The Regulatory Effects of Licochalcone A on the Intestinal Epithelium and Gut Microbiota in Murine Colitis
Abstract
:1. Introduction
2. Results
2.1. LA Protected against DSS-Induced UC
2.2. LA Suppressed Colonic Inflammation
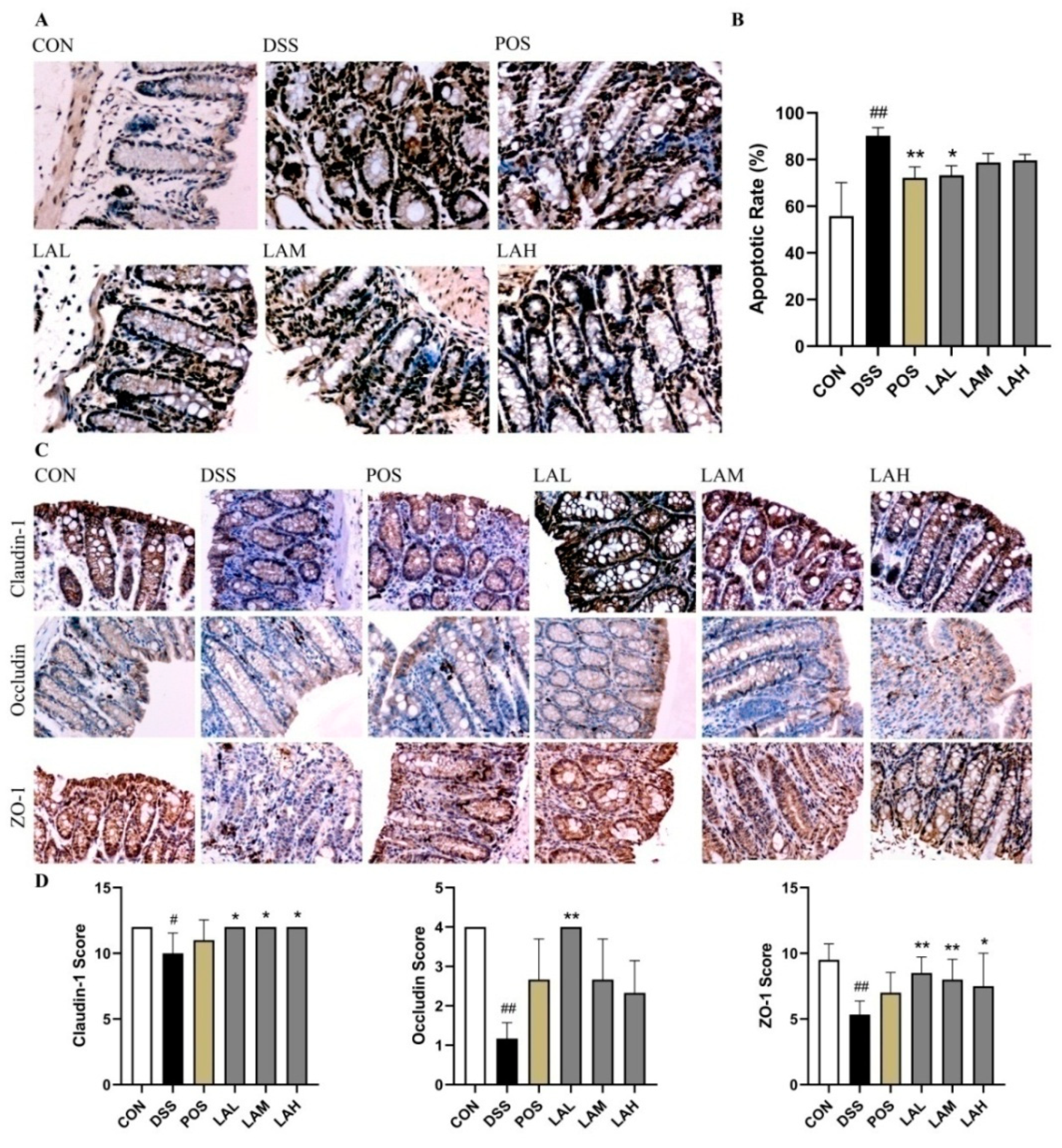
2.3. LA Preserved the Gut Barrier Integrity
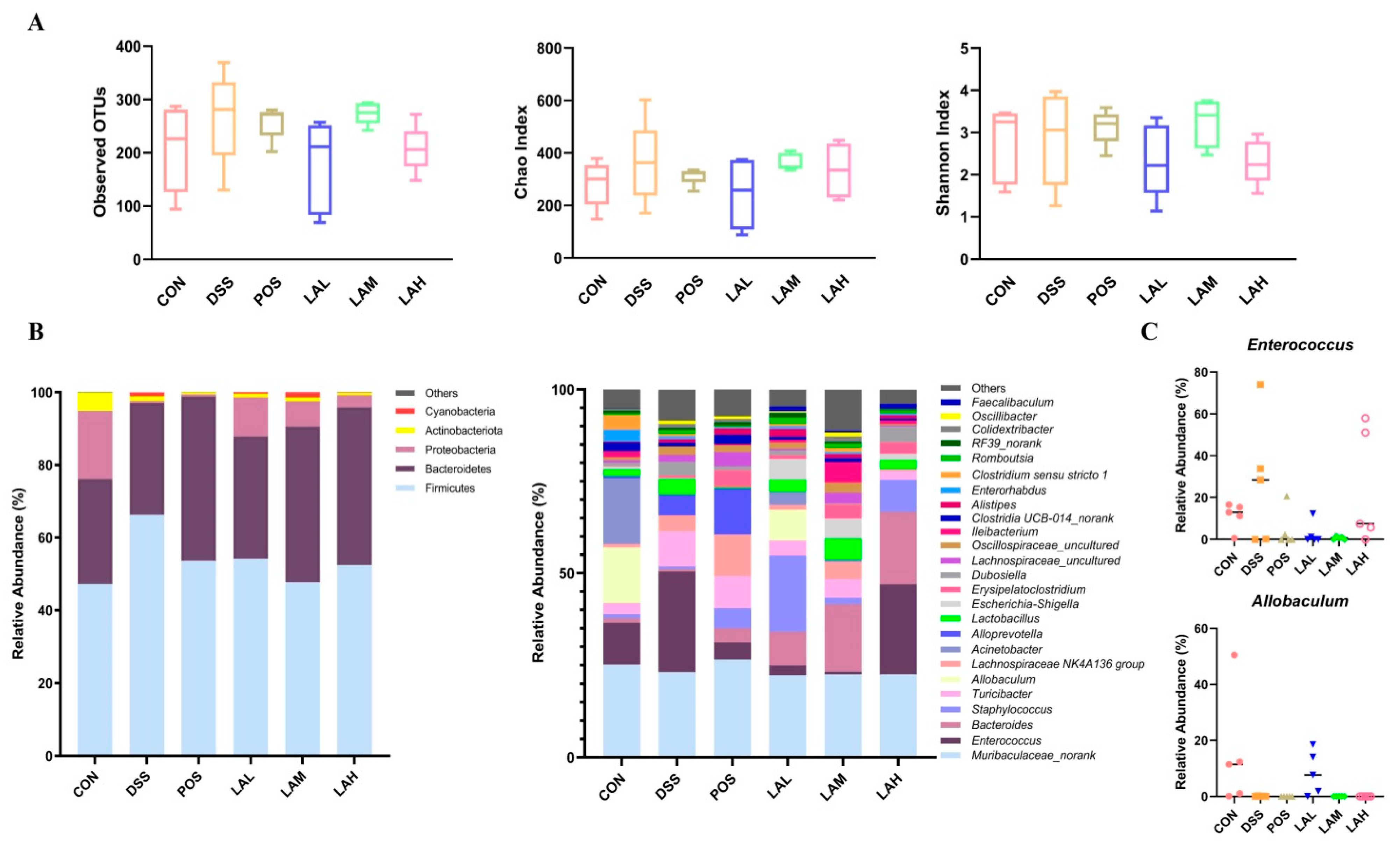
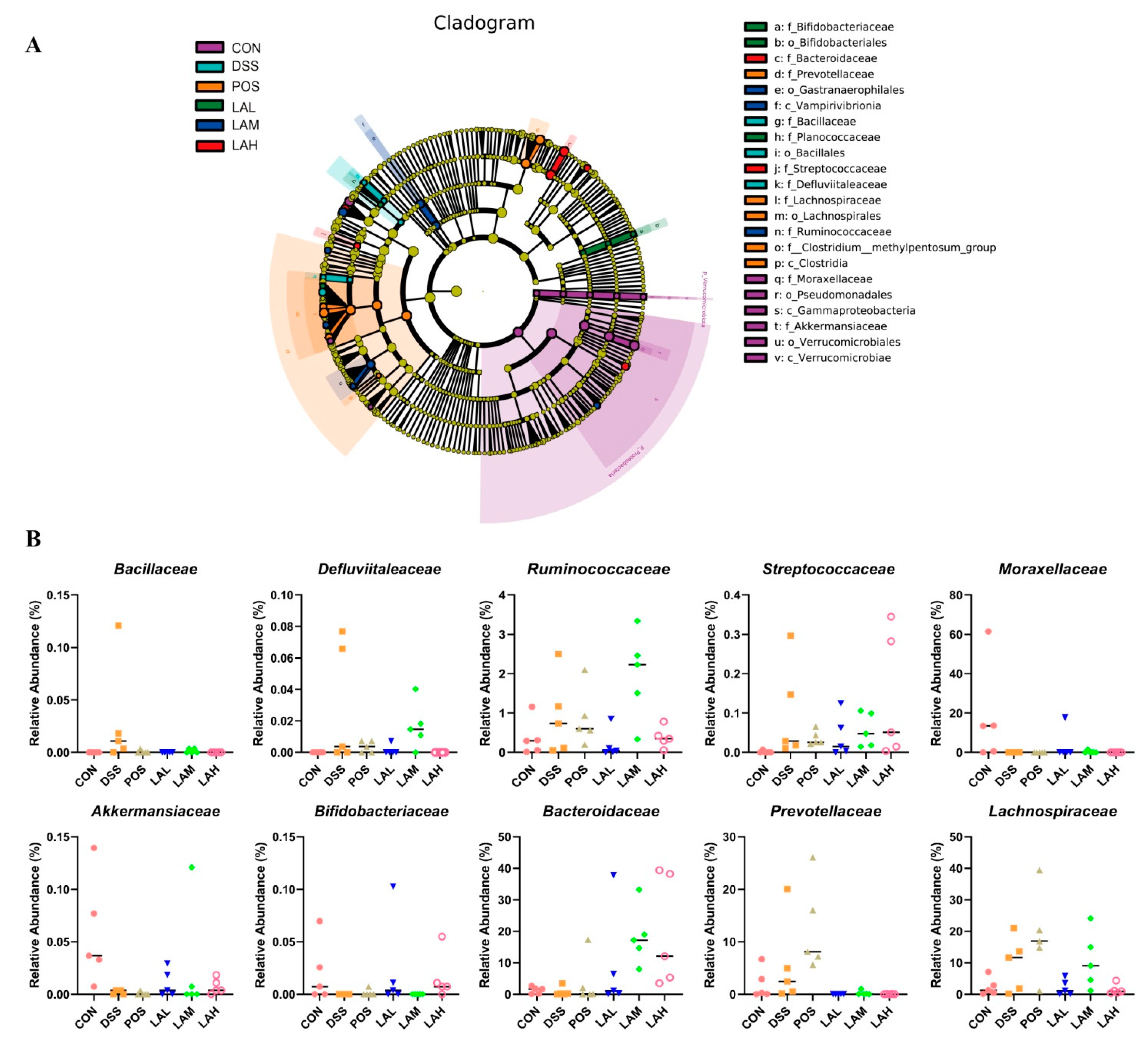
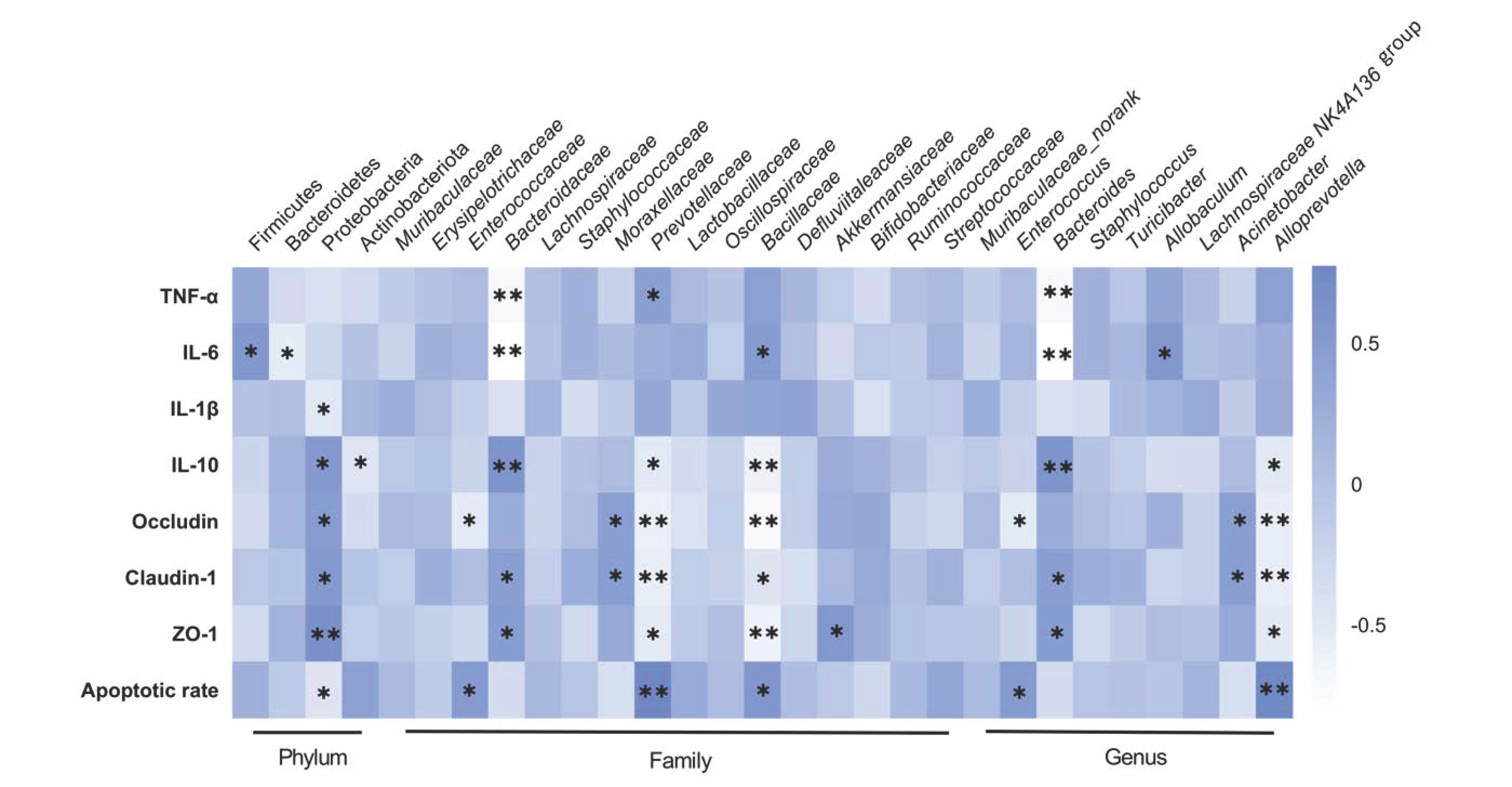
2.4. LA Reshaped the Intestinal Microbiota
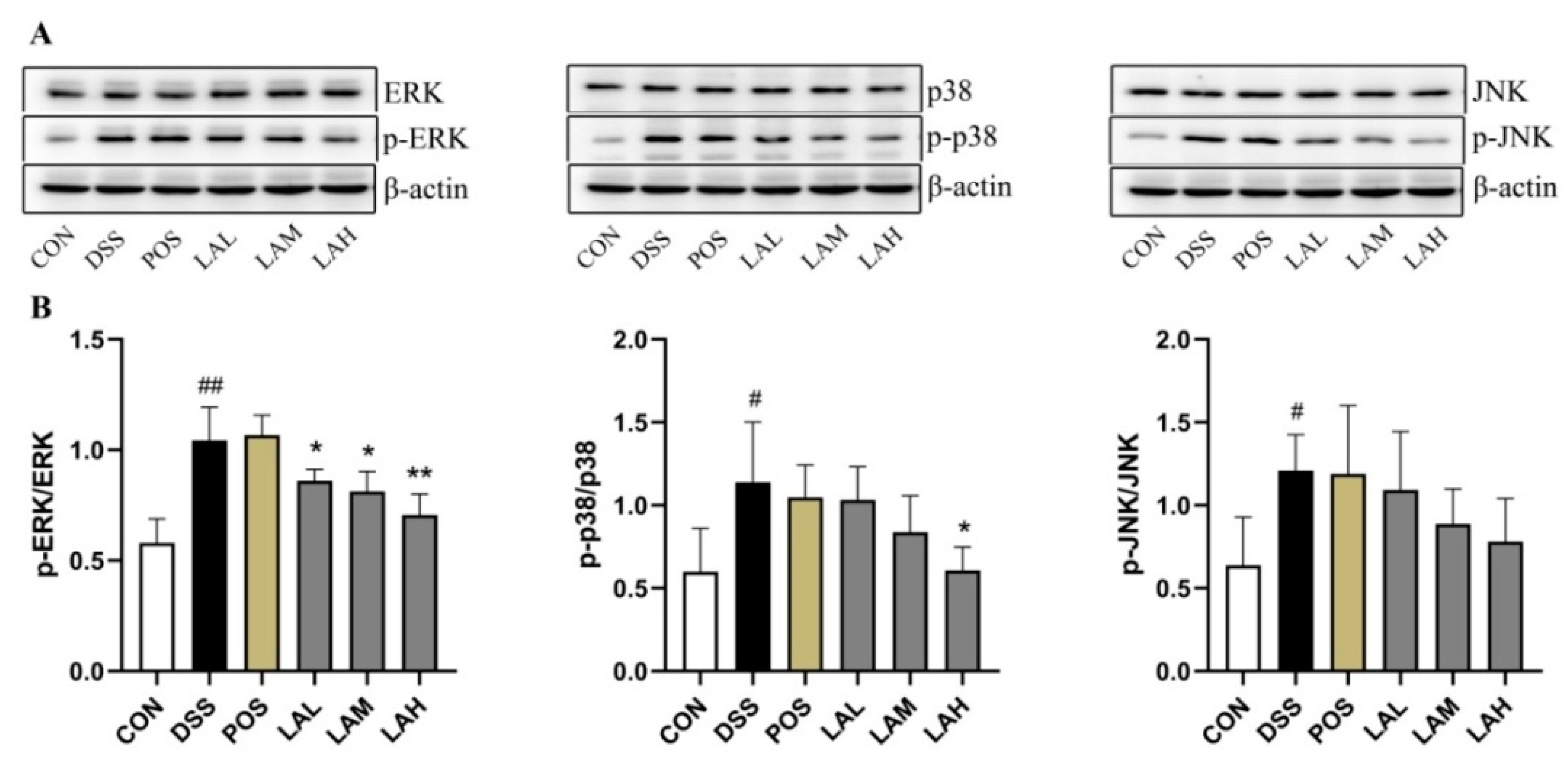
2.5. LA Blocked the Mitogen-Activated Protein Kinase (MAPK) Pathway
3. Discussion
4. Materials and Methods
4.1. Chemicals and Animals
4.2. DSS-Induced Colitis
4.3. Assessment of Disease Activity
4.4. Evaluation of Histology
4.5. ELISA Assay
4.6. TUNEL Assay
4.7. IHC
4.8. 16S rRNA Analysis
4.9. Western Blot
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Guo, T.; Lin, Q.; Li, X.; Nie, Y.; Wang, L.; Shi, L.; Xu, W.; Hu, T.; Guo, T.; Luo, F. Octacosanol attenuates inflammation in both RAW264.7 macrophages and a mouse model of colitis. J. Agric. Food Chem. 2017, 65, 3647–3658. [Google Scholar] [CrossRef] [PubMed]
