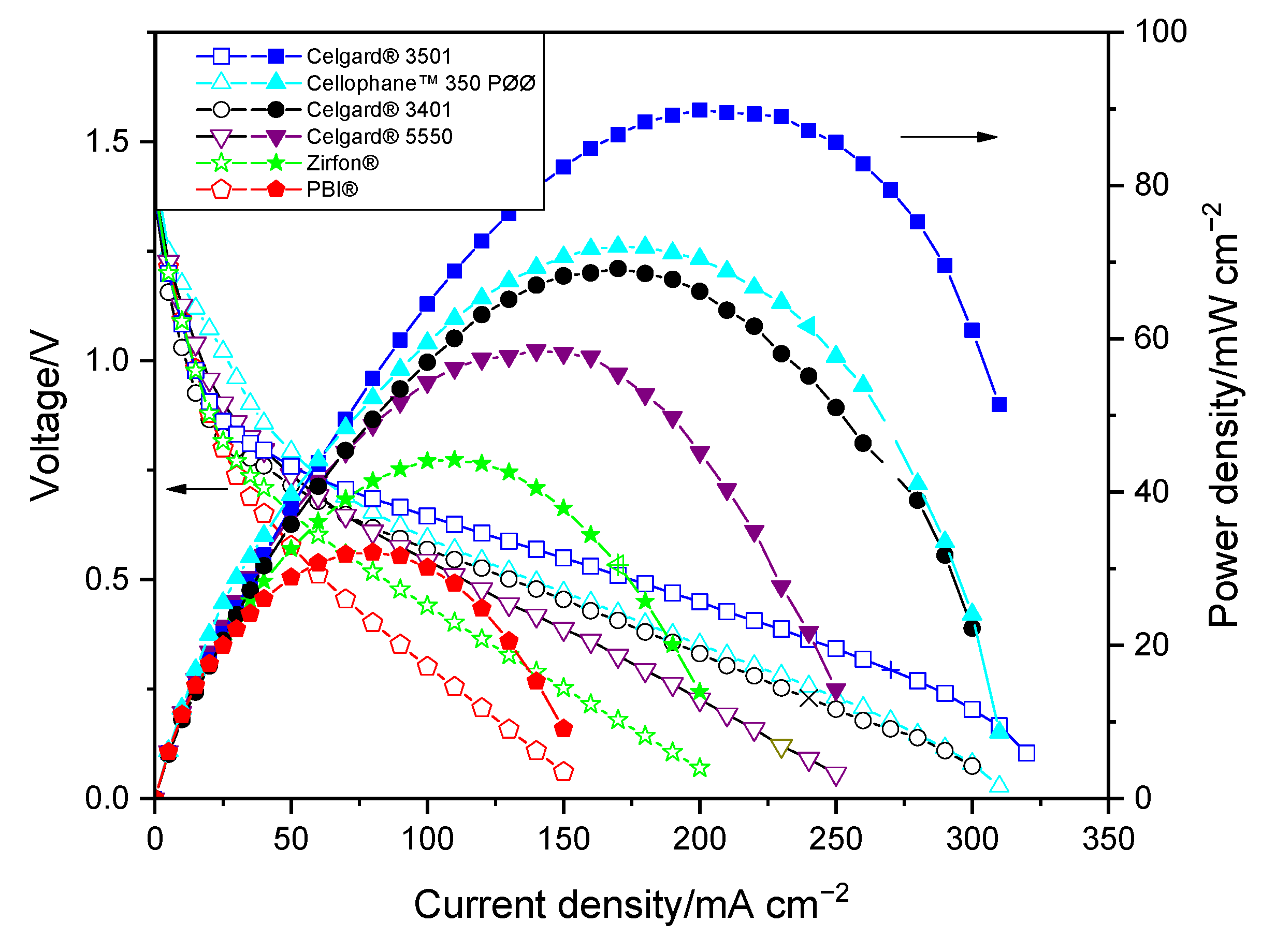
2.1.3. Zn Slurry–Air Flow Battery Performance
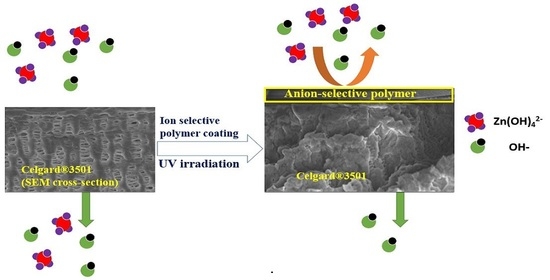
2.2. Improving the Selectivity of Porous Membrane by Ion-Selective Polymers Coating
2.2.1. Polymer and Cation Preparation
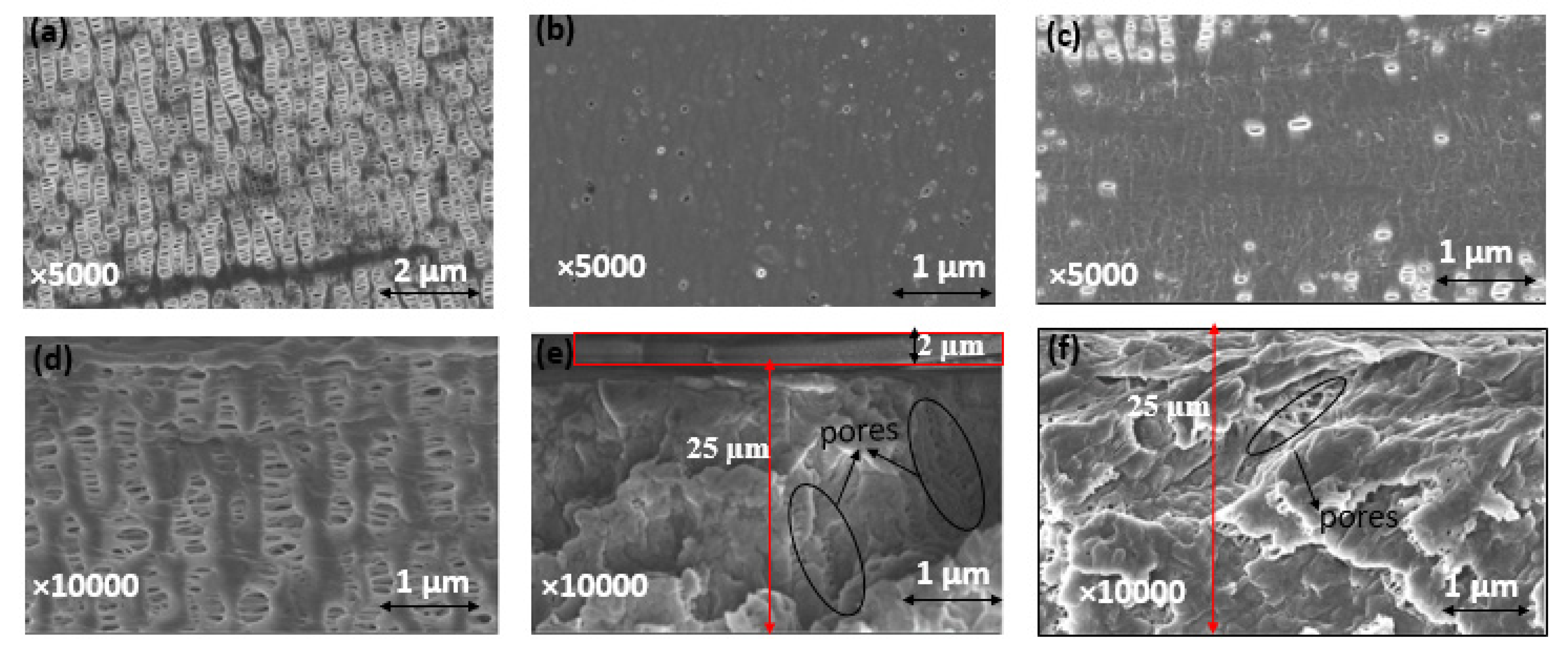
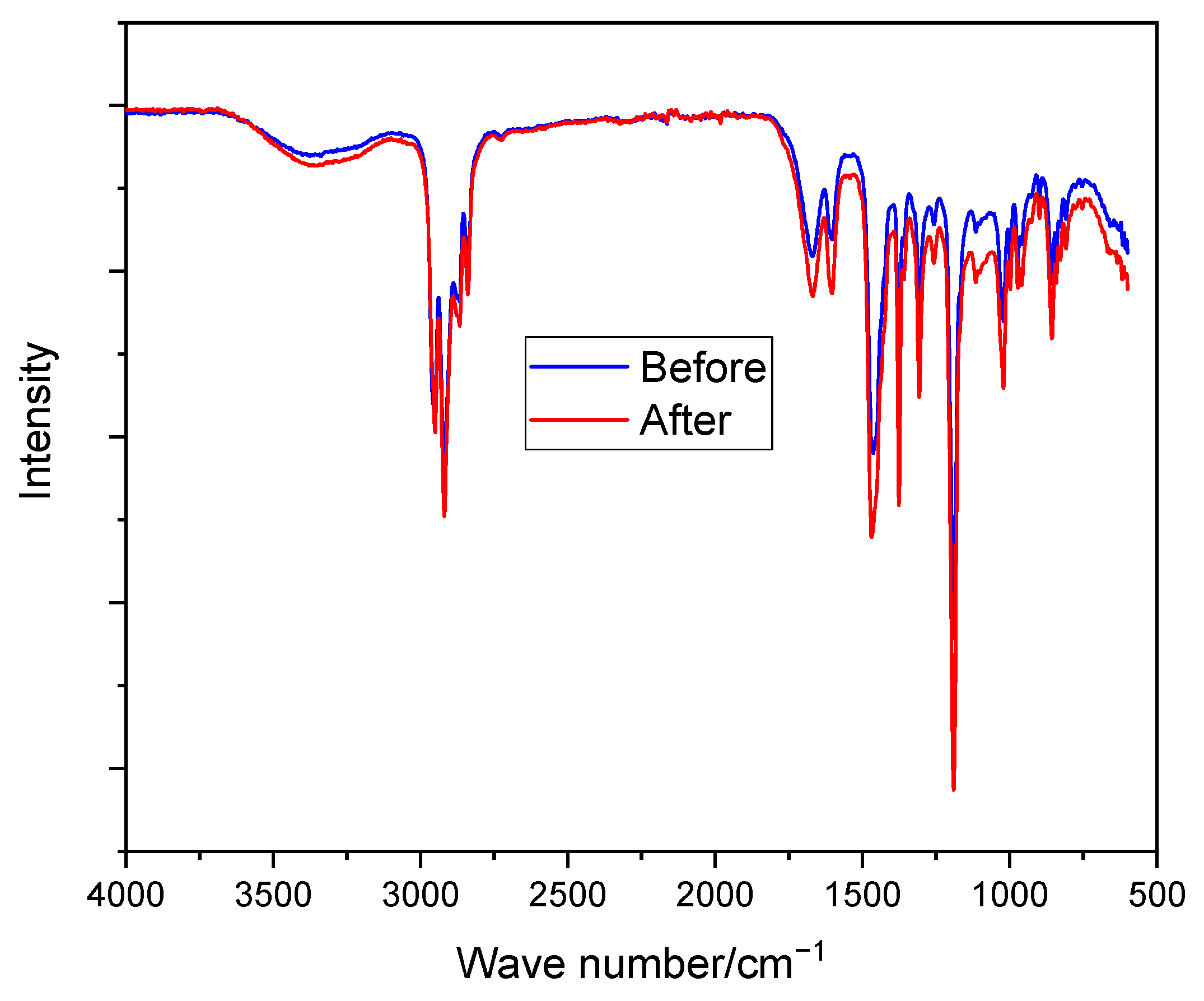
2.2.2. Modified Membrane Structural Characterization
2.2.3. Electrolyte Uptake and Ion Conductivity
2.2.4. Alkaline Stability
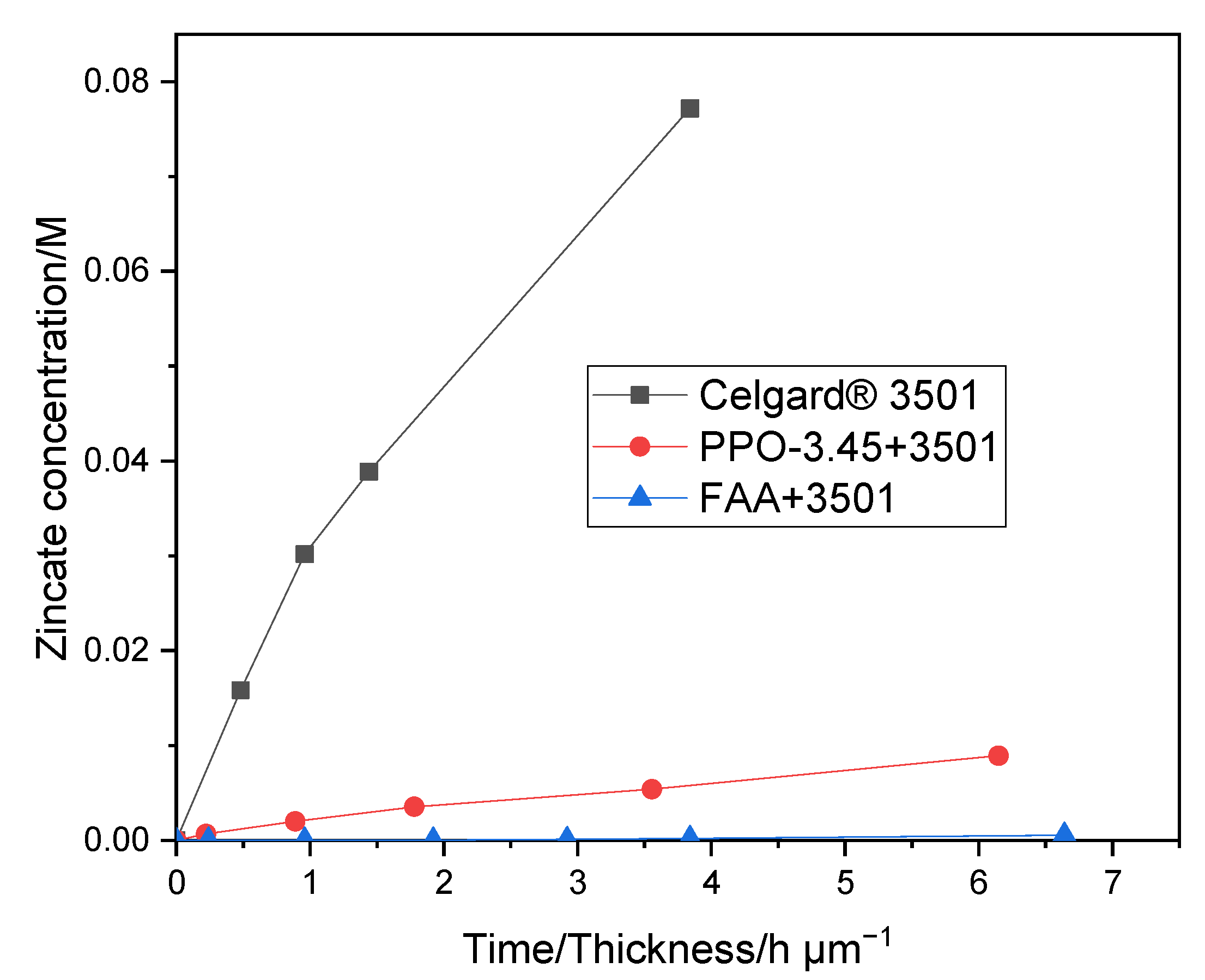
2.2.5. Zincate Ions Crossover
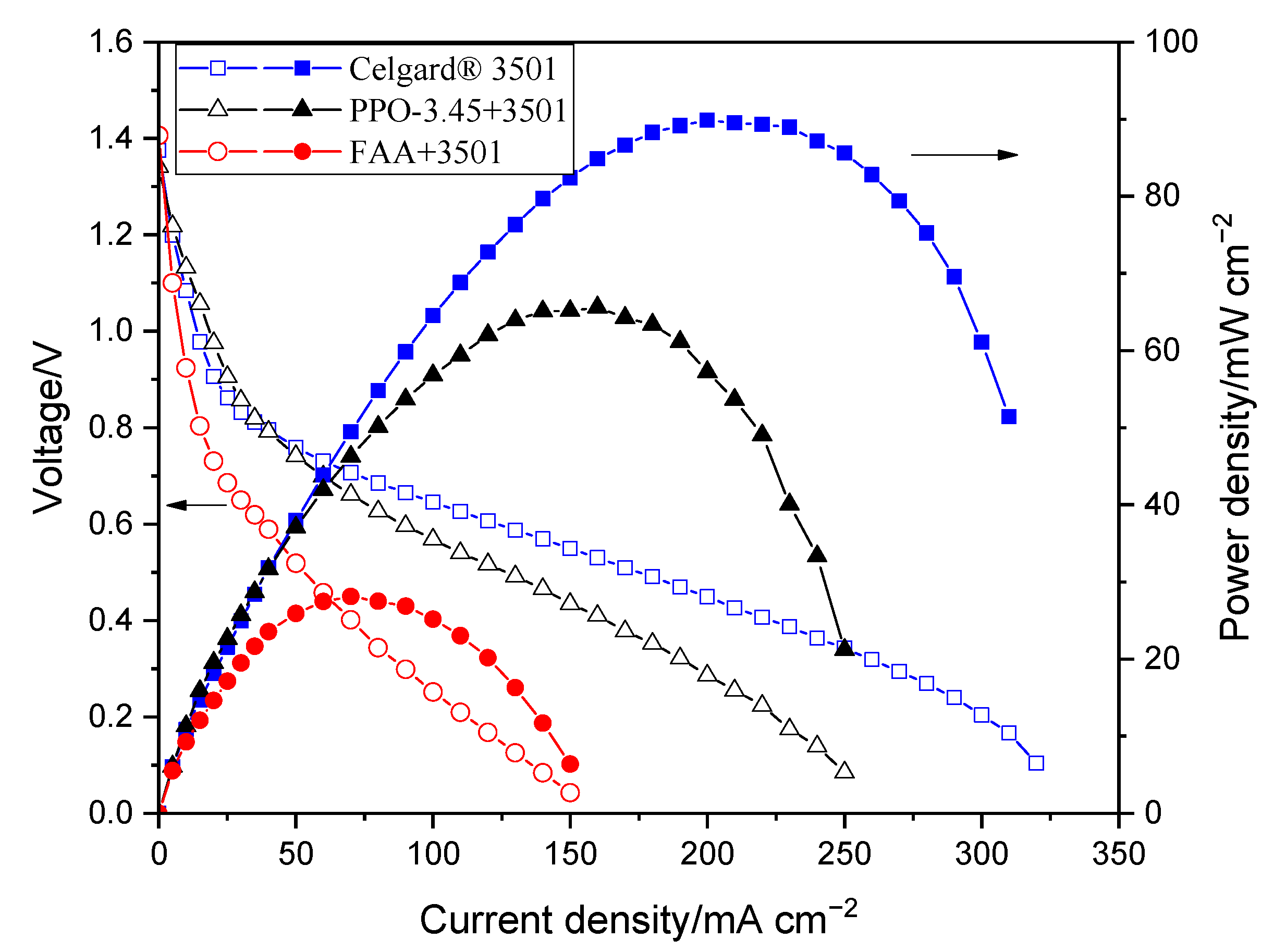
2.2.6. Zn Slurry–Air Flow Battery Discharge Performance
3. Materials and Methods
3.1. Materials
3.2. Polymer and Cation Preparation
3.3. Membrane Preparation
3.4. Characterization
3.4.1. Structural Characterization
3.4.2. Electrolyte Uptake
3.4.3. Ionic Conductivity
3.4.4. Rheometry
3.4.5. Membrane Density
3.4.6. Mercury Porosimetry
3.4.7. Alkaline Stability
3.4.8. Zincate Ion Crossover
3.4.9. Single Cell Assembly and Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Luo, Q.; Li, B.; Wei, X.; Li, L.; Yang, Z. Recent Progress in Redox Flow Battery Research and Development. Adv. Funct. Mater. 2013, 23, 970–986. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F. Redox flow batteries for the storage of renewable energy: A review. Renew. Sustain. Energy Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Rychcik, M.; Skyllas-Kazacos, M. Characteristics of a new all-vanadium redox flow battery. J. Power Sources 1988, 22, 59–67. [Google Scholar] [CrossRef]
- Li, L.; Kim, S.; Wang, W.; Vijayakumar, M.; Nie, Z.; Chen, B.; Zhang, J.; Xia, G.; Hu, J.; Graff, G.; et al. A stable vanadium redox-flow battery with high energy density for large-scale energy storage. Adv. Energy Mater. 2011, 1, 394–400. [Google Scholar] [CrossRef]
- Sun, B.; Skyllas-Kazacos, M. Modification of graphite electrode materials for vanadium redox flow battery application-I. Thermal treatment. Electrochim. Acta 1992, 37, 1253–1260. [Google Scholar] [CrossRef]
- Hosseini, S.; Lao-atiman, W.; Han, S.J.; Arpornwichanop, A.; Yonezawa, T.; Kheawhom, S. Discharge Performance of Zinc-Air Flow Batteries Under the Effects of Sodium Dodecyl Sulfate and Pluronic F-127. Sci. Rep. 2018, 8, 14909. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.; Jang, H.; Park, J.; Yoo, Y.; Park, M.; Cho, J. Seed-mediated atomic-scale reconstruction of silver manganate nanoplates for oxygen reduction towards high-energy aluminum-air flow batteries. Nat. Commun. 2018, 9, 3715. [Google Scholar] [CrossRef]
