Melatonin, Its Metabolites and Their Interference with Reactive Nitrogen Compounds
Abstract
:1. Introduction
2. Nitric Oxide, Its Congeners and Reactive Products
2.1. Formation of NO Redox Congeners
2.2. Nitric Oxide by Reduction of Nitrate and Nitrite: External Sources and Metabolite Recycling
2.3. Properties of the NO Congeners
2.4. Other NO-Derived Reactive Nitrogen Species and Generation of Secondary Radicals
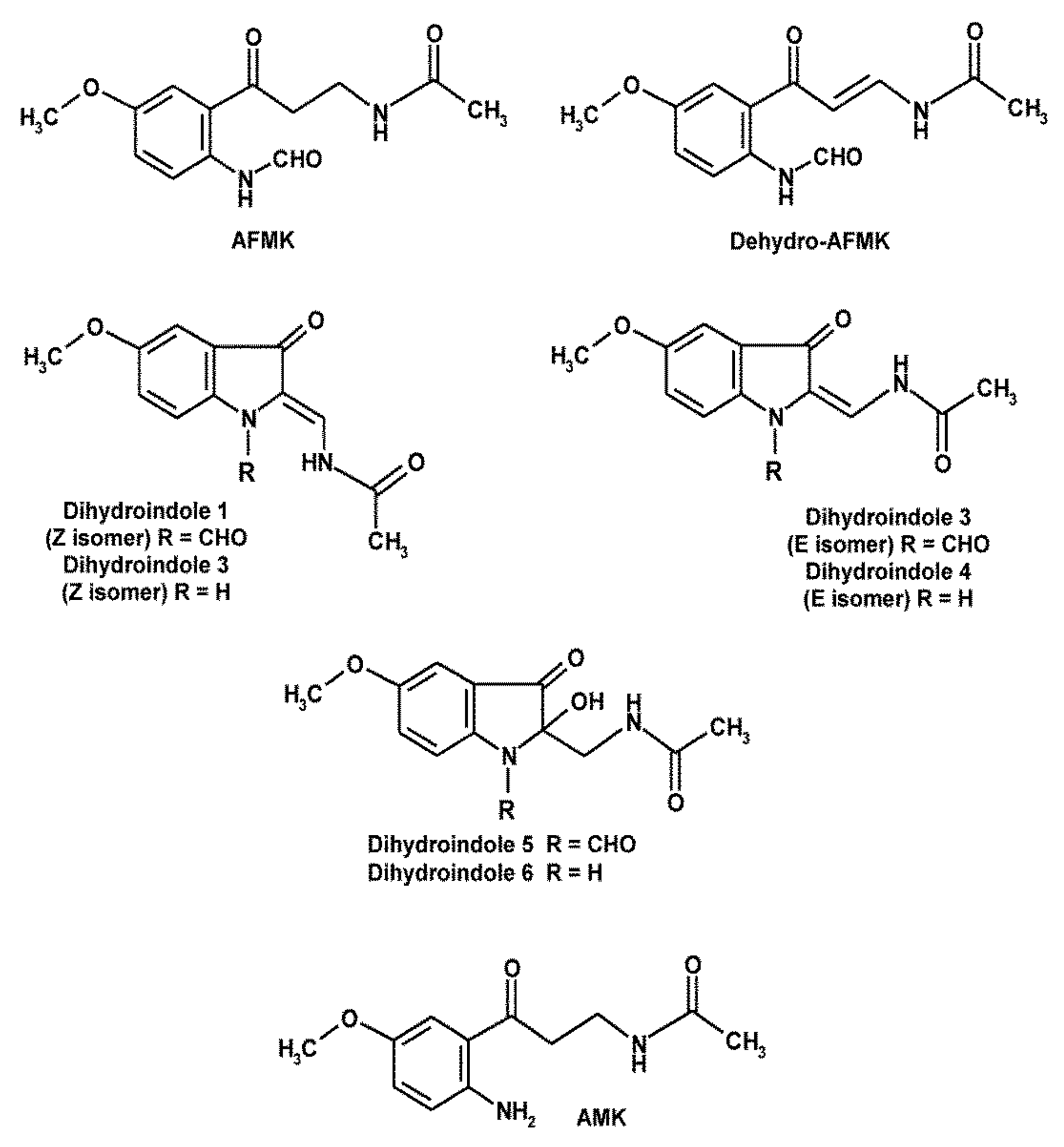
3. Melatonin’s Oxidatively Formed Metabolites
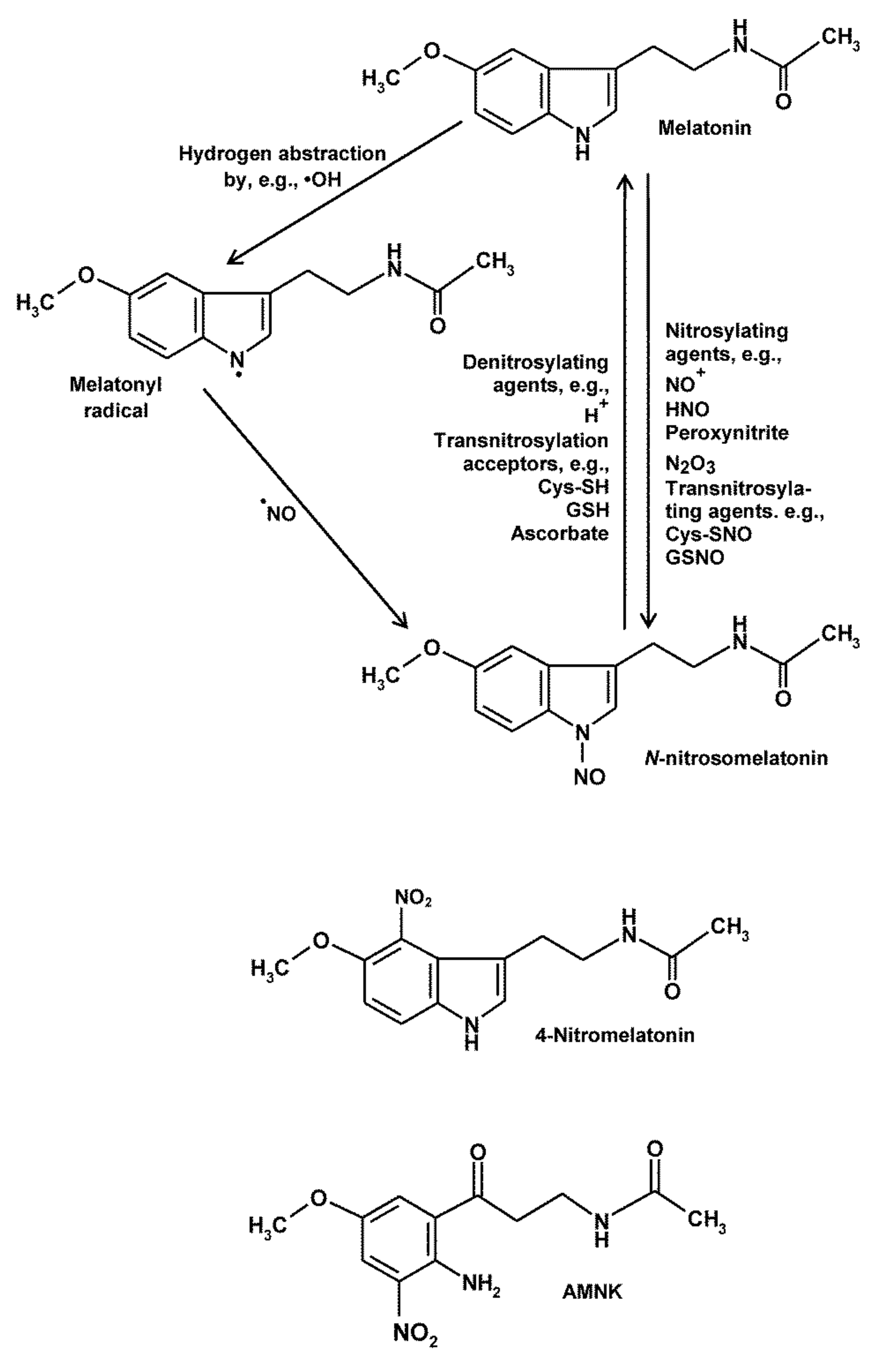
4. Nitrosylation, Nitration and RNS-Mediated Oxidation of Melatonin and Its Metabolites
5. Peculiarities of AMK
6. Pathophysiological Relevance of Actions against Nitration, RNS-Mediated Oxidation and Nitrosylation
7. Consequences to Inflammation and Mitochondrial Integrity
8. Conclusions
Funding
Conflicts of Interest
References
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef] [Green Version]
- Pandi-Perumal, S.R.; Srinivasan, V.; Maestroni, G.J.M.; Cardinali, D.P.; Poeggeler, B.; Hardeland, R. Melatonin—Nature’s most versatile biological signal? FEBS J. 2006, 273, 2813–2838. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Divergent importance of chronobiological considerations in high- and low-dose melatonin therapies. Diseases 2021, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintela, T.; Gonçalves, I.; Silva, M.; Duarte, A.C.; Guedes, P.; Andrade, K.; Freitas, F.; Talhada, D.; Albuquerque, T.; Tavares, S.; et al. Choroid plexus is an additional source of melatonin in the brain. J. Pineal Res. 2018, 65, e12528. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuña-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 2013, 54, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Chen, L.-D.; Poeggeler, B.; Manchester, L.C.; Reiter, R.J. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr. J. 1993, 1, 57–60. [Google Scholar]
- Tan, D.-X.; Reiter, R.J.; Manchester, L.C.; Yan, M.T.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, R.J.; Tan, D.-X.; Mayo, J.C.; Sainz, R.M.; Leon, J.; Czarnocki, Z. Melatonin as an antioxidant: Biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003, 50, 1129–1146. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Brown, G.M.; Spence, D.W.; Bharti, V.K.; Kaur, C.; Hardeland, R.; Cardinali, D.P. Melatonin antioxidative defense: Therapeutical implications for aging and neurodegenerative processes. Neurotox. Res. 2013, 23, 267–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Entrena, A.; Camacho, M.E.; Carrión, M.D.; López-Cara, L.C.; Velasco, G.; León, J.; Escames, G.; Acuña-Castroviejo, D.; Tapias, V.; Gallo, M.A.; et al. Kynurenamines as neural nitric oxide synthase inhibitors. J. Med. Chem. 2005, 48, 8174–8181. [Google Scholar] [CrossRef]
- León, J.; Escames, G.; Rodríguez, M.I.; López, L.C.; Tapias, V.; Entrena, A.; Camacho, E.; Carrión, M.D.; Gallo, M.A.; Espinosa, A.; et al. Inhibition of neuronal nitric oxide synthase activity by N1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J. Neurochem. 2006, 98, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; López, L.C.; Ortíz, F.; Ros, E.; Acuña-Castroviejo, D. Age-dependent lipopolysaccharide-induced iNOS expression and multiorgan failure in rats: Effects of melatonin treatment. Exp. Gerontol. 2006, 41, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; López, L.C.; Tapias, V.; Utrilla, P.; Reiter, R.J.; Hitos, A.B.; León, J.; Rodríguez, M.I.; Acuña-Castroviejo, D. Melatonin counteracts inducible mitochondrial nitric oxide synthase-dependent mitochondrial dysfunction in skeletal muscle of septic mice. J. Pineal Res. 2006, 40, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Pandi-Perumal, S.R.; Cardinali, D.P.; Poeggeler, B.; Hardeland, R. Melatonin in Alzheimer’s disease and other neurodegenerative disorders. Behav. Brain Funct. 2006, 2, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escames, G.; López, L.C.; Ortíz, F.; López, A.; García, J.A.; Ros, E.; Acuña-Castroviejo, D. Attenuation of cardiac mitochondrial dysfunction by melatonin in septic mice. FEBS J. 2007, 274, 2135–2147. [Google Scholar] [CrossRef]
- Hardeland, R. Neuroprotection by radical avoidance: Search for suitable agents. Molecules 2009, 14, 5054–5102. [Google Scholar] [CrossRef]
- Hardeland, R.; Coto-Montes, A. New vistas on oxidative damage and aging. Open Biol. J. 2010, 3, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R.; Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin and brain inflammaging. Prog. Neurobiol. 2015, 127, 46–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardeland, R. Melatonin and inflammation—Story of a double-edged blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R. Melatonin, mitochondrial electron flux and leakage: Recent findings and resolution of contradictory results. Adv. Stud. Biol. 2009, 1, 207–230. [Google Scholar]
- Kim, T.-K.; Lin, Z.; Tidwell, W.J.; Li, W.; Slominski, A.T. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol. Cell. Endocrinol. 2015, 404, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.-K.; Lin, Z.; Li, W.; Reiter, R.J.; Slominski, A.T. N1-Acetyl-5-Methoxykynuramine (AMK) is produced in the human epidermis and shows antiproliferative effects. Endocrinology 2015, 156, 1630–1636. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Semak, I.; Fischer, T.W.; Kim, T.-K.; Kleszczyński, K.; Hardeland, R.; Reiter, R.J. Metabolism of melatonin in the skin: Why is it important? Exp. Dermatol. 2017, 26, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.L.; Schmidt, S.I.; Laatsch, H.; Fotso, S.; Ness, H.; Ressmeyer, A.-R.; Poeggeler, B.; Hardeland, R. Reactions of the melatonin metabolite AMK (N1-acetyl-5-methoxykynuramine) with reactive nitrogen species: Formation of novel compounds, 3-acet-amidomethyl-6-methoxycinnolinone and 3-nitro-AMK. J. Pineal Res. 2005, 39, 251–260. [Google Scholar] [CrossRef]
- Rosen, J.; Than, N.N.; Koch, D.; Poeggeler, B.; Laatsch, H.; Hardeland, R. Interactions of melatonin and its metabolites with the ABTS cation radical: Extension of the radical scavenger cascade and formation of a novel class of oxidation products, C2-substituted 3-indolinones. J. Pineal Res. 2006, 41, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin, hormone of darkness and more: Occurrence, control mechanisms, actions and bioactive metabolites. Cell. Mol. Life Sci. 2008, 65, 2001–2018. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R.; Tan, D.-X.; Reiter, R.J. Kynuramines, metabolites of melatonin and other indoles: The resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 2009, 47, 109–126. [Google Scholar] [CrossRef]
- Omar, S.A.; Webb, A.J. Nitrite reduction and cardiovascular protection. J. Mol. Cell. Cardiol. 2014, 73, 57–69. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Q.; Zhu, D. An update on hydrogen sulfide and nitric oxide interactions in the cardiovascular system. Oxid. Med. Cell. Longev. 2018, 2018, 4579140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; Von Schwarz, E.R. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.R.; Flitney, F.W.; Williams, D.L.H. NO, nitrosonium ions, nitroxide ions and iron-nitrosyls in biology: A chemist’s perspective. Trends Pharmacol. Sci. 1995, 16, 18–22. [Google Scholar] [CrossRef]
- Hardeland, R.; Backhaus, C.; Fadavi, A. Reactions of the NO redox forms NO+, •NO and HNO (protonated NO−) with the melatonin metabolite N1-acetyl-5-methoxykynuramine. J. Pineal Res. 2007, 43, 382–388. [Google Scholar] [CrossRef]
- Nagababu, E.; Ramasamy, S.; Abernethy, D.R.; Rifkind, J.M. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J. Biol. Chem. 2003, 278, 46349–46356. [Google Scholar] [CrossRef] [Green Version]
- Kashiwagi, S.; Kajimura, M.; Yoshimura, Y.; Suematsu, M. Nonendothelial source of nitric oxide in arterioles but not in venules: Alternative source revealed in vivo by diaminofluorescein microfluorography. Circ. Res. 2002, 91, e55–e64. [Google Scholar] [CrossRef] [Green Version]