- Alavala, S.; Sangaraju, R.; Nalban, N.; Sahu, B.D.; Jerald, M.K.; Kilari, E.K.; Sistla, R. Stevioside, a diterpenoid glycoside, shows anti-inflammatory property against dextran sulphate sodium-induced ulcerative colitis in mice. Eur. J. Pharmacol. 2019, 855, 192–201. [Google Scholar] [CrossRef]
- Shastri, S.; Shinde, T.; Sohal, S.S.; Gueven, N.; Eri, R. Idebenone protects against acute murine colitis via antioxidant and anti-Inflammatory mechanisms. Int. J. Mol. Sci. 2020, 21, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Hu, Y.; Li, X.; Zheng, X.; Wang, Y.; Zhang, J.; Fu, C.; Geng, F. Periplaneta Americana ameliorates dextran sulfate sodium-induced ulcerative colitis in rats by Keap1/Nrf-2 activation, intestinal barrier function, and gut microbiota regulation. Front. Pharmacol. 2018, 9, 944. [Google Scholar] [CrossRef]
- Cao, H.; Liu, J.; Shen, P.; Cai, J.; Han, Y.; Zhu, K.; Fu, Y.; Zhang, N.; Zhang, Z.; Cao, Y. Protective effect of naringin on DSS-induced ulcerative colitis in mice. J. Agric. Food Chem. 2018, 66, 13133–13140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Wang, S.N.; Miao, C.Y. Influence of microbiota on intestinal immune system in ulcerative colitis and its intervention. Front. Immunol. 2017, 8, 1674. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Piao, X.; Niu, W.; Zhang, Q.; Ma, C.; Wu, T.; Gu, Q.; Cui, T.; Li, S. Kuijieyuan decoction improved intestinal barrier injury of ulcerative colitis by affecting TLR4-dependent PI3K/AKT/NF-κB oxidative and inflammatory signaling and gut microbiota. Front. Pharmacol. 2020, 11, 1036. [Google Scholar] [CrossRef]
- Zhao, B.; Xia, B.; Li, X.; Zhang, L.; Liu, X.; Shi, R.; Kou, R.; Liu, Z.; Liu, X. Sesamol supplementation attenuates DSS-induced colitis via mediating gut barrier integrity, inflammatory responses, and reshaping gut microbiome. J. Agric. Food Chem. 2020, 68, 10697–10708. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Ruan, Z.; Li, J.; Zhang, L.; Lu, H.; Xu, Z. Puerarin rebuilding the mucus layer and regulating mucin-utilizing bacteria to relieve ulcerative colitis. J. Agric. Food Chem. 2020, 68, 11402–11411. [Google Scholar] [CrossRef]
- Shen, F.; Feng, J.; Wang, X.; Qi, Z.; Shi, X.; An, Y.; Zhang, Q.; Wang, C.; Liu, M.; Liu, B.; et al. Vinegar treatment prevents the development of murine experimental colitis via inhibition of inflammation and apoptosis. J. Agric. Food Chem. 2016, 64, 1111–1121. [Google Scholar] [CrossRef]
- Ren, Q.; Yang, B.; Zhang, H.; Ross, R.P.; Stanton, C.; Chen, H.; Chen, W. c9, t11, c15-CLNA and t9, t11, c15-CLNA from Lactobacillus plantarum ZS2058 ameliorate dextran sodium sulfate-induced colitis in mice. J. Agric. Food Chem. 2020, 68, 3758–3769. [Google Scholar] [CrossRef]