- Yu, W.; Shang, W.; Tan, P.; Chen, B.; Wu, Z.; Xu, H.; Shao, Z.; Liu, M.; Ni, M. Toward a new generation of low cost, efficient, and durable metal–air flow batteries. J. Mater. Chem. A 2019, 7, 26744–26768. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Pei, P.; Wang, K.; Xiao, Y.; Zhang, C.; Chen, C. A Dendrite-Resistant Zinc-Air Battery. iScience 2020, 23, 101169. [Google Scholar] [CrossRef]
- Gu, P.; Zheng, M.; Zhao, Q.; Xiao, X.; Xue, H.; Pang, H. Rechargeable zinc-air batteries: A promising way to green energy. J. Mater. Chem. A 2017, 5, 7651–7666. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically Rechargeable Zinc–Air Batteries: Progress, Challenges, and Perspectives. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Liang, P.; Liu, X.; Wu, K.; Liu, Y.; Wang, Y.; Xia, Y.; Zhang, J. Challenges, mitigation strategies and perspectives in development of zinc-electrode materials and fabrication for rechargeable zinc-air batteries. Energy Environ. Sci. 2018, 11, 3075–3095. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.W.; Sathiyanarayanan, K.; Eom, S.W.; Yun, M.S. Novel alloys to improve the electrochemical behavior of zinc anodes for zinc/air battery. J. Power Sources 2006, 160, 1436–1441. [Google Scholar] [CrossRef]
- Schmid, M.; Willert-Porada, M. Electrochemical behavior of zinc particles with silica based coatings as anode material for zinc air batteries with improved discharge capacity. J. Power Sources 2017, 351, 115–122. [Google Scholar] [CrossRef]
- Stock, D.; Dongmo, S.; Miyazaki, K.; Abe, T.; Janek, J.; Schröder, D. Towards zinc-oxygen batteries with enhanced cycling stability: The benefit of anion-exchange ionomer for zinc sponge anodes. J. Power Sources 2018, 395, 195–204. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, Y.; Sun, S.; Yan, S.; Miao, H.; Liu, Z. Facile synthesis of ternary spinel Co–Mn–Ni nanorods as efficient bi-functional oxygen catalysts for rechargeable zinc-air batteries. J. Power Sources 2019, 435, 226761. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Chen, J.; Wang, Q. Polyoxometalate on rice paper derived 3D mesoporous carbon paper: An electrocatalyst as cathode for asymmetric Zn-air battery. J. Power Sources 2019, 430, 201–209. [Google Scholar] [CrossRef]