- Filipović, M.R.; Stanić, D.; Raicević, S.; Spasić, M.; Niketić, V. Consequences of MnSOD interactions with nitric oxide: Nitric oxide dismutation and the generation of peroxynitrite and hydrogen peroxide. Free Radic. Res. 2007, 41, 62–72. [Google Scholar] [CrossRef]
- Fukuto, J.M. A recent history of nitroxyl chemistry, pharmacology and therapeutic potential. Br. J. Pharmacol. 2019, 176, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Cheong, E.; Tunbev, V.; Abramson, J.; Salama, G.; Stoyanovsky, D.A. Nitroxyl triggers Ca2+ release from skeletal and cardiac sarcoplasmic reticulum by oxidizing ryanodine receptors. Cell Calcium 2005, 37, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Tocchetti, C.G.; Wang, W.; Froehlich, J.P.; Huke, S.; Aon, M.A.; Wilson, G.M.; Di Benedetto, G.; O’Rourke, B.; Gao, W.D.; Wink, D.A.; et al. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ. Res. 2007, 100, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Jon, O.; Lundberg, J.O.; Weitzberg, E.; Cole, J.A.; Benjamin, N. Nitrate, bacteria and human health. Nat. Rev. Microbiol. 2004, 2, 593–602. [Google Scholar]
- Hobbs, D.A.; Goulding, M.G.; Nguyen, A.; Malaver, T.; Walker, C.F.; George, T.W.; Methven, L.; Lovegrove, J.A. Acute ingestion of beetroot bread increases endothelium-independent vasodilation and lowers diastolic blood pressure in healthy men: A randomized controlled trial. J. Nutr. 2013, 143, 1399–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira-Paula, G.H.; Pinheiro, L.C.; Tanus-Santos, J.E. Mechanisms impairing blood pressure responses to nitrite and nitrate. Nitric Oxide 2019, 85, 35–43. [Google Scholar] [CrossRef]
- Jansson, E.A.; Huang, L.; Malkey, R.; Govoni, M.; Nihlén, C.; Olsson, A.; Stensdotter, M.; Petersson, J.; Holm, L.; Weitzberg, E.; et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat. Chem. Biol. 2008, 4, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Fini, H.; Kerman, K. Revisiting the nitrite reductase activity of hemoglobin with differential pulse voltammetry. Anal. Chim. Acta 2020, 1104, 38–46. [Google Scholar] [CrossRef]
- Lim, Y.J.; Foo, T.C.; Yeung, A.W.S.; Tu, X.; Ma, Y.; Hawkins, C.L.; Witting, P.K.; Jameson, G.N.L.; Terentis, A.C.; Thomas, S.R. Human Indoleamine 2,3-Dioxygenase 1 Is an Efficient Mammalian Nitrite Reductase. Biochemistry 2019, 58, 974–986. [Google Scholar] [CrossRef]
- Gherasim, C.; Yadav, P.K.; Kabil, O.; Niu, W.-N.; Banerjee, R. Nitrite reductase activity and inhibition of H2S biogenesis by human cystathionine β-synthase. PLoS ONE 2014, 9, e85544. [Google Scholar] [CrossRef] [Green Version]
- Erdal, S. Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant. Cell Rep. 2019, 38, 1001–1012. [Google Scholar] [CrossRef]
- Gattullo, D.; Pagliaro, P.; Marsh, N.A.; Losano, G. New insights into nitric oxide and coronary circulation. Life Sci. 1999, 65, 2167–2174. [Google Scholar] [CrossRef]
- Cherian, L.; Hlatky, R.; Robertson, C.S. Nitric oxide in traumatic brain injury. Brain Pathol. 2004, 14, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Baylis, C. Changes in renal hemodynamics and structure in the aging kidney; sexual dimorphism and the nitric oxide system. Exp. Gerontol. 2005, 40, 271–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mónica, F.Z.; Bian, K.; Murad, F. The endothelium-dependent nitric oxide-cGMP pathway. Adv. Pharmacol. 2016, 77, 1–27. [Google Scholar] [PubMed]
- Hardeland, R. Melatonin and the theories of aging: A critical appraisal of melatonin’s role in antiaging mechanisms. J. Pineal Res. 2013, 55, 325–356. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Kayahara, M.; Joashi, U.; Mazarakis, N.D.; Sarraf, C.; Edwards, A.D.; Hughes, M.N.; Mehmet, H. Differential induction of apoptosis in Swiss 3T3 cells by nitric oxide and the nitrosonium cation. J. Cell Sci. 1997, 110, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Kayahara, M.; Felderhoff, U.; Pocock, J.; Hughes, M.N.; Mehmet, H. Nitric oxide (NO•) and the nitrosonium cation (NO+) reduce mitochondrial membrane potential and trigger apoptosis in neuronal PC12 cells. Biochem. Soc. Trans. 1998, 26, S340. [Google Scholar] [CrossRef]
- Kim, S.; Ponka, P. Role of nitric oxide in cellular iron metabolism. Biometals 2003, 16, 125–135. [Google Scholar] [CrossRef]
- Mikhael, M.; Kim, S.F.; Schranzhofer, M.; Soe-Lin, S.; Sheftel, A.D.; Mullner, E.W.; Ponka, P. Iron regulatory protein-independent regulation of ferritin synthesis by nitrogen monoxide. FEBS J. 2006, 273, 3828–3836. [Google Scholar] [CrossRef]
- Wink, D.A.; Miranda, K.M.; Katori, T.; Mancardi, D.; Thomas, D.D.; Ridnour, L.; Espey, M.G.; Feelisch, M.; Colton, C.A.; Fukuto, J.M.; et al. Orthogonal properties of the redox siblings nitroxyl and nitric oxide in the cardiovascular system: A novel redox paradigm. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2264–H2276. [Google Scholar] [CrossRef] [Green Version]
- Paolocci, N.; Katori, T.; Champion, H.C.; St John, M.E.; Miranda, K.M.; Fukuto, J.M.; Wink, D.A.; Kass, D.A. Positive inotropic and lusitropic effects of HNO/NO- in failing hearts: Independence from beta-adrenergic signaling. Proc. Natl. Acad. Sci. USA 2003, 100, 5537–5542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagliaro, P.; Mancardi, D.; Rastaldo, R.; Penna, C.; Gattullo, D.; Miranda, K.M.; Feelisch, M.; Wink, D.A.; Kass, D.A.; Paolocci, N. Nitroxyl affords thiol-sensitive myocardial protective effects akin to early preconditioning. Free Radic. Biol. Med. 2003, 34, 33–43. [Google Scholar] [CrossRef]
- Miranda, K.M.; Paolocci, N.; Katori, T.; Thomas, D.D.; Ford, E.; Bartberger, M.D.; Espey, M.G.; Kass, D.A.; Feelisch, M.; Fukuto, J.M.; et al. A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc. Natl. Acad. Sci. USA 2003, 100, 9196–9201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wink, D.A.; Feelisch, M.; Fukuto, J.; Chistodoulou, D.; Jourd’heuil, D.; Grisham, M.B.; Vodovotz, Y.; Cook, J.A.; Krishna, M.; DeGraff, W.G.; et al. The cytotoxicity of nitroxyl: Possible implications for the pathophysiological role of NO. Arch. Biochem. Biophys. 1998, 351, 66–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiva, S.; Crawford, J.H.; Ramachandran, A.; Ceaser, E.K.; Hillson, T.; Brookes, P.S.; Patel, R.P.; Darley-Usmar, V.M. Mechanisms of the interaction of nitroxyl with mitochondria. Biochem. J. 2004, 379, 359–366. [Google Scholar] [CrossRef]
- Sharpe, M.A.; Cooper, C.E. Reactions of nitric oxide with mitochondrial cytochrome c: A novel mechanism for the formation of nitroxyl anion and peroxynitrite. Biochem. J. 1998, 332, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Zhang, X.; He, N.; Wang, Y.; Kang, Q.; Shen, D.; Yu, F.; Chen, L. Imaging of anti-inflammatory effects of HNO via a near-infrared fluorescent probe in cells and in rat gouty arthritis model. J. Mater. Chem. B 2019, 7, 305–313. [Google Scholar] [CrossRef]
- Peyrot, F.; Houée-Levin, C.; Ducrocq, C. Melatonin nitrosation promoted by NO*2; comparison with the peroxynitrite reaction. Free Radic. Res. 2006, 40, 910–920. [Google Scholar] [CrossRef]
- Kirsch, M.; Groot, H. Detection of N-nitrosomelatonin and other N-nitrosotryptophan derivatives by transnitrosation of APF and DAF-2. J. Pineal Res. 2006, 40, 10–17. [Google Scholar] [CrossRef]
- Nedospasov, A.A. Is N2O3 the main nitrosating intermediate in aerated nitric oxide (NO) solutions in vivo? If so, where, when, and which one? J. Biochem. Mol. Toxicol. 2002, 16, 109–120. [Google Scholar] [CrossRef]