- Ren, X.; Zhu, Y.; Gamallat, Y.; Ma, S.; Chiwala, G.; Meyiah, A.; Xin, Y.E. coli O124 K72 alters the intestinal barrier and the tight junctions proteins of guinea pig intestine. Biomed. Pharmacother. 2017, 94, 468–473. [Google Scholar] [CrossRef]
- Wang, L.; An, J.; Song, S.; Mei, M.; Li, W.; Ding, F.; Liu, S. Electroacupuncture preserves intestinal barrier integrity through modulating the gut microbiota in DSS induced chronic colitis. Life Sci. 2020, 261, 118473. [Google Scholar] [CrossRef]
- Cai, X.; Han, Y.; Gu, M.; Song, M.; Wu, X.; Li, Z.; Li, F.; Goulette, T.; Xiao, H. Dietary cranberry suppressed colonic inflammation and alleviated gut microbiota dysbiosis in dextran sodium sulfate-treated mice. Food Funct. 2019, 10, 6331–6341. [Google Scholar] [CrossRef]
- Sheng, K.; Zhang, G.; Sun, M.; He, S.; Kong, X.; Wang, J.; Zhu, F.; Zha, X.; Wang, Y. Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation. Food Funct. 2020, 11, 7817–7829. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, H.; Hu, X.; Dong, W. Hesperetin ameliorates DSS-induced colitis by maintaining the epithelial barrier via blocking RIPK3/MLKL necroptosis signaling. Eur. J. Pharmacol. 2020, 873, 172992. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, C.; Guo, C.; Li, X. Chitosan ameliorates DSS-induced ulcerative colitis mice by enhancing intestinal barrier function and improving microflora. Int. J. Mol. Sci. 2019, 20, 5751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Wu, J.; Li, J.; Bai, Y.; Luo, Y.; Ji, B.; Xia, B.; Liu, Z.; Tan, X.; Lv, J.; et al. Lycopene alleviates DSS-induced colitis and behavioral disorders via mediating microbes-gut–brain axis balance. J. Agric. Food Chem. 2020, 68, 3963–3975. [Google Scholar] [CrossRef] [PubMed]
- Rizzato, G.; Scalabrin, E.; Radaelli, M.; Capodaglio, G.; Piccolo, O. A new exploration of licorice metabolome. Food Chem. 2017, 221, 959–968. [Google Scholar] [CrossRef]
- Liu, D.; Huo, X.; Gao, L.; Zhang, J.; Ni, H.; Cao, L. NF-κB and Nrf2 pathways contribute to the protective effect of Licochalcone A on dextran sulphate sodium-induced ulcerative colitis in mice. Biomed. Pharmacother. 2018, 102, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Rupa, P.; Kovacs-Nolan, J.; Turner, P.V.; Matsui, T.; Mine, Y. Oral administration of hen egg white ovotransferrin attenuates the development of colitis induced by dextran sodium sulfate in mice. J. Agric. Food Chem. 2015, 63, 1532–1539. [Google Scholar] [CrossRef]