- Hosseini, S.; Han, S.J.; Arponwichanop, A.; Yonezawa, T.; Kheawhom, S. Ethanol as an electrolyte additive for alkaline zinc-air flow batteries. Sci. Rep. 2018, 8, 11273. [Google Scholar] [CrossRef] [Green Version]
- Jiratchayamaethasakul, C.; Srijaroenpramong, N.; Bunyangyuen, T.; Arpavate, W.; Wongyao, N.; Therdthianwong, A.; Therdthianwong, S. Effects of anode orientation and flow channel design on performance of refuelable zinc-air fuel cells. J. Appl. Electrochem. 2014, 44, 1205–1218. [Google Scholar] [CrossRef]
- Kupsch, C.; Feierabend, L.; Nauber, R.; Lars, B. Ultrasound flow investigations at a zinc-air flow battery model. In Proceedings of the 5th International Conference on Experimental Fluid Mechanics ICEFM 2018 Munich, Munich, Germany, 2–4 July 2018. [Google Scholar]
- Ma, H.; Wang, B.; Fan, Y.; Hong, W. Development and Characterization of an Electrically Rechargeable Zinc-Air Battery Stack. Energies 2014, 7, 6549–6557. [Google Scholar] [CrossRef] [Green Version]
- Choi, N.H.; del Olmo, D.; Milian, D.; El Kissi, N.; Fischer, P.; Pinkwart, K.; Tübke, J. Use of Carbon Additives towards Rechargeable Zinc Slurry Air Flow Batteries. Energies 2020, 13, 4482. [Google Scholar] [CrossRef]
- Mele, C.; Bilotta, A.; Bocchetta, P.; Bozzini, B. Characterization of the particulate anode of a laboratory flow Zn–air fuel cell. J. Appl. Electrochem. 2017, 47, 877–888. [Google Scholar] [CrossRef]
- Puapattanakul, A.; Therdthianwong, S.; Therdthianwong, A.; Wongyao, N. Improvement of zinc-air fuel cell performance by gelled koh. Energy Procedia 2013, 34, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Liu, B.; Fan, X.; Liu, X.; Ding, J.; Hu, W.; Zhong, C. An easily assembled boltless zinc–air battery configuration for power systems. J. Power Sources 2020, 458, 228061. [Google Scholar] [CrossRef]
- Tsehaye, M.T.; Alloin, F.; Iojoiu, C. Prospects for Anion-Exchange Membranes in Alkali Metal–Air Batteries. Energies 2019, 12, 4702. [Google Scholar] [CrossRef] [Green Version]
- Tsehaye, M.T.; Alloin, F.; Iojoiu, C.; Tufa, R.A.; Aili, D.; Fischer, P.; Velizarov, S. Membranes for zinc-air batteries: Recent progress, challenges and perspectives. J. Power Sources 2020, 475, 228689. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhang, H.; Li, X. Ion conducting membranes for aqueous flow battery systems. Chem. Commun. 2018, 54, 7570–7588. [Google Scholar] [CrossRef]
