Synthesis and Evaluation of Chalcone-Quinoline Based Molecular Hybrids as Potential Anti-Malarial Agents
Abstract
:1. Introduction
2. Results
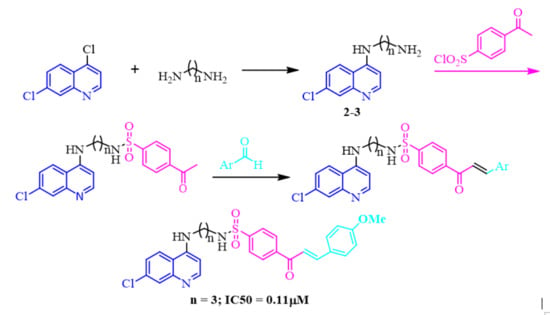
2.1. Chemistry
2.2. In-Vitro Antiplasmodial Activity Evaluation
3. Discussion
4. Materials and Methods
4.1. General
4.2. Experimental Section
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mueller, R.; Reddy, V.; Nchinda, A.T.; Mebrahtu, F.; Taylor, D.; Lawrence, N.; Tanner, L.; Barnabe, M.; Eyermann, C.J.; Zou, B.; et al. Lerisetron Analogues with Antimalarial Properties: Synthesis, Structure–Activity Relationship Studies, and Biological Assessment. ACS Omega 2020, 5, 6967–6982. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.K.; Sharma, R.; Murthy, R.; Bhardwaj, T. Design, synthesis and evaluation of antimalarial potential of polyphosphazene linked combination therapy of primaquine and dihydroartemisinin. Eur. J. Pharm. Sci. 2015, 66, 123–137. [Google Scholar] [CrossRef]
- Rudrapal, M.; Chetia, D. Endoperoxide antimalarials: Development, structural diversity and pharmacodynamic aspects with reference to 1,2,4-trioxane-based structural scaffold. Drug Des. Dev. Ther. 2016, 10, 3575–3590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. The “World Malaria Report 2020” at a Glance. 2020. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2020 (accessed on 25 May 2021).
- WHO. The “World Malaria Report 2019” at a Glance. 2019. Available online: https://www.who.int/news-room/feature-stories/detail/world-malaria-report-2019 (accessed on 25 May 2021).
- Sherman, I.W. Malaria: Parasite Biology, Pathogenesis and Protection, in Malaria: Parasite Biology, Pathogenesis and Protection; ASM Press: Washington, DC, USA, 1998. [Google Scholar]
- Pinheiro, L.C.D.S.; Boechat, N.; Ferreira, M.D.L.G.; Júnior, C.C.; Jesus, A.M.; Leite, M.M.; Souza, N.B.; Krettli, A.U. Anti- Plasmodium falciparum activity of quinoline–sulfonamide hybrids. Bioorgan. Med. Chem. 2015, 23, 5979–5984. [Google Scholar] [CrossRef] [PubMed]
- Guantai, E.M.; Ncokazi, K.; Egan, T.J.; Gut, J.; Rosenthal, P.J.; Smith, P.J.; Chibale, K. Design, synthesis and in vitro antimalarial evaluation of triazole-linked chalcone and dienone hybrid compounds. Bioorgan. Med. Chem. 2010, 18, 8243–8256. [Google Scholar] [CrossRef] [PubMed]
- Smit, F.J.; Van Biljon, R.; Birkholtz, L.-M.; N’Da, D.D. Synthesis and in vitro biological evaluation of dihydroartemisinyl-chalcone esters. Eur. J. Med. Chem. 2015, 90, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Muregi, F.W.; Ishih, A. Next-generation antimalarial drugs: Hybrid molecules as a new strategy in drug design. Drug Dev. Res. 2009, 71, 20–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viegas-Junior, C.; Danuello, A.; da Silva Bolzani, V.; Barreiro, E.J.; Fraga, C.A.M. Molecular Hybridization: A Useful Tool in the Design of New Drug Prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef]
- Vandekerckhove, S.; D’Hooghe, M. Quinoline-based antimalarial hybrid compounds. Bioorgan. Med. Chem. 2015, 23, 5098–5119. [Google Scholar] [CrossRef]
- Lopez, S.N.; Castelli, M.V.; Zacchino, S.A.; Domınguez, J.N.; Lobo, G.; Charris-Charris, J.; Cortes, J.G.; Ribas, J.C.; Devia, C.; Rodrıguez, A.M.; et al. In vitro antifungal evaluation and structure–activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorgan. Med. Chem. 2001, 9, 1999–2013. [Google Scholar] [CrossRef]
- Konieczny, M.T.; Horowska, B.; Kunikowski, A.; Konopa, J.; Wierzba, K.; Yamada, Y.; Asao, T. Synthesis of Polyhydroxylated Derivatives of Phenyl Vinyl Sulfone as Structural Analogs of Chalcones. Synthesis 2001, 2001, 1363–1367. [Google Scholar] [CrossRef]
- Go, M.L.; Wu, X.; Liu, X.L. Chalcones: An Update on Cytotoxic and Chemoprotective Properties. Curr. Med. Chem. 2005, 12, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Barfod, L.; Kemp, K.; Hansen, M.; Kharazmi, A. Chalcones from Chinese liquorice inhibit proliferation of T cells and production of cytokines. Int. Immunopharmacol. 2002, 2, 545–555. [Google Scholar] [CrossRef]
- Tomar, V.; Bhattacharjee, G.; Kamaluddin; Rajakumar, S.; Srivastava, K.; Puri, S. Synthesis of new chalcone derivatives containing acridinyl moiety with potential antimalarial activity. Eur. J. Med. Chem. 2010, 45, 745–751. [Google Scholar] [CrossRef]
- Ducki, S.; Forrest, R.; Hadfield, J.A.; Kendall, A.; Lawrence, N.J.; McGown, A.T.; Rennison, D. Potent antimitotic and cell growth inhibitory properties of substituted chalcones. Bioorgan. Med. Chem. Lett. 1998, 8, 1051–1056. [Google Scholar] [CrossRef]
- Parmar, V.S.; Sharma, N.K.; Husain, M.; Watterson, A.C.; Kumar, J.; A Samuelson, L.; Cholli, A.L.; Prasad, A.K.; Malhotra, S.; Kumar, N.; et al. Synthesis, characterization and in vitro anti-invasive activity screening of polyphenolic and heterocyclic compounds. Bioorgan. Med. Chem. 2003, 11, 913–929. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kumar, V.; Prasad, A.K.; Raj, H.G.; Bracke, M.E.; Olsen, C.E.; Jain, S.C.; Parmar, V.S. Synthetic and biological activity evaluation studies on novel 1,3-diarylpropenones. Bioorgan. Med. Chem. 2001, 9, 337–345. [Google Scholar] [CrossRef]
- Hsieh, H.-K.; Tsao, L.-T.; Wang, J.-P.; Lin, C.-N. Synthesis and Anti-inflammatory Effect of Chalcones. J. Pharm. Pharmacol. 2010, 52, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.; Lee, T.; Wang, J.; Wang, J.; Lin, C. Synthesis and Anti-inflammatory Effect of Chalcones and Related Compounds. Pharm. Res. 1998, 15, 39–46. [Google Scholar] [CrossRef]
- Liu, M.; Wilairat, P.; Go, M.-L. Antimalarial Alkoxylated and Hydroxylated Chalones: Structure−Activity Relationship Analysis. J. Med. Chem. 2001, 44, 4443–4452. [Google Scholar] [CrossRef]
- Gutteridge, C.E.; Nichols, D.A.; Curtis, S.M.; Thota, D.S.; Vo, J.V.; Gerena, L.; Montip, G.; Asher, C.O.; Diaz, D.S.; DiTusa, C.A.; et al. In vitro and in vivo efficacy and in vitro metabolism of 1-phenyl-3-aryl-2-propen-1-ones against Plasmodium falciparum. Bioorgan. Med. Chem. Lett. 2006, 16, 5682–5686. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Dixit, S.K.; Bhattacharya, A.; Mishra, L.C.; Sharma, M.; Awasthi, S.K.; Bhasin, V.K. Antimalarial Activity of Newly Synthesized Chalcone Derivatives In Vitro. Chem. Biol. Drug Des. 2012, 80, 340–347. [Google Scholar] [CrossRef]
- Bondock, S.; Gieman, H.; El-Shafei, A. Selective synthesis, structural studies and antitumor evaluation of some novel unsymmetrical 1-hetaryl-4-(2-chloroquinolin-3-yl)azines. J. Saudi Chem. Soc. 2016, 20, 695–702. [Google Scholar] [CrossRef] [Green Version]
- Aminake, M.N.; Mahajan, A.; Kumar, V.; Hans, R.; Wiesner, L.; Taylor, D.; de Kock, C.; Grobler, A.; Smith, P.J.; Kirschner, M.; et al. Synthesis and evaluation of hybrid drugs for a potential HIV/AIDS-malaria combination therapy. Bioorgan. Med. Chem. 2012, 20, 5277–5289. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; Coughlan, D.; Heneghan, N.; Gaynor, C.; Bell, A. A novel artemisinin–quinine hybrid with potent antimalarial activity. Bioorgan. Med. Chem. Lett. 2007, 17, 3599–3602. [Google Scholar] [CrossRef]
- Nqoro, X.; Tobeka, N.; Aderibigbe, B.A. Quinoline-Based Hybrid Compounds with Antimalarial Activity. Molecules 2017, 22, 2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, S.; Deshpande, S.; Pandey, S.; Agarwal, P.; Saxena, J.K.; Srivastava, K.; Chauhan, P.M.S.; Prabhakar, Y.S. N-(7-Chloroquinolinyl-4-aminoalkyl)arylsulfonamides as antimalarial agents: Rationale for the activity with reference to inhibition of hemozoin formation. RSC Adv. 2016, 6, 25584–25593. [Google Scholar] [CrossRef]
- Soares, R.R.; da Silva, J.M.F.; Carlos, B.C.; da Fonseca, C.C.; de Souza, L.S.A.; Lopes, F.V.; Dias, R.M.D.P.; Moreira, P.O.L.; Abramo, C.; Viana, G.H.R.; et al. New quinoline derivatives demonstrate a promising antimalarial activity against Plasmodium falciparum in vitro and Plasmodium berghei in vivo. Bioorgan. Med. Chem. Lett. 2015, 25, 2308–2313. [Google Scholar] [CrossRef]
- Fisher, G.M.; Tanpure, R.P.; Douchez, A.; Andrews, K.T.; Poulsen, S.-A. Synthesis and Evaluation of Antimalarial Properties of Novel 4-Aminoquinoline Hybrid Compounds. Chem. Biol. Drug Des. 2014, 84, 462–472. [Google Scholar] [CrossRef]
- Salahuddin, A.; Inam, A.; Van Zyl, R.; Heslop, D.C.; Chen, C.-T.; Avecilla, F.; Agarwal, S.M.; Azam, A. Synthesis and evaluation of 7-chloro-4-(piperazin-1-yl)quinoline-sulfonamide as hybrid antiprotozoal agents. Bioorgan. Med. Chem. 2013, 21, 3080–3089. [Google Scholar] [CrossRef]
- Pandey, S.; Agarwal, P.; Srivastava, K.; Rajakumar, S.; Puri, S.K.; Verma, P.; Saxena, J.; Sharma, A.; Lal, J.; Chauhan, P.M. Synthesis and bioevaluation of novel 4-aminoquinoline-tetrazole derivatives as potent antimalarial agents. Eur. J. Med. Chem. 2013, 66, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Hashimoto, Y.J. Improvement in Aqueous Solubility in Small Molecule Drug Discovery Programs by Disruption of Molecular Planarity and Symmetry. Med. Chem. 2011, 54, 1539–1554. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, L.; Tilton, S.; Wang, J. Development of a high throughput equilibrium solubility assay using miniaturized shake-flask method in early drug discovery. J. Pharm. Sci. 2007, 96, 3052–3071. [Google Scholar] [CrossRef] [PubMed]
- Makler, M.T.; Piper, R.; Williams, J.A.; Gibbins, B.L.; Hinrichs, D.J.; Ries, J.M.; Bancroft, J.E. Parasite Lactate Dehydrogenase as an Assay for Plasmodium falciparum Drug Sensitivity. Am. J. Trop. Med. Hyg. 1993, 48, 739–741. [Google Scholar] [CrossRef] [PubMed]



 | |||
|---|---|---|---|
| Compound | n | Ar | Yield (%) |
| 4 | 2 | - | 29 |
| 5 | 3 | - | 41 |
| 6 | 3 | Ph | 72 |
| 7 | 3 | 4-Me-Ph | 59 |
| 8 | 3 | 2-Br-Ph | 78 |
| 9 | 3 | 4-F-Ph | 61 |
| 10 | 3 | 2,6-F2-Ph | 70 |
| 11 | 3 | 4-Cl-Ph | 35 |
| 12 | 3 | 4-OMe-Ph | 20 |
| 13 | 3 | 3,4-F2-Ph | 65 |
| 14 | 3 | 3,4-(OMe)2-Ph | 11 |
| 15 | 3 | 2-F-Ph | 66 |
| 16 | 3 | 3,4,5-(OMe)3-Ph | 76 |
| 17 | 2 | 4-OMe-Ph | 89 |
| 18 | 3 | 2,4-F2-Ph | 38 |
| 19 | 2 | 3,4-(OMe)2-Ph | 45 |
| 20 | 3 | 2,5-F2-Ph | 66 |
| 21 | 3 | 2-furanyl | 50 |
| 22 | 2 | 4-Me-Ph | 56 |
| 23 | 3 | ferrocenyl | 31 |
| 24 | 2 | 4-F-Ph | 71 |
| 25 | 2 | 2-Cl-Ph | 39 |
| 26 | 3 | 3,4-OCH2O-Ph | 66 |
| 27 | 3 | 4-OCF3-Ph | 96 |
 | ||||||
|---|---|---|---|---|---|---|
| Compound | n | Ar | PfNF54 IC50 (μM) a | Solubility b (μM) pH 6.5 | CHO IC50 (μM) c | SI d |
| Chloroquine | - | - | 0.011 | - | - | |
| Emetine | 0.0034 | |||||
| 4 | 2 | - | 0.45 | 200 | 26.32 | 58.4 |
| 5 | 3 | - | 0.48 | <5 | >50 | >104 |
| 6 | 3 | Ph | 1.67 | ND | >50 | >29 |
| 7 | 3 | 4-Me-Ph | 0.37 | 130 | 25.08 | 67.8 |
| 8 | 3 | 2-Br-Ph | 0.10 | ND | 43.25 | 435 |
| 9 | 3 | 4-F-Ph | 0.28 | <5 | 14.65 | 52.5 |
| 10 | 3 | 2,6-F2-Ph | 0.54 | <5 | 27.62 | 51.1 |
| 11 | 3 | 4-Cl-Ph | 0.10 | ND | 41.2 | 416.2 |
| 12 | 3 | 4-OMe-Ph | 0.11 | ND | 43.3 | 393.6 |
| 13 | 3 | 3,4-F2-Ph | 2.58 | <5 | >300 | >116 |
| 14 | 3 | 3,4-(OMe)2-Ph | 0.32 | ND | 43.4 | 135.6 |
| 15 | 3 | 2-F-Ph | 0.86 | 5 | 45.53 | 52.9 |
| 16 | 3 | 2,3,4-(OMe)3-Ph | 0.58 | 15 | 22.62 | 39.0 |
| 17 | 2 | 4-OMe-Ph | 0.49 | 185 | 17.53 | 35.7 |
| 18 | 3 | 2,4-F2-Ph | 0.50 | 130 | 22.44 | 45.2 |
| 19 | 2 | 3,4-(OMe)2-Ph | 0.33 | ND | 45.1 | 136.7 |
| 20 | 3 | 2,5-F2-Ph | 0.79 | 43.7 | 55.3 | |
| 21 | 3 | 2-furanyl | 4.45 | 200 | >50 | >11 |
| 22 | 2 | 4-Me-Ph | 0.39 | 190 | 24.89 | 63.8 |
| 23 | 3 | ferrocenyl | 1.53 | ND | ND | |
| 24 | 2 | 4-F-Ph | 0.57 | <5 | 14.54 | 25.5 |
| 25 | 2 | 2-Cl-Ph | 0.37 | 19 | 35.41 | 95.7 |
| 26 | 3 | 3,4-OCH2O-Ph | 0.69 | 200 | 34.13 | 49.5 |
| 27 | 3 | 4-OCF3-Ph | 0.51 | ND | 44.04 | 86.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinindwa, B.; Dziwornu, G.A.; Masamba, W. Synthesis and Evaluation of Chalcone-Quinoline Based Molecular Hybrids as Potential Anti-Malarial Agents. Molecules 2021, 26, 4093. https://doi.org/10.3390/molecules26134093
Vinindwa B, Dziwornu GA, Masamba W. Synthesis and Evaluation of Chalcone-Quinoline Based Molecular Hybrids as Potential Anti-Malarial Agents. Molecules. 2021; 26(13):4093. https://doi.org/10.3390/molecules26134093
Chicago/Turabian StyleVinindwa, Bonani, Godwin Akpeko Dziwornu, and Wayiza Masamba. 2021. "Synthesis and Evaluation of Chalcone-Quinoline Based Molecular Hybrids as Potential Anti-Malarial Agents" Molecules 26, no. 13: 4093. https://doi.org/10.3390/molecules26134093
APA StyleVinindwa, B., Dziwornu, G. A., & Masamba, W. (2021). Synthesis and Evaluation of Chalcone-Quinoline Based Molecular Hybrids as Potential Anti-Malarial Agents. Molecules, 26(13), 4093. https://doi.org/10.3390/molecules26134093