- Yan, F.; Wang, L.; Shi, Y.; Cao, H.; Liu, L.; Washington, M.K.; Chaturvedi, R.; Israel, D.A.; Cao, H.; Wang, B.; et al. Berberine promotes recovery of colitis and inhibits inflammatory responses in colonic macrophages and epithelial cells in DSS-treated mice. Am. J. Physiol. Gastrointest Liver Physiol. 2012, 302, G504–G514. [Google Scholar] [CrossRef]
- Gupta, J.; del Barco Barrantes, I.; Igea, A.; Sakellarious, S.; Pateras, I.S.; Gorgoulis, V.G.; Nebreda, A.R. Dual function of p38α MAPK in colon cancer suppression of colitis-associated tumor initiation but requirement for cancer cell survival. Cancer Cell 2014, 25, 484–500. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.W.; Choi, S.; Kim, S.Y.; Yoon, Y.S.; Kang, J.H.; Oh, S.H. Allyl isothiocyanate ameliorates dextran sodium sulfate-induced colitis in mouse by enhancing tight junction and mucin expression. Int. J. Mol. Sci. 2018, 19, 2025. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, S.; Cao, H.; Shen, P.; Liu, J.; Fu, Y.; Cao, Y.; Zhang, N. The protective role of phloretin against dextran sulfate sodium-induced ulcerative colitis in mice. Food Funct. 2019, 10, 422–431. [Google Scholar] [CrossRef]