- Abbasi, A.; Hosseini, S.; Somwangthanaroj, A.; Mohamad, A.A.; Kheawhom, S. Poly(2,6-Dimethyl-1,4-Phenylene Oxide)-Based Hydroxide Exchange Separator Membranes for Zinc–Air Battery. Int. J. Mol. Sci. 2019, 20, 3678. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Dai, H. Recent advances in zinc–air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [Green Version]
- Dewi, E.L.; Oyaizu, K.; Nishide, H.; Tsuchida, E. Cationic polysulfonium membrane as separator in zinc–air cell. J. Power Sources 2003, 115, 149–152. [Google Scholar] [CrossRef]
- Kiros, Y. Separation and permeability of zincate ions through membranes. J. Power Sources 1996, 62, 117–119. [Google Scholar] [CrossRef]
- Krejčí, I.; Vanýsek, P.; Trojánek, A. Transport of Zn(OH) 4 2—Ions across a Polyolefin Microporous Membrane. J. Electrochem. Soc. 1993, 140, 2279–2283. [Google Scholar] [CrossRef]
- Hwang, H.J.; Chi, W.S.; Kwon, O.; Lee, J.G.; Kim, J.H.; Shul, Y.G. Selective ion transporting polymerized ionic liquid membrane separator for enhancing cycle stability and durability in secondary zinc-air battery systems. ACS Appl. Mater. Interfaces 2016, 8, 26298–26308. [Google Scholar] [CrossRef]
- Strasser, D.J.; Graziano, B.J.; Knauss, D.M. Base stable poly(diallylpiperidinium hydroxide) multiblock copolymers for anion exchange membranes. J. Mater. Chem. A 2017, 5, 9627–9640. [Google Scholar] [CrossRef]
- Olsson, J.S.; Pham, T.H.; Jannasch, P. Poly(N,N-diallylazacycloalkane)s for Anion-Exchange Membranes Functionalized with N -Spirocyclic Quaternary Ammonium Cations. Macromolecules 2017, 50, 2784–2793. [Google Scholar] [CrossRef]
- Wang, Z.; Parrondo, J.; Sankarasubramanian, S.; Bhattacharyya, K.; Ghosh, M.; Ramani, V. Alkaline Stability of Pure Aliphatic-based Anion Exchange Membranes Containing Cycloaliphatic Quaternary Ammonium Cations. J. Electrochem. Soc. 2020, 167, 124504. [Google Scholar] [CrossRef]
- Celgard LLC. Celgard High Performance Battery Separators 2200; Celgard LLC: Charlotte, NC, USA, 2009. [Google Scholar]
- Rodríguez, J.; Palmas, S.; Sánchez-Molina, M.; Amores, E.; Mais, L.; Campana, R. Simple and precise approach for determination of Ohmic contribution of diaphragms in alkaline water electrolysis. Membranes 2019, 9, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AGFA. Technical Data Sheet ZIRFON PERL UTP 500; AGFA: Mortsel, Belgium, 2020. [Google Scholar]
- Shi, C.; Dai, J.; Li, C.; Shen, X.; Peng, L.; Zhang, P.; Wu, D.; Sun, D.; Zhao, J. A modified ceramic-coating separator with high-temperature stability for lithium-ion battery. Polymers 2017, 9, 159. [Google Scholar] [CrossRef]
- Xu, R.; Huang, H.; Tian, Z.; Xie, J.; Lei, C. Effects of Coated Separator Surface Morphology on Electrolyte Interfacial Wettability and Corresponding Li–Ion Battery Performance. Polymers 2020, 12, 117. [Google Scholar] [CrossRef] [Green Version]
- Kirchhöfer, M.; von Zamory, J.; Paillard, E.; Passerini, S. Separators for Li-Ion and Li-Metal Battery Including Ionic Liquid Based Electrolytes Based on the TFSI− and FSI− Anions. Int. J. Mol. Sci. 2014, 15, 14868–14890. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.W.; Lim, J.M.; Lee, H.J.; Eom, S.W.; Hong, Y.T.; Lee, S.Y. Artificially engineered, bicontinuous anion-conducting/-repelling polymeric phases as a selective ion transport channel for rechargeable zinc-air battery separator membranes. J. Mater. Chem. A 2016, 4, 3711–3720. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, C.; Lee, J.H.; Kim, S.-K.; Cho, H.-S.; Kim, M.; Cho, W.C.; Joo, J.H.; Kim, C.-H. Cerium Oxide–Polysulfone Composite Separator for an Advanced Alkaline Electrolyzer. Polymers 2020, 12, 2821. [Google Scholar] [CrossRef]
- Liu, S.J.; Cui, S.P.; Qin, Z.P.; Fei, C.J.; Wang, Y.L.; Guo, H.X. A Novel Way to Modify PTFE Membrane into Hydrophilicity. Mater. Sci. Forum 2017, 898, 1892–1895. [Google Scholar] [CrossRef]
- Van der Bruggen, B. Chemical modification of polyethersulfone nanofiltration membranes: A review. J. Appl. Polym. Sci. 2009, 114, 630–642. [Google Scholar] [CrossRef]
- Vermeiren, P.; Leysen, R.; Beckers, H.; Moreels, J.P.; Claes, A. The influence of manufacturing parameters on the properties of macroporous Zirfon® separators. J. Porous Mater. 2008, 15, 259–264. [Google Scholar] [CrossRef]
- Park, J.-H.; Park, J.-S. KOH-doped Porous Polybenzimidazole Membranes for Solid Alkaline Fuel Cells. Energies 2020, 13, 525. [Google Scholar] [CrossRef] [Green Version]
- Kolesnichenko, I.V.; Arnot, D.J.; Lim, M.B.; Yadav, G.G.; Nyce, M.; Huang, J.; Banerjee, S.; Lambert, T.N. Zincate-Blocking-Functionalized Polysulfone Separators for Secondary Zn–MnO2 Batteries. ACS Appl. Mater. Interfaces 2020, 12, 50406–50417. [Google Scholar] [CrossRef]
- Turney, D.E.; Gallaway, J.W.; Yadav, G.G.; Ramirez, R.; Nyce, M.; Banerjee, S.; Chen-Wiegart, Y.C.K.; Wang, J.; D’Ambrose, M.J.; Kolhekar, S.; et al. Rechargeable Zinc Alkaline Anodes for Long-Cycle Energy Storage. Chem. Mater. 2017, 29, 4819–4832. [Google Scholar] [CrossRef]
- Seymour, W.B. The preparation of cellophane membranes of graded permeability. J. Biol. Chem. 1940, 134, 701–707. [Google Scholar]
- Lee, H.J.; Lim, J.M.; Kim, H.W.; Jeong, S.H.; Eom, S.W.; Hong, Y.T.; Lee, S.Y. Electrospun polyetherimide nanofiber mat-reinforced, permselective polyvinyl alcohol composite separator membranes: A membrane-driven step closer toward rechargeable zinc-air batteries. J. Memb. Sci. 2016, 499, 526–537. [Google Scholar] [CrossRef]
- Duay, J.; Lambert, T.N.; Aidun, R. Stripping Voltammetry for the Real Time Determination of Zinc Membrane Diffusion Coefficients in High pH: Towards Rapid Screening of Alkaline Battery Separators. Electroanalysis 2017, 29, 2261–2267. [Google Scholar] [CrossRef]
- Cao, W.; Li, Y.; Fitch, B.; Shih, J.; Doung, T.; Zheng, J. Strategies to optimize lithium-ion supercapacitors achieving high-performance: Cathode configurations, lithium loadings on anode, and types of separator. J. Power Sources 2014, 268, 841–847. [Google Scholar] [CrossRef]
- Dzieciuch, M.A.; Gupta, N.; Wroblowa, H.S. Rechargeable Cells with Modified MnO2 Cathodes. J. Electrochem. Soc. 2019, 135, 2415–2418. [Google Scholar] [CrossRef]
- Djian, D.; Alloin, F.; Martinet, S.; Lignier, H.; Sanchez, J.Y. Lithium-ion batteries with high charge rate capacity: Influence of the porous separator. J. Power Sources 2007, 172, 416–421. [Google Scholar] [CrossRef]
- Marino, M.G.; Kreuer, K.D. Alkaline Stability of Quaternary Ammonium Cations for Alkaline Fuel Cell Membranes and Ionic Liquids. ChemSusChem 2015, 8, 513–523. [Google Scholar] [CrossRef]
- Gu, L.; Dong, H.; Sun, Z.; Li, Y.; Yan, F. Spirocyclic quaternary ammonium cations for alkaline anion exchange membrane applications: An experimental and theoretical study. RSC Adv. 2016, 6, 94387–94398. [Google Scholar] [CrossRef]
- Tongwen, X.; Weihua, Y. Fundamental studies of a new series of anion exchange membranes: Membrane preparation and characterization. J. Memb. Sci. 2001, 190, 159–166. [Google Scholar] [CrossRef]
- Xu, T.; Wu, D.; Wu, L. Poly(2,6-dimethyl-1,4-phenylene oxide) (PPO)-A versatile starting polymer for proton conductive membranes (PCMs). Prog. Polym. Sci. 2008, 33, 894–915. [Google Scholar] [CrossRef]
- Sun, C.; Chen, J.; Zhang, H.; Han, X.; Luo, Q. Investigations on transfer of water and vanadium ions across Nafion membrane in an operating vanadium redox flow battery. J. Power Sources 2010, 195, 890–897. [Google Scholar] [CrossRef]
- Choi, N.H.; Del Olmo, D.; Fischer, P.; Pinkwart, K.; Tübke, J. Development of flow fields for zinc slurry air flow batteries. Batteries 2020, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Xu, Y.; Ye, N.; Zhang, D.; Yang, J.; He, R. Alkali Resistant Anion Exchange Membranes Based on Saturated Heterocyclic Quaternary Ammonium Cations Functionalized Poly(2,6-dimethyl-1,4-phenylene oxide)s. J. Electrochem. Soc. 2018, 165, F350–F356. [Google Scholar] [CrossRef]
- Msomi, P.F.; Nonjola, P.; Ndungu, P.G.; Ramonjta, J. Quaternized poly(2.6 dimethyl-1.4 phenylene oxide)/polysulfone blend composite membrane doped with ZnO-nanoparticles for alkaline fuel cells. J. Appl. Polym. Sci. 2018, 135, 45959. [Google Scholar] [CrossRef]
- Zhu, L.; Zimudzi, T.J.; Wang, Y.; Yu, X.; Pan, J.; Han, J.; Kushner, D.I.; Zhuang, L.; Hickner, M.A. Mechanically Robust Anion Exchange Membranes via Long Hydrophilic Cross-Linkers. Macromolecules. 2017, 50, 2329–2337. [Google Scholar] [CrossRef]
- Patel, A.M.; Patel, R.G.; Patel, M.P. Nickel and copper removal study from aqueous solution using new cationic poly[acrylamide/ N,N -DAMB/ N,N -DAPB] super absorbent hydrogel. J. Appl. Polym. Sci. 2011, 119, 2485–2493. [Google Scholar] [CrossRef]

| Membrane | Material | Structure | Pore Size (nm) | Porosity (%) | Thickness (µm) | Ref. |
|---|---|---|---|---|---|---|
| Celgard® 3501 | PP | Monolayer and surfactant-coated | 64 | 55 | 25 | [40] |
| Celgard® 3401 | PP | Monolayer and surfactant-coated | 43 | 41 | 25 | |
| Celgard® 5550 | PP | Laminated and surfactant-coated | 64 | 55 | 70 | |
| Cellophane™ 350 PØØ | Cellulose | Negatively charged | - | - | 86 | |
| PBI® | Polybenzimidazole | - | - | 8 | ||
| Zirfon® | Polysulfone and ZrO2 | Porous composite diaphragm | 150 ± 50 | 55 ± 10 | 500 ± 50 | [41,42] |
| Membrane | Electrolyte Uptake (wt. %) | Percent (%) of Porosity Filled with Electrolyte * | Swelling Degree: |
|---|---|---|---|
| Celgard® 3501 | 98 ± 2 | 76 | 3 |
| Celgard® 3401 | 49 ± 2 | 63 | 3.6 |
| Celgard® 5550 | 113 ± 3 | 82 | 4.1 |
| Cellophane™ 350 PØØ | 129 ± 3 | ** | 3.2 |
| PBI® | 36 ± 0.4 | ** | 1.2 |
| Zirfon® | 51 ± 0.5 | 89 | 3.1 |
| Membrane | D Zn(OH)42− (m2 s−1) | Ref |
|---|---|---|
| Celgard® 3501 | 9.2 × 10−12 | This work |
| Celgard® 3401 | 6.6 × 10−12 | |
| Celgard® 5550 | 1.4 × 10−11 | |
| Cellophane™ 350 PØØ | 1.3 × 10−11 | |
| Zirfon® | 6.6 × 10−11 | |
| PBI® | ND * | |
| Celgard® 3501 | 3.2 × 10−11 | [31] |
| 1.3 × 10−11 | [52] | |
| 9.5 × 10−12 | [53] | |
| Cellophane™ 350 PØØ | 3.8 × 10−12 | [54] |
| 6.7 × 10−12 | [52] | |
| 3.3 × 10−12 | [53] |
| Membrane | Electrolyte Uptake (wt.%) | Ion Conductivity (mS cm−1) |
|---|---|---|
| Celgard® 3501 | 98 ± 2 | 17 ± 2.5 |
| PPO-3.45 +3501 | 55 ± 1.9 | 12 ± 0.9 |
| FAA + 3501 | 46 ± 2.1 | 1 ± 0.7 |
| Membrane | Diffusion Coefficient (m2 s−1) | Ref. |
|---|---|---|
| Celgard® 3501 | 9.2 × 10−12 | This work |
| PPO-3.45 + 3501 | 5.2 × 10−13 | |
| FAA + 3501 | 3.3 × 10−14 | |
| Two Celgard® 3401 coated with Mn(OH)2 | 6.0 × 10−15 | [34] |
| Chemicals | Mass Fraction (wt. %) |
|---|---|
| Zn | 33.8 |
| ZnO | 4 |
| Carbopol | 0.7 |
| KOH + H2O | 61.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsehaye, M.T.; Teklay Gebreslassie, G.; Heon Choi, N.; Milian, D.; Martin, V.; Fischer, P.; Tübke, J.; El Kissi, N.; Donten, M.L.; Alloin, F.; et al. Pristine and Modified Porous Membranes for Zinc Slurry–Air Flow Battery. Molecules 2021, 26, 4062. https://doi.org/10.3390/molecules26134062
Tsehaye MT, Teklay Gebreslassie G, Heon Choi N, Milian D, Martin V, Fischer P, Tübke J, El Kissi N, Donten ML, Alloin F, et al. Pristine and Modified Porous Membranes for Zinc Slurry–Air Flow Battery. Molecules. 2021; 26(13):4062. https://doi.org/10.3390/molecules26134062
Chicago/Turabian StyleTsehaye, Misgina Tilahun, Getachew Teklay Gebreslassie, Nak Heon Choi, Diego Milian, Vincent Martin, Peter Fischer, Jens Tübke, Nadia El Kissi, Mateusz L. Donten, Fannie Alloin, and et al. 2021. "Pristine and Modified Porous Membranes for Zinc Slurry–Air Flow Battery" Molecules 26, no. 13: 4062. https://doi.org/10.3390/molecules26134062
APA StyleTsehaye, M. T., Teklay Gebreslassie, G., Heon Choi, N., Milian, D., Martin, V., Fischer, P., Tübke, J., El Kissi, N., Donten, M. L., Alloin, F., & Iojoiu, C. (2021). Pristine and Modified Porous Membranes for Zinc Slurry–Air Flow Battery. Molecules, 26(13), 4062. https://doi.org/10.3390/molecules26134062