- Basu, S.; Grubina, R.; Huang, J.; Conradie, J.; Huang, Z.; Jeffers, A.; Jiang, A.; He, X.; Azarov, I.; Seibert, R.; et al. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat. Chem. Biol. 2007, 3, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Goetz, B.I.; Shields, H.W.; Basu, S.; Wang, P.; King, S.B.; Hogg, N.; Gladwin, M.T.; Kim-Shapiro, D.B. An electron paramagnetic resonance study of the affinity of nitrite for methemoglobin. Nitric Oxide 2010, 22, 149–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardeland, R. Melatonin and the electron transport chain. Cell. Mol. Life Sci. 2017, 74, 3883–3896. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. The underrated carbonate radical (CO3•−)—Detoxification and reduced formation by melatonin. Biomed. J. Sci. Tech. Res. 2017, 1, 264. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin metabolism in the central nervous system. Curr. Neuropharmacol. 2010, 8, 168–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardeland, R. Taxon- and site-specific melatonin catabolism. Molecules 2017, 22, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, D.-X.; Hardeland, R.; Back, K.; Manchester, L.C.; Alatorre-Jimenez, M.A.; Reiter, R.J. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: Comparisons across species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.-X.; Manchester, L.C.; Reiter, R.J.; Plummer, B.F.; Hardies, L.J.; Weintraub, S.T.; Shepherd, A.M. A novel melatonin metabolite, cyclic 3-hydroxymelatonin: A biomarker of in vivo hydroxyl radical generation. Biochem. Biophys. Res. Commun. 1998, 253, 614–620. [Google Scholar] [CrossRef]
- Hardeland, R.; Poeggeler, B. Melatonin beyond its classical functions. Open Physiol. J. 2008, 1, 1–23. [Google Scholar] [CrossRef]
- Pähkla, R.; Zilmer, M.; Kullisaar, T.; Rägo, L. Comparison of the antioxidant activity of melatonin and pinoline in vitro. J. Pineal Res. 1998, 24, 96–101. [Google Scholar] [CrossRef]
- Dose, A.; Poeggeler, P.; Schoenke, M.; Zsizsik, B.K.; Hardeland, R. Pinoline [6-methoxy-1,2,3,4-tetrahydro-9H-pyrido-(3,4-b)-indole] as a radical scavenger. I. Scavenging properties and multiplicity of products. In Actions and Redox Properties of Melatonin and Other Aromatic Amino Acid Metabolites; Hardeland, R., Ed.; Cuvillier: Göttingen, Germany, 2001; pp. 101–106. [Google Scholar]
- Thuermann, S.; Hardeland, R.; Poeggeler, B. Pinoline [6-methoxy-1,2,3,4-tetra-hydro-9H-pyrido-(3,4-b)-indole] as a radical scavenger. II. Chemiluminescence during oxidation. In Actions and Redox Properties of Melatonin and Other Aromatic Amino Acid Metabolites; Hardeland, R., Ed.; Cuvillier: Göttingen, Germany, 2001; pp. 107–113. [Google Scholar]
- Piñol-Ripoll, G.; Fuentes-Broto, L.; Millán-Plano, S.; Reyes-Gonzáles, M.; Mauri, J.A.; Martínez-Ballarín, E.; Reiter, R.J.; García, J.J. Protective effect of melatonin and pinoline on nitric oxide-induced lipid and protein peroxidation in rat brain homogenates. Neurosci. Lett. 2006, 405, 89–93. [Google Scholar] [CrossRef]
- Diem, S.; Gutsche, B.; Herderich, M. Degradation of tetrahydro-β-carbolines in the presence of nitrite: HPLC-MS analysis of the reaction products. J. Agric. Food Chem. 2001, 49, 5993–5998. [Google Scholar] [CrossRef]
- Herraiz, T.; Galisteo, J. Nitrosative deamination of 2′-deoxyguanosine and DNA by nitrite, and antinitrosating activity of β-carboline alkaloids and antioxidants. Food Chem. Toxicol. 2018, 112, 282–289. [Google Scholar] [CrossRef]
- Burkhardt, S.; Reiter, R.J.; Tan, D.-X.; Hardeland, R.; Cabrera, J.; Karbownik, M. DNA oxidatively damaged by chromium(III) and H2O2 is protected by melatonin, N1-acetyl-N2-formyl-5-methoxykynuramine, resveratrol and uric acid. Int. J. Biochem. Cell Biol. 2001, 33, 775–783. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.-X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Ressmeyer, A.-R.; Mayo, J.C.; Zelosko, V.; Sáinz, R.M.; Tan, D.-X.; Poeggeler, B.; Antolín, I.; Zsizsik, B.K.; Reiter, R.J.; Hardeland, R. Antioxidant properties of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK): Scavenging of free radicals and prevention of protein destruction. Redox Rep. 2003, 8, 205–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardeland, R.; Ressmeyer, A.-R.; Zelosko, V.; Burkhardt, S.; Poeggeler, B. Metabolites of melatonin: Formation and properties of the methoxylated kynuramines AFMK and AMK. In Recent Advances in Endocrinology and Reproduction: Evolutionary, Biotechnological and Clinical Applications; Haldar, C., Singh, S.S., Eds.; Banaras Hindu University: Varanasi, India, 2004; pp. 21–38. [Google Scholar]
- Silva, S.O.; Rodrigues, M.R.; Carvalho, S.R.Q.; Catalani, L.H.; Campa, A.; Ximenes, V.F. Oxidation of melatonin and its catabolites, N1-acetyl-N2 -formyl-5-methoxykynuramine and N1-acetyl-5-methoxykynuramine, by activated leukocytes. J. Pineal Res. 2004, 37, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Burkhardt, S.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Shohami, E.; Huo, Y.-S.; Hardeland, R.; Reiter, R.J. N1-Acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 2001, 15, 2294–2296. [Google Scholar] [CrossRef]
- Collin, F.; Bonnefont-Rousselot, D.; Yous, S.; Marchetti, C.; Jore, D.; Gardès-Albert, M. Online H/D exchange liquid chromatography as a support for the mass spectrometric identification of the oxidation products of melatonin. J. Mass Spectrom. 2009, 44, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Seever, K.; Hardeland, R. Novel pathway for N1-acetyl-5-methoxykynuramine: UVB-induced liberation of carbon monoxide from precursor N1-acetyl-N2-formyl-5-methoxykynuramine. J. Pineal Res. 2008, 44, 450–455. [Google Scholar] [CrossRef]
- Hirata, F.; Hayaishi, O.; Tokuyama, T.; Senoh, S. In vitro and in vivo formation of two new metabolites of melatonin. J. Biol. Chem. 1974, 249, 1311–1313. [Google Scholar] [CrossRef]
- Niu, S.; Li, F.; Tan, D.-X.; Zhang, L.; Idle, J.R.; Gonzalez, F.J.; Ma, X. Analysis of N1-acetyl-N2-formyl-5-methoxykynuramine/N1-acetyl-5-methoxy-kynuramine formation from melatonin in mice. J. Pineal Res. 2010, 49, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, M.; Hardeland, R. The melatonin metabolite N1-acetyl-5-methoxykynuramine is a potent singlet oxygen scavenger. J. Pineal Res. 2009, 46, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Mori, A.; Liburdy, R.; Packer, L. Melatonin and its precursors scavenge nitric oxide. J. Pineal Res. 1999, 27, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, B.; Pompon, D.; Ducrocq, C. Nitrosation of melatonin by nitric oxide and peroxynitrite. J. Pineal Res. 2000, 29, 184–192. [Google Scholar] [CrossRef]
- Turjanski, A.G.; Sáenz, D.A.; Doctorovich, F.; Estrin, D.A.; Rosenstein, R.E. Nitrosation of melatonin by nitric oxide: A computational study. J. Pineal Res. 2001, 31, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Turjanski, A.; Chaia, Z.D.; Doctorovich, F.; Estrin, D.; Rosenstein, R.; Piro, O.E. N-nitrosomelatonin. Acta Crystallogr. C 2000, 56, 682–683. [Google Scholar] [CrossRef]
- Kirsch, M.; de Groot, H. First insights into regiospecific transnitrosation reactions between tryptophan derivatives: Melatonin as an effective target. J. Pineal Res. 2005, 38, 247–523. [Google Scholar] [CrossRef]
- Peyrot, F.; Fernandez, B.O.; Bryan, N.S.; Feelisch, M.; Ducrocq, C. N-Nitroso products from the reaction of indoles with Angeli’s salt. Chem. Res. Toxicol. 2006, 19, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.-X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Singh, N.; Kaur, H.; Yadav, S.; Bhatla, S.C. Does N-nitrosomelatonin compete with S-nitrosothiols as a long distance nitric oxide carrier in plants? Biochem. Anal. Biochem. 2016, 5, 262. [Google Scholar] [CrossRef] [Green Version]
- Berchner-Pfannschmidt, U.; Tug, S.; Trinidad, B.; Becker, M.; Oehme, F.; Flamme, I.; Fandrey, J.; Kirsch, M. The impact of N-nitrosomelatonin as nitric oxide donor in cell culture experiments. J. Pineal Res. 2008, 45, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and its metabolites as anti-nitrosating and anti-nitrating agents. J. Exp. Integ. Med. 2011, 1, 67–81. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S. Insights into nitric oxide-melatonin crosstalk and N-nitrosomelatonin functioning in plants. J. Exp. Bot. 2019, 70, 6035–6047. [Google Scholar] [CrossRef] [PubMed]
- Blanchard-Fillion, B.; Servy, C.; Ducrocq, C. 1-Nitrosomelatonin is a spontaneous NO-releasing compound. Free Radic. Res. 2001, 35, 857–866. [Google Scholar] [CrossRef]
- Peyrot, F.; Grillon, C.; Vergely, C.; Rochette, L.; Ducrocq, C. Pharmacokinetics of 1-nitrosomelatonin and detection by EPR using iron dithiocarbamate complex in mice. Biochem. J. 2005, 387, 473–478. [Google Scholar] [CrossRef] [PubMed]
- De Biase, P.M.; Turjanski, A.G.; Estrin, D.A.; Doctorovich, F. Mechanisms of NO release by N1-nitrosomelatonin: Nucleophilic attack versus reducing pathways. J. Org. Chem. 2005, 70, 5790–5798. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, M.; de Groot, H. N-nitrosomelatonin outcompetes S-nitrosocysteine in inhibiting glyceraldehyde 3-phosphate dehydrogenase: First evidence that N-nitrosomelatonin can modify protein function. J. Pineal Res. 2008, 44, 244–249. [Google Scholar] [CrossRef]
- Kirsch, M.; de Groot, H. N-nitrosomelatonin: Synthesis, chemical properties, potential prodrug. J. Pineal Res. 2009, 46, 121–127. [Google Scholar] [CrossRef]
- Suzuki, T.; Mower, H.F.; Friesen, M.D.; Gilibert, I.; Sawa, T.; Ohshima, H. Nitration and nitrosation of N-acetyl-L-tryptophan and tryptophan residues in proteins by various reactive nitrogen species. Free Radic. Biol. Med. 2004, 37, 671–681. [Google Scholar] [CrossRef]
- Peyrot, F.; Ducrocq, C. Potential role of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species. J. Pineal Res. 2008, 45, 235–426. [Google Scholar] [CrossRef]
- Lehnig, M.; Kirsch, M. 15N-CIDNP investigations during tryptophan, N-acetyl-L-tryptophan, and melatonin nitration with reactive nitrogen species. Free Radic. Res. 2007, 41, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Squadrito, G.L.; Pryor, W.A. The reaction of melatonin with peroxynitrite: Formation of melatonin radical cation and absence of stable nitrated products. Biochem. Biophys. Res. Commun. 1998, 51, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Uppu, R.M.; Squadrito, G.L.; Pryor, W.A. Acceleration of peroxynitrite oxidations by carbon dioxide. Arch. Biochem. Biophys. 1996, 327, 335–343. [Google Scholar] [CrossRef]
- Hensley, K.; Maidt, M.L.; Yu, Z.; Sang, H.; Markesbery, W.R.; Floyd, R.A. Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer brain indicates region-specific accumulation. J. Neurosci. 1998, 18, 8126–8132. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, G.L.; Pryor, W.A. Oxidative chemistry of nitric oxide: The roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic. Biol. Med. 1998, 25, 392–403. [Google Scholar] [CrossRef]
- Lehnig, M. Radical mechanisms of the decomposition of peroxynitrite and the peroxynitrite-CO2 adduct and of reactions with L-tyrosine and related compounds as studied by 15N chemically induced dynamic nuclear polarization. Arch. Biochem. Biophys. 1999, 368, 303–318. [Google Scholar] [CrossRef]
- Santos, C.X.; Bonini, M.G.; Augusto, O. Role of the carbonate radical anion in tyrosine nitration and hydroxylation by peroxynitrite. Arch. Biochem. Biophys. 2000, 377, 146–152. [Google Scholar] [CrossRef]
- Hardeland, R.; Poeggeler, B.; Niebergall, R.; Zelosko, V. Oxidation of melatonin by carbonate radicals and chemiluminescence emitted during pyrrole ring cleavage. J. Pineal Res. 2003, 34, 17–25. [Google Scholar] [CrossRef]
- Kuesel, J.T.; Hardeland, R.; Pfoertner, H.; Aeckerle, N. Reactions of the melatonin metabolite N1-acetyl-5-methoxykynuramine with carbamoyl phosphate and related compounds. J. Pineal Res. 2010, 48, 47–54. [Google Scholar] [CrossRef]
- Krotzky, M.; Hardeland, R. Metabolism of the melatonin metabolite N1-acetyl-N2-formyl-5-methoxykynuramine in Saccharomyces cerevisiae. Cytologia 2008, 73, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R.; Backhaus, C.; Fadavi, A.; Hess, M. N1-acetyl-5-methoxykynuramine contrasts with other tryptophan metabolites by a peculiar type of NO scavenging: Cyclization to a cinnolinone prevents formation of unstable nitrosamines. J. Pineal Res. 2007, 43, 104–105. [Google Scholar] [CrossRef] [PubMed]
- Than, N.N.; Heer, C.; Laatsch, H.; Hardeland, R. Reactions of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK) with the ABTS cation radical: Identification of new oxidation products. Redox Rep. 2006, 11, 15–24. [Google Scholar] [CrossRef]
- Koehler, A. Investigations on the Redox Behavior of the Melatonin Metabolite N1-acetyl-5-methoxykynuramine (AMK). Master’s Thesis, University of Goettingen, Biological Faculty, Goettingen, Germany, 2007. (In German). [Google Scholar]
- Nowak, A.; Rahman, H.; Heer, C.; Schueth, A.; Laatsch, H.; Hardeland, R. Reactions of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK) with the tyrosine side-chain fragment, 4-ethylphenol. Redox Rep. 2008, 13, 102–108. [Google Scholar] [CrossRef]
- Nakazawa, H.; Fukuyama, N.; Takizawa, S.; Tsuji, C.; Yoshitake, M.; Ishida, H. Nitrotyrosine formation and its role in various pathological conditions. Free Radic. Res. 2000, 33, 771–784. [Google Scholar] [CrossRef]
- Deng, G.; Vaziri, N.D.; Jabbari, B.; Ni, Z.; Yan, X.X. Increased tyrosine nitration of the brain in chronic renal insufficiency: Reversal by antioxidant therapy and angiotensin-converting enzyme inhibition. J. Am. Soc. Nephrol. 2001, 12, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Kharitonov, S.A.; Barnes, P.J. Nitric oxide, nitrotyrosine, and nitric oxide modulators in asthma and chronic obstructive pulmonary disease. Curr. Allergy Asthma Rep. 2003, 3, 121–129. [Google Scholar] [CrossRef]
- Mohiuddin, I.; Chai, H.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Nitrotyrosine and chlorotyrosine: Clinical significance and biological functions in the vascular system. J. Surg. Res. 2006, 133, 143–149. [Google Scholar] [CrossRef]
- Topkaya, S.N.; Ozyurt, V.H.; Cetin, A.E.; Otles, S. Nitration of tyrosine and its effect on DNA hybridization. Biosens. Bioelectron. 2018, 102, 464–469. [Google Scholar] [CrossRef]
- Ahmad, R.; Hussain, A.; Ahsan, H. Peroxynitrite: Cellular pathology and implications in autoimmunity. J. Immunoass. Immunochem. 2019, 40, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Campolo, N.; Issoglio, F.M.; Estrin, D.A.; Bartesaghi, S.; Radi, R. 3-Nitrotyrosine and related derivatives in proteins: Precursors, radical intermediates and impact in function. Essays Biochem. 2020, 64, 111–133. [Google Scholar] [CrossRef]
- Ceriello, A.; Quagliaro, L.; D’Amico, M.; Di Filippo, C.; Marfella, R.; Nappo, F.; Berrino, L.; Rossi, F.; Giugliano, D. Acute hyperglycemia induces nitrotyrosine formation and apoptosis in perfused heart from rat. Diabetes 2002, 51, 1076–1082. [Google Scholar] [CrossRef] [Green Version]
- Eisele, H.J.; Markart, P.; Schulz, R. Obstructive sleep apnea, oxidative stress, and cardiovascular disease: Evidence from human studies. Oxid. Med. Cell. Longev. 2015, 2015, 608438. [Google Scholar] [CrossRef] [Green Version]
- Thomson, L. 3-Nitrotyrosine modified proteins in atherosclerosis. Dis. Markers 2015, 2015, 708282. [Google Scholar] [CrossRef] [PubMed]
- Pialoux, V.; Poulin, M.J.; Hemmelgarn, B.R.; Muruve, D.A.; Chirico, E.N.; Faes, C.; Sola, D.Y.; Ahmed, S.B. Cyclooxygenase-2 inhibition limits angiotensin II-induced DNA oxidation and protein nitration in humans. Front. Physiol. 2017, 8, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butterfield, D.A. Proteomics: A new approach to investigate oxidative stress in Alzheimer’s disease brain. Brain Res. 2004, 1000, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.; Ozben, T. Reactive oxygen and nitrogen species in Alzheimer’s disease. Curr. Alzheimer Res. 2004, 1, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Bombeiro, A.L.; D’Império Lima, M.R.; Chadi, G.; Alvarez, J.M. Neurodegeneration and increased production of nitrotyrosine, nitric oxide synthase, IFN-γ and S100β protein in the spinal cord of IL-12p40-deficient mice infected with Trypanosoma cruzi. Neuroimmunomodulation 2010, 17, 67–78. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Reed, T.; Sultana, R. Roles of 3-nitrotyrosine- and 4-hydroxynonenal-modified brain proteins in the progression and pathogenesis of Alzheimer’s disease. Free Radic. Res. 2011, 45, 59–72. [Google Scholar] [CrossRef]
- D’Amico, E.; Factor-Litvak, P.; Santella, R.M.; Mitsumoto, H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2013, 65, 509–527. [Google Scholar] [CrossRef] [Green Version]
- Bandookwala, M.; Sengupta, P. 3-Nitrotyrosine: A versatile oxidative stress biomarker for major neurodegenerative diseases. Int. J. Neurosci. 2020, 130, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.I.; Wang, D.; Leu, F.J.; Chen, C.F.; Chen, H.I. Ischemia and reperfusion of liver induces eNOS and iNOS expression: Effects of a NO donor and NOS inhibitor. Chin. J. Physiol. 2004, 47, 121–127. [Google Scholar] [PubMed]
- Yanagisawa, D.; Kitamura, Y.; Inden, M.; Takata, K.; Taniguchi, T.; Morikawa, S.; Morita, M.; Inubushi, T.; Tooyama, I.; Taira, T.; et al. DJ-1 protects against neurodegeneration caused by focal cerebral ischemia and reperfusion in rats. J. Cereb. Blood Flow Metab. 2008, 28, 563–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosche, A.; Freeman, D.E.; Morton, A.J.; Polyak, M.M.; Matyjaszek, S.A. Effects of ischemia and reperfusion on production of nitrotyrosine, activation of eosinophils, and apoptosis in the large colonic mucosa of horses. Am. J. Vet. Res. 2012, 73, 53–61. [Google Scholar] [CrossRef]
- Gao, L.; Chen, X.; Peng, T.; Yang, D.; Wang, Q.; Lv, Z.; Shen, J. Caveolin-1 protects against hepatic ischemia/reperfusion injury through ameliorating peroxynitrite-mediated cell death. Free Radic Biol. Med. 2016, 95, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Tatarkova, Z.; Kovalska, M.; Sivonova, M.K.; Racay, P.; Lehotsky, J.; Kaplan, P. Tyrosine nitration of mitochondrial proteins during myocardial ischemia and reperfusion. J. Physiol. Biochem. 2019, 75, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Torisu, K.; Tomita, K.; Kawai, Y.; Tsuruya, K.; Nakano, T.; Kitazono, T. Arginase 2 is a mediator of ischemia-reperfusion injury in the kidney through regulation of nitrosative stress. Kidney Int. 2020, 98, 673–685. [Google Scholar] [CrossRef]
- Chen, H.; Guan, B.; Wang, B.; Pu, H.; Bai, X.; Chen, X.; Liu, J.; Li, C.; Qiu, J.; Yang, D.; et al. Glycyrrhizin prevents hemorrhagic transformation and improves neurological outcome in ischemic stroke with delayed thrombolysis through targeting peroxynitrite-mediated HMGB1 signaling. Transl. Stroke Res. 2020, 11, 967–982. [Google Scholar] [CrossRef]
- Tuo, J.; Liu, L.; Poulsen, H.E.; Weimann, A.; Svendsen, O.; Loft, S. Importance of guanine nitration and hydroxylation in DNA in vitro and in vivo. Free Radic. Biol. Med. 2000, 29, 147–155. [Google Scholar] [CrossRef]
- Ohshima, H.; Sawa, T.; Akaike, T. 8-Nitroguanine, a product of nitrative DNA damage caused by reactive nitrogen species: Formation, occurrence, and implications in inflammation and carcinogenesis. Antioxid. Redox Signal. 2006, 8, 1033–1045. [Google Scholar] [CrossRef]
- Kim, J. Spermidine is protective against kidney ischemia and reperfusion injury through inhibiting DNA nitration and PARP1 activation. Anat. Cell Biol. 2017, 50, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Murata, M. Inflammation and cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef] [Green Version]
- Alexander, K.J.; McConville, M.; Williams, K.R.; Luzyanin, K.V.; O’Neil, I.A.; Cosstick, R. Chemistry of the 8-nitroguanine DNA lesion: Reactivity, labelling and repair. Chemistry 2018, 24, 3013–3020. [Google Scholar] [CrossRef]
- Caulfield, J.L.; Wishnok, J.S.; Tannenbaum, S.R. Nitric oxide-induced deamination of cytosine and guanine in deoxynucleosides and oligonucleotides. J. Biol. Chem. 1998, 273, 12689–12695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.J.; Wu, S.B.; Chang, C.M. Biological and dietary antioxidants protect against DNA nitration induced by reaction of hypochlorous acid with nitrite. Arch. Biochem. Biophys. 2003, 415, 109–116. [Google Scholar] [CrossRef]
- Hardeland, R.; Poeggeler, B.; Pappolla, M.A. Mitochondrial actions of melatonin—An endeavor to identify their adaptive and cytoprotective mechanisms. Proc. Saxon Acad. Sci. 2009, 65, 14–31. [Google Scholar]
- Stomberski, C.T.; Hess, D.T.; Stamler, J.S. Protein S-nitrosylation: Determinants of specificity and enzymatic regulation of S-nitrosothiol-based signaling. Antioxid. Redox Signal. 2019, 30, 1331–1351. [Google Scholar] [CrossRef] [PubMed]
- Fernhoff, N.B.; Derbyshire, E.R.; Underbakke, E.S.; Marletta, M.A. Heme-assisted S-nitrosation desensitizes ferric soluble guanylate cyclase to nitric oxide. J. Biol. Chem. 2012, 287, 43053–43062. [Google Scholar] [CrossRef] [Green Version]
- Foster, M.W.; Hess, D.T.; Stamler, J.S. Protein S-nitrosylation in health and disease: A current perspective. Trends Mol. Med. 2009, 15, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Aranda, E.; López-Pedrera, C.; De La Haba-Rodriguez, J.R.; Rodriguez-Ariza, A. Nitric oxide and cancer: The emerging role of S-nitrosylation. Curr. Mol. Med. 2012, 12, 50–67. [Google Scholar] [CrossRef]
- Wang, Z. Protein S-nitrosylation and cancer. Cancer Lett. 2012, 320, 123–129. [Google Scholar] [CrossRef]
- Bignon, E.; Allega, M.F.; Lucchetta, M.; Tiberti, M.; Papaleo, E. Computational structural biology of S-nitrosylation of cancer targets. Front. Oncol. 2018, 8, 272. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Li, Y.; Huang, X.; Wei, M.; Huang, Y.; Tang, Z.; Huang, H.; Zhou, W.; Wang, Y.; Hu, J. Extensive protein S-nitrosylation associated with human pancreatic ductal adenocarcinoma pathogenesis. Cell Death Dis. 2019, 10, 914. [Google Scholar] [CrossRef] [Green Version]
- Mishra, D.; Patel, V.; Banerjee, D. Nitric oxide and S-nitrosylation in cancers: Emphasis on breast cancer. Breast Cancer 2020, 14. [Google Scholar] [CrossRef]
- Xu, P.; Ye, S.; Li, K.; Huang, M.; Wang, Q.; Zeng, S.; Chen, X.; Gao, W.; Chen, J.; Zhang, Q.; et al. NOS1 inhibits the interferon response of cancer cells by S-nitrosylation of HDAC2. J. Exp. Clin. Cancer Res. 2019, 38, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizza, S.; Filomeni, G. Exploiting S-nitrosylation for cancer therapy: Facts and perspectives. Biochem. J. 2020, 477, 3649–3672. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Guo, Y.; Song, Y.; Chen, L.; Ruan, Q.; Wang, Y.; Sun, L.; Hu, Y.; Zhou, J.; et al. Regulation of ezrin tension by S-nitrosylation mediates non-small cell lung cancer invasion and metastasis. Theranostics 2019, 9, 2555–2571. [Google Scholar] [CrossRef]
- Ehrenfeld, P.; Cordova, F.; Duran, W.N.; Sanchez, F.A. S-nitrosylation and its role in breast cancer angiogenesis and metastasis. Nitric Oxide 2019, 87, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Switzer, C.H.; Cheng, R.Y.; Ridnour, L.A.; Glynn, S.A.; Ambs, S.; Wink, D.A. Ets-1 is a transcriptional mediator of oncogenic nitric oxide signaling in estrogen receptor-negative breast cancer. Breast Cancer Res. 2012, 14, R125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, H.E.; Foster, M.W. S-nitrosylation of Ras in breast cancer. Breast Cancer Res. 2012, 14, 113. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Anjomani-Virmouni, S.; Koundouros, N.; Dimitriadi, M.; Choo-Wing, R.; Valle, A.; Zheng, Y.; Chiu, Y.H.; Agnihotri, S.; Zadeh, G.; et al. PARK2 depletion connects energy and oxidative stress to PI3K/Akt activation via PTEN S-nitrosylation. Mol. Cell 2017, 65, 999–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Zheng, K.; Ma, C.; Li, J.; Zhuo, L.; Huang, W.; Chen, T.; Jiang, Y. PTPS facilitates compartmentalized LTBP1 S-nitrosylation and promotes tumor growth under hypoxia. Mol. Cell 2020, 77, 95–107. [Google Scholar] [CrossRef]
- Monteiro, H.P.; Costa, P.E.; Reis, A.K.; Stern, A. Nitric oxide: Protein tyrosine phosphorylation and protein S-nitrosylation in cancer. Biomed. J. 2015, 38, 380–388. [Google Scholar]
- Switzer, C.H.; Glynn, S.A.; Cheng, R.Y.; Ridnour, L.A.; Green, J.E.; Ambs, S.; Wink, D.A. S-nitrosylation of EGFR and Src activates an oncogenic signaling network in human basal-like breast cancer. Mol. Cancer Res. 2012, 10, 1203–1215. [Google Scholar] [CrossRef] [Green Version]
- Basudhar, D.; Somasundaram, V.; de Oliveira, G.A.; Kesarwala, A.; Heinecke, J.L.; Cheng, R.Y.; Glynn, S.A.; Ambs, S.; Wink, D.A.; Ridnour, L.A. Nitric oxide synthase-2-derived nitric oxide drives multiple pathways of breast cancer progression. Antioxid. Redox Signal. 2017, 26, 1044–1058. [Google Scholar] [CrossRef]
- Jin, L.; Cao, Y.; Zhang, T.; Wang, P.; Ji, D.; Liu, X.; Shi, H.; Hua, L.; Yu, R.; Gao, S. Effects of ERK1/2 S-nitrosylation on ERK1/2 phosphorylation and cell survival in glioma cells. Int. J. Mol. Med. 2018, 41, 1339–1348. [Google Scholar] [CrossRef] [Green Version]
- Romagny, S.; Bouaouiche, S.; Lucchi, G.; Ducoroy, P.; Bertoldo, J.B.; Terenzi, H.; Bettaieb, A.; Plenchette, S. S-nitrosylation of cIAP1 switches cancer cell fate from TNFalpha/TNFR1-mediated cell survival to cell death. Cancer Res. 2018, 78, 1948–1957. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Sun, C.; Xiao, G.; Shan, H.; Tang, L.; Yi, Y.; Yu, W.; Gu, Y. S-nitrosylation of the Peroxiredoxin-2 promotes S-nitrosoglutathione-mediated lung cancer cells apoptosis via AMPK-SIRT1 pathway. Cell Death Dis. 2019, 10, 329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardeland, R. Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J. Pineal Res. 2017, 62, e12377. [Google Scholar] [CrossRef] [PubMed]
- Jung-Hynes, B.; Schmit, T.L.; Reagan-Shaw, S.R.; Siddiqui, I.A.; Mukhtar, H.; Ahmad, N. Melatonin, a novel Sirt1 inhibitor, imparts antiproliferative effects against prostate cancer in vitro in culture and in vivo in TRAMP model. J. Pineal Res. 2011, 50, 140–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardeland, R. Melatonin, noncoding RNAs, messenger RNA stability and epigenetics—Evidence, hints, gaps and perspectives. Int. J. Mol. Sci. 2014, 15, 18221–18252. [Google Scholar] [CrossRef]
- Hardeland, R. Sirtuins, melatonin, and the relevance of circadian oscillators. In Sirtuin Biology in Medicine. Targeting New Avenues of Care in Development, Aging, and Disease; Maiese, K., Ed.; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2021; pp. 137–151. [Google Scholar]
- Hardeland, R. Dysregulation of sirtuins in cancer. Oncol. Res. Rev. 2019, 2, 1–4. [Google Scholar] [CrossRef]
- Lanoix, D.; Lacasse, A.A.; Reiter, R.J.; Vaillancourt, C. Melatonin: The smart killer: The human trophoblast as a model. Mol. Cell. Endocrinol. 2012, 348, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bizarri, M.; Proietti, S.; Cucina, A.; Reiter, R.J. Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: A review. Expert Opin. Ther. Targets 2013, 17, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.K.; Dawson, T.M.; Dawson, V.L. Nitric oxide, S-nitrosylation and neurodegeneration. Cell. Mol. Biol. 2005, 51, 247–254. [Google Scholar] [PubMed]
- Chung, K.K. Say NO to neurodegeneration: Role of S-nitrosylation in neurodegenerative disorders. Neurosignals 2006, 15, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Tsang, A.H.; Lee, Y.I.; Ko, H.S.; Savitt, J.M.; Pletnikova, O.; Troncoso, J.C.; Dawson, V.L.; Dawson, T.M.; Chung, K.K. S-nitrosylation of XIAP compromises neuronal survival in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 4900–4905. [Google Scholar] [CrossRef] [Green Version]
- Di Giacomo, G.; Rizza, S.; Montagna, C.; Filomeni, G. Established principles and emerging concepts on the interplay between mitochondrial physiology and S-(de)nitrosylation: Implications in cancer and neurodegeneration. Int. J. Cell Biol. 2012, 2012, 361872. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.W.; Sunico, C.R.; Nakamura, T.; Lipton, S.A. Redox regulation of protein function via cysteine S-nitrosylation and its relevance to neurodegenerative diseases. Int. J. Cell Biol. 2012, 2012, 463756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, S.; Nakamura, T.; Cieplak, P.; Chan, S.F.; Kalashnikova, E.; Liao, L.; Saleem, S.; Han, X.; Clemente, A.; Nutter, A.; et al. S-nitrosylation-mediated redox transcriptional switch modulates neurogenesis and neuronal cell death. Cell Rep. 2014, 8, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Conway, M.E.; Harris, M. S-nitrosylation of the thioredoxin-like domains of protein disulfide isomerase and its role in neurodegenerative conditions. Front. Chem. 2015, 3, 27. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.W.; Sanz-Blasco, S.; Dolatabadi, N.; Parker, J.; Chon, K.; Lee, M.S.; Soussou, W.; McKercher, S.R.; Ambasudhan, R.; Nakamura, T.; et al. Elevated glucose and oligomeric β-amyloid disrupt synapses via a common pathway of aberrant protein S-nitrosylation. Nat. Commun. 2016, 7, 10242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montagna, C.; Rizza, S.; Maiani, E.; Piredda, L.; Filomeni, G.; Cecconi, F. To eat, or NOt to eat: S-nitrosylation signaling in autophagy. FEBS J. 2016, 283, 3857–3869. [Google Scholar] [CrossRef] [Green Version]
- Ni, C.L.; Seth, D.; Fonseca, F.V.; Wang, L.; Xiao, T.S.; Gruber, P.; Sy, M.S.; Stamler, J.S.; Tartakoff, A.M. Polyglutamine tract expansion increases S-nitrosylation of huntingtin and ataxin-1. PLoS ONE 2016, 11, e0163359. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Veremeyko, T.; Wong, A.H.; El Fatimy, R.; Wei, Z.; Cai, W.; Krichevsky, A.M. Downregulation of miR-132/212 impairs S-nitrosylation balance and induces tau phosphorylation in Alzheimer’s disease. Neurobiol. Aging 2017, 51, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Fominykh, V.; Vorobyeva, A.; Onufriev, M.V.; Brylev, L.; Zakharova, M.N.; Gulyaeva, N.V. Interleukin-6, S-nitrosothiols, and neurodegeneration in different central nervous system demyelinating disorders: Is there a relationship? J. Clin. Neurol. 2018, 14, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Crunfli, F.; Mazucanti, C.H.; de Moraes, R.C.M.; Costa, A.P.; Rodrigues, A.C.; Scavone, C.; Torrão, A.S. NO-dependent Akt inactivation by S-nitrosylation as a possible mechanism of STZ-induced neuronal insulin resistance. J. Alzheimers Dis. 2018, 65, 1427–1443. [Google Scholar] [CrossRef]
- Wilkaniec, A.; Lenkiewicz, A.M.; Czapski, G.A.; Jęśko, H.M.; Hilgier, W.; Brodzik, R.; Gąssowska-Dobrowolska, M.; Culmsee, C.; Adamczyk, A. Extracellular alpha-synuclein oligomers induce parkin S-nitrosylation: Relevance to sporadic Parkinson’s disease etiopathology. Mol. Neurobiol. 2019, 56, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Wijasa, T.S.; Sylvester, M.; Brocke-Ahmadinejad, N.; Schwartz, S.; Santarelli, F.; Gieselmann, V.; Klockgether, T.; Brosseron, F.; Heneka, M.T. Quantitative proteomics of synaptosome S-nitrosylation in Alzheimer’s disease. J. Neurochem. 2020, 152, 710–726. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Li, X.; Du, X.; Bi, M.; Ma, F.; Xie, J.; Jiang, H. The S-nitrosylation of parkin attenuated the ubiquitination of divalent metal transporter 1 in MPP+-treated SH-SY5Y cells. Sci. Rep. 2020, 10, 15542. [Google Scholar] [CrossRef]
- Montagna, C.; Cirotti, C.; Rizza, S.; Filomeni, G. When S-nitrosylation gets to mitochondria: From signaling to age-related diseases. Antioxid. Redox Signal. 2020, 32, 884–905. [Google Scholar] [CrossRef]
- Cho, D.H.; Nakamura, T.; Fang, J.; Cieplak, P.; Godzik, A.; Gu, Z.; Lipton, S.A. S-nitrosylation of Drp1 mediates β-amyloid-related mitochondrial fission and neuronal injury. Science 2009, 324, 102–105. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Cieplak, P.; Cho, D.H.; Godzik, A.; Lipton, S.A. S-nitrosylation of Drp1 links excessive mitochondrial fission to neuronal injury in neurodegeneration. Mitochondrion 2010, 10, 573–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Liu, L.; Jiang, X.; Zhai, S.; Xing, D. The essential role of Drp1 and its regulation by S-mitrosylation of parkin in dopaminergic neurodegeneration: Implications for Parkinson’s disease. Antioxid. Redox Signal. 2016, 25, 609–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, Y.D.; Ma, T.; Diao, S.; Zhang, X.; Chen, Y.; Hsu, J.; Lipton, S.A.; Masliah, E.; Xu, H.; Liao, F.F. NO signaling and S-nitrosylation regulate PTEN inhibition in neurodegeneration. Mol. Neurodegener. 2010, 5, 49. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Lipton, S.A. Emerging roles of S-nitrosylation in protein misfolding and neurodegenerative diseases. Antioxid. Redox Signal. 2008, 10, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Halloran, M.; Parakh, S.; Atkin, J.D. The role of S-nitrosylation and S-glutathionylation of protein disulphide isomerase in protein misfolding and neurodegeneration. Int. J. Cell Biol. 2013, 2013, 797914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, Y.; Oguro, A.; Yagi, E.; Mitani, A.; Kudoh, S.N.; Imaoka, S. Bisphenol A and rotenone induce S-nitrosylation of protein disulfide isomerase (PDI) and inhibit neurite outgrowth of primary cultured cells of the rat hippocampus and PC12 cells. J. Toxicol. Sci. 2020, 45, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Song, B.J.; Abdelmegeed, M.A.; Henderson, L.E.; Yoo, S.H.; Wan, J.; Purohit, V.; Hardwick, J.P.; Moon, K.H. Increased nitroxidative stress promotes mitochondrial dysfunction in alcoholic and nonalcoholic fatty liver disease. Oxid. Med. Cell. Longev. 2013, 2013, 781050. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Calay, E.S.; Fan, J.; Arduini, A.; Kunz, R.C.; Gygi, S.P.; Yalcin, A.; Fu, S.; Hotamisligil, G.S. S-Nitrosylation links obesity-associated inflammation to endoplasmic reticulum dysfunction. Science 2015, 349, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Qian, Q.; Zhang, Z.; Orwig, A.; Chen, S.; Ding, W.X.; Xu, Y.; Kunz, R.C.; Lind, N.R.L.; Stamler, J.S.; Yang, L. S-nitrosoglutathione reductase dysfunction contributes to obesity-associated hepatic insulin resistance via regulating autophagy. Diabetes 2018, 67, 193–207. [Google Scholar] [CrossRef] [Green Version]
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019, 568, 351–356. [Google Scholar]
- Hegyi, B.; Bers, D.M.; Bossuyt, J. CaMKII signaling in heart diseases: Emerging role in diabetic cardiomyopathy. J. Mol. Cell. Cardiol. 2019, 127, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, T.; Tokunaga, E.; Ota, H.; Sugita, H.; Martyn, J.A.; Kaneki, M. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J. Biol. Chem. 2005, 280, 7511–7518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneki, M.; Shimizu, N.; Yamada, D.; Chang, K. Nitrosative stress and pathogenesis of insulin resistance. Antioxid. Redox Signal. 2007, 9, 319–329. [Google Scholar] [CrossRef]
- Pérez-Gallardo, R.V.; Noriega-Cisneros, R.; Esquivel-Gutiérrez, E.; Calderón-Cortés, E.; Cortés-Rojo, C.; Manzo-Avalos, S.; Campos-García, J.; Salgado-Garciglia, R.; Montoya-Pérez, R.; Boldogh, I.; et al. Effects of diabetes on oxidative and nitrosative stress in kidney mitochondria from aged rats. J. Bioenerg. Biomembr. 2014, 46, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wu, J.; Jin, Z.; Yan, L.J. Protein modifications as manifestations of hyperglycemic glucotoxicity in diabetes and its complications. Biochem. Insights 2016, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visiedo, F.; Santos-Rosendo, C.; Mateos-Bernal, R.M.; Gil-Sánchez, M.D.; Bugatto, F.; Aguilar-Diosdado, M.; Segundo, C.; López-Tinoco, C. Characterization of NO-induced nitrosative status in human placenta from pregnant women with gestational diabetes mellitus. Oxid. Med. Cell. Longev. 2017, 2017, 5629341. [Google Scholar] [CrossRef]
- Yanar, K.; Atayik, M.C.; Simsek, B.; Çakatay, U. Novel biomarkers for the evaluation of aging-induced proteinopathies. Biogerontology 2020, 21, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Goligorsky, M.S.; Rabelink, T. Meeting report: ISN forefronts in nephrology on endothelial biology and renal disease: From bench to prevention. Kidney Int. 2006, 70, 258–264. [Google Scholar] [CrossRef] [Green Version]
- López-Sánchez, L.M.; Corrales, F.J.; Barcos, M.; Espejo, I.; Muñoz-Castañeda, J.R.; Rodríguez-Ariza, A. Inhibition of nitric oxide synthesis during induced cholestasis ameliorates hepatocellular injury by facilitating S-nitrosothiol homeostasis. Lab. Investig. 2010, 90, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Montagna, C.; Di Giacomo, G.; Rizza, S.; Cardaci, S.; Ferraro, E.; Grumati, P.; De Zio, D.; Maiani, E.; Muscoli, C.; Lauro, F.; et al. S-nitrosoglutathione reductase deficiency-induced S-nitrosylation results in neuromuscular dysfunction. Antioxid. Redox Signal. 2014, 21, 570–587. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Gomes, S.A.; Rangel, E.B.; Paulino, E.C.; Fonseca, T.L.; Li, J.; Teixeira, M.B.; Gouveia, C.H.; Bianco, A.C.; Kapiloff, M.S.; et al. S-nitrosoglutathione reductase-dependent PPARγ denitrosylation participates in MSC-derived adipogenesis and osteogenesis. J. Clin. Investig. 2015, 125, 1679–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, L.C.; Escames, G.; Tapias, V.; Utrilla, P.; León, J.; Acuña-Castroviejo, D. Identification of an inducible nitric oxide synthase in diaphragm mitochondria from septic mice: Its relation with mitochondrial dysfunction and prevention by melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.I.; Escames, G.; López, L.C.; García, J.A.; Ortiz, F.; López, A.; Acuña-Castroviejo, D. Melatonin administration prevents cardiac and diaphragmatic mitochondrial oxidative damage in senescence-accelerated mice. J. Endocrinol. 2007, 194, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, M.I.; Carretero, M.; Escames, G.; López, L.C.; Maldonado, M.D.; Tan, D.-X.; Reiter, R.J.; Acuña-Castroviejo, D. Chronic melatonin treatment prevents age-dependent cardiac mitochondrial dysfunction in senescence-accelerated mice. Free Radic. Res. 2007, 41, 15–24. [Google Scholar] [CrossRef]
- Rodríguez, M.I.; Escames, G.; López, L.C.; López, A.; García, J.A.; Ortiz, F.; Sánchez, V.; Romeu, M.; Acuña-Castroviejo, D. Improved mitochondrial function and increased life span after chronic melatonin treatment in senescent prone mice. Exp. Gerontol. 2008, 43, 749–756. [Google Scholar] [CrossRef] [Green Version]
- Carretero, M.; Escames, G.; López, L.C.; Venegas, C.; Dayoub, J.C.; García, L.; Acuña-Castroviejo, D. Long-term melatonin administration protects brain mitochondria from aging. J. Pineal Res. 2009, 47, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, Y.; Wu, X.; Zhao, G.; Zhang, K.; Yang, C.S.; Reiter, R.J.; Zhang, J. Melatonin and (-)-epigallocatechin-3-gallate: Partners in fighting cancer. Cells 2019, 8, 745. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R. Melatonin and neuroinflammation: Encouraging findings vs. fundamental problems. In Pineal Gland: Research Advances and Clinical Challenges; Català, A., Ed.; Nova Science: Hauppauge, NY, USA, 2017; pp. 163–204. [Google Scholar]
- Favero, G.; Franceschetti, L.; Bonomini, F.; Rodella, L.F.; Rezzani, R. Melatonin as an anti-inflammatory agent modulating inflammasome activation. Int. J. Endocrinol. 2017, 2017, 1835195. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhou, F.; Dou, Y.; Tian, X.; Liu, C.; Li, H.; Shen, H.; Chen, G. Melatonin alleviates intracerebral hemorrhage-induced secondary brain injury in rats via suppressing apoptosis, inflammation, oxidative stress, DNA damage, and mitochondria injury. Transl. Stroke Res. 2018, 9, 74–91. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R. Recent findings in melatonin research and their relevance to the CNS. Cent. Nerv. Syst. Agents Med. Chem. 2018, 18, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Aging, melatonin and the pro- and anti-inflammatory networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitimus, D.M.; Popescu, M.R.; Voiculescu, S.E.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Zagrean, A.M. Melatonin’s impact on antioxidative and anti-inflammatory reprogramming in homeostasis and disease. Biomolecules 2020, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, I.J.; Huang, C.C.; Liu, S.C.; Tang, C.H. Reconsidering the Role of Melatonin in Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 2877. [Google Scholar] [CrossRef] [PubMed]
- Martín Giménez, V.M.; de Las Heras, N.; Ferder, L.; Lahera, V.; Reiter, R.J.; Manucha, W. Potential effects of melatonin and micronutrients on mitochondrial dysfunction during a cytokine storm typical of oxidative/inflammatory diseases. Diseases 2021, 9, 30. [Google Scholar] [CrossRef]
- Ma, S.; Chen, J.; Feng, J.; Zhang, R.; Fan, M.; Han, D.; Li, X.; Li, C.; Ren, J.; Wang, Y.; et al. Melatonin ameliorates the progression of atherosclerosis via mitophagy activation and NLRP3 inflammasome inhibition. Oxid. Med. Cell. Longev. 2018, 2018, 9286458. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Dos Santos, M.; Veronese, F.V.; Rezzani, R. NLRP3 inflammasome modulation by melatonin aupplementation in chronic pristane-lnduced lupus nephritis. Int. J. Mol. Sci. 2019, 20, 3466. [Google Scholar] [CrossRef] [Green Version]
- Arioz, B.I.; Tastan, B.; Tarakcioglu, E.; Tufekci, K.U.; Olcum, M.; Ersoy, N.; Bagriyanik, A.; Genc, K.; Genc, S. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Front. Immunol. 2019, 10, 1511. [Google Scholar] [CrossRef]
- Wu, X.; Ji, H.; Wang, Y.; Gu, C.; Gu, W.; Hu, L.; Zhu, L. Melatonin alleviates eadiation-induced lung injury via regulation of miR-30e/NLRP3 axis. Oxid. Med. Cell. Longev. 2019, 2019, 4087298. [Google Scholar]
- Wu, H.M.; Zhao, C.C.; Xie, Q.M.; Xu, J.; Fei, G.H. TLR2-melatonin feedback loop regulates the activation of NLRP3 inflammasome in murine allergic airway inflammation. Front. Immunol. 2020, 11, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Ge, J.; Gao, H.; Pan, Y.; Hao, Y.; Li, J. Melatonin attenuates AFB1-induced cardiotoxicity via the NLRP3 signalling pathway. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Su, L.Y.; Sun, C.; Jiao, L.; Miao, Y.; Xu, M.; Luo, R.; Zuo, X.; Zhou, R.; Zheng, P.; et al. Melatonin alleviates morphine analgesic tolerance in mice by decreasing NLRP3 inflammasome activation. Redox Biol. 2020, 34, 101560. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ortiz, M.; Sayed, R.K.A.; Fernández-Martínez, J.; Cionfrini, A.; Aranda-Martínez, P.; Escames, G.; de Haro, T.; Acuña-Castroviejo, D. Melatonin/Nrf2/NLRP3 connection in mouse heart mitochondria during aging. Antioxidants 2020, 9, 1187. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Gao, S.; Wu, H.; Xu, W.; Pan, Y.; Fang, Y.; Wang, X.; Zhang, J. Melatonin ameliorates hemorrhagic transformation via suppression of ROS-induced NLRP3 activation after cerebral ischemia in gyperglycemic rats. Oxid. Med. Cell. Longev. 2021, 2021, 6659282. [Google Scholar] [CrossRef]
- Hardeland, R. Noncoding RNAs: Bridging regulation of circadian rhythms and inflammation. Adv. Neuroimmune Biol. 2019, 7, 155–177. [Google Scholar] [CrossRef]
- Sayed, R.K.; Fernández-Ortiz, M.; Fernández-Martínez, J.; Aranda Martínez, P.; Guerra-Librero, A.; Rodríguez-Santana, C.; de Haro, T.; Escames, G.; Acuña-Castroviejo, D.; Rusanova, I. The impact of melatonin and NLRP3 inflammasome on the expression of microRNAs in aged muscle. Antioxidants 2021, 10, 524. [Google Scholar] [CrossRef]
- Mayo, J.C.; Sainz, R.M.; González Menéndez, P.; Cepas, V.; Tan, D.-X.; Reiter, R.J. Melatonin and sirtuins: A “not-so unexpected” relationship. J. Pineal Res. 2017, 62, e12391. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R. Brain inflammaging: Roles of melatonin, circadian clocks and sirtuins. J. Clin. Cell. Immunol. 2018, 9, 543. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R. Extended signaling by melatonin. Cell Cell. Life Sci. J. 2018, 3, 000123. [Google Scholar]
- Xia, Y.; Zeng, S.; Zhao, Y.; Zhu, C.; Deng, B.; Zhu, G.; Yin, Y.; Wang, W.; Hardeland, R.; Ren, W. Melatonin in macrophage biology: Current understanding and future perspectives. J. Pineal Res. 2019, 66, e12547. [Google Scholar] [CrossRef] [Green Version]
- Crespo, E.; Macías, M.; Pozo, D.; Escames, G.; Martín, M.; Vives, F.; Guerrero, J.M.; Acuña-Castroviejo, D. Melatonin inhibits expression of the inducible NO synthase II in liver and lung and prevents endotoxemia in lipopolysaccharide-induced multiple organ dysfunction syndrome in rats. FASEB J. 1999, 13, 1537–1546. [Google Scholar] [CrossRef] [Green Version]
- Escames, G.; León, J.; Macías, M.; Khaldy, H.; Acuña-Castroviejo, D. Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxide synthase in rats. FASEB J. 2003, 17, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; Acuña-Castroviejo, D.; López, L.C.; Tan, D.-X.; Maldonado, M.D.; Sánchez-Hidalgo, M.; León, J.; Reiter, R.J. Pharmacological utility of melatonin in the treatment of septic shock: Experimental and clinical evidence. J. Pharm. Pharmacol. 2006, 58, 1153–1165. [Google Scholar] [CrossRef]
- Gitto, E.; Marseglia, L.; Manti, S.; D’Angelo, G.; Barberi, I.; Salpietro, C.; Reiter, R.J. Protective role of melatonin in neonatal diseases. Oxid. Med. Cell. Longev. 2013, 2013, 980374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Angelo, G.; Marseglia, L.; Reiter, R.J.; Buonocore, G.; Gitto, E. Melatonin and neonatal sepsis: A promising antioxidant adjuvant agent. Am. J. Perinatol. 2017, 34, 1382–1388. [Google Scholar]
- El-Gendy, F.M.; El-Hawy, M.A.; Hassan, M.G. Beneficial effect of melatonin in the treatment of neonatal sepsis. J. Matern. Fetal Neonatal. Med. 2018, 31, 2299–2303. [Google Scholar] [CrossRef]
- Henderson, R.; Kim, S.; Lee, E. Use of melatonin as adjunctive therapy in neonatal sepsis: A systematic review and meta-analysis. Complement. Ther. Med. 2018, 39, 131–136. [Google Scholar] [CrossRef]
- Tan, D.-X.; Hardeland, R. Potential utility of melatonin in deadly infectious diseases related to the overreaction of innate immune response and destructive inflammation: Focus on COVID-19. Melatonin Res. 2020, 3, 120–143. [Google Scholar] [CrossRef]
- Hardeland, R.; Tan, D.-X. Protection by melatonin in respiratory diseases: Valuable information for the treatment of COVID-19. Melatonin Res. 2020, 3, 264–275. [Google Scholar] [CrossRef]
- Tan, D.-X.; Hardeland, R. Estimated doses of melatonin for treating deadly virus infections: Focus on COVID-19. Melatonin Res. 2020, 3, 276–296. [Google Scholar] [CrossRef]
- Tan, D.-X.; Hardeland, R. Targeting host defense system and rescuing compromised mitochondria to increase tolerance against pathogens by melatonin may impact outcome of deadly virus infection pertinent to COVID-19. Molecules 2020, 25, 4410. [Google Scholar] [CrossRef] [PubMed]
- Tapias, V.; Escames, G.; López, L.C.; López, A.; Camacho, E.; Carrión, M.D.; Entrena, A.; Gallo, M.A.; Espinosa, A.; Acuña-Castroviejo, D. Melatonin and its brain metabolite N1-acetyl-5-methoxykynuramine prevent mitochondrial nitric oxide synthase induction in parkinsonian mice. J. Neurosci. Res. 2009, 87, 3002–3010. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Tan, D.-X.; Hardeland, R.; Leon, J.; Rodriguez, C.; Reiter, R.J. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol. 2005, 165, 139–149. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hardeland, R. Melatonin, Its Metabolites and Their Interference with Reactive Nitrogen Compounds. Molecules 2021, 26, 4105. https://doi.org/10.3390/molecules26134105
Hardeland R. Melatonin, Its Metabolites and Their Interference with Reactive Nitrogen Compounds. Molecules. 2021; 26(13):4105. https://doi.org/10.3390/molecules26134105
Chicago/Turabian StyleHardeland, Rüdiger. 2021. "Melatonin, Its Metabolites and Their Interference with Reactive Nitrogen Compounds" Molecules 26, no. 13: 4105. https://doi.org/10.3390/molecules26134105
APA StyleHardeland, R. (2021). Melatonin, Its Metabolites and Their Interference with Reactive Nitrogen Compounds. Molecules, 26(13), 4105. https://doi.org/10.3390/molecules26134105