- You, B.H.; Chae, H.S.; Song, J.; Ko, H.W.; Chin, Y.W.; Choi, Y.H. α-Mangostin ameliorates dextran sulfate sodium-induced colitis through inhibition ofNF-κBand MAPK pathways. Int. Immunopharmacol. 2017, 49, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Meeker, S.; Seamons, A.; Paik, J.; Treuting, P.M.; Brabb, T.; Grady, W.M.; Maggio-Price, L. Increased dietary vitamin D suppresses MAPK signaling colitis and colon cancer. Cancer Res. 2014, 74, 4398–4408. [Google Scholar] [CrossRef] [Green Version]
- Lang, Y.N.; Yu, J.G.; Zhang, D.B.; Zhang, Z.; Ren, L.L.; Li, L.H.; Wang, Z.; Tang, Z.S. Indigo Naturalis ameliorates dextran sulfate sodium-induced colitis in mice by modulating the intestinal microbiota community. Molecules 2019, 24, 4086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Fan, H.; Liang, R.; Zhang, R.; Zhang, J.; Zhu, J. Taraxacum officinale extract ameliorates dextran sodium sulphate-induced colitis by regulating fatty acid degradation and microbial dysbiosis. J. Cell Mol. Med. 2019, 23, 8161–8172. [Google Scholar] [CrossRef] [PubMed]
- Munyaka, P.M.; Rabbi, M.F.; Khafipour, E.; Ghia, J.E. Acute dextran sulfate sodium (DSS)-induced colitis promotes gut microbial dysbiosis in mice. J. Basic Microbiol. 2016, 56, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, W.; Wang, H.; Ma, Y.; Zhao, X.; Zhang, X.; Yang, H.; Qian, J.; Li, J. Saccharomyces boulardii alleviates ulcerative colitis carcinogenesis in mice by reducing TNF-α and IL-6 levels and functions and by rebalancing intestinal microbiota. BMC Microbiol. 2019, 19, 246. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Wang, H.; Ma, Y.; Zhao, X.; Yang, H.; Qian, J.; Li, J. VSL#3 can prevent ulcerative colitis-associated carcinogenesis in mice. World J. Gastroenterol. 2018, 24, 4254–4262. [Google Scholar]
- Fan, T.J.; Goeser, L.; Naziripour, A.; Redinbo, M.R.; Hansen, J.J. Enterococcus faecalis gluconate phosphotransferase system accelerates experimental colitis and bacterial killing by macrophages. Infect. Immun. 2019, 87, e00080-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.; Lv, H.; Wang, H.; Yang, H.; Li, Y.; Qian, J. Aging increases the severity of colitis and the related changes to the gut barrier and gut microbiota in humans and mice. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Tonkonogy, S.L.; Albright, C.A.; Tsang, J.; Balish, E.J.; Braun, J.; Huycke, M.M.; Sartor, R.B. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology 2005, 128, 891–906. [Google Scholar] [CrossRef]
- Cao, H.; Li, C.; Lei, L.; Wang, X.; Liu, S.; Liu, Q.; Huan, Y.; Sun, S.; Shen, Z. Stachyose improves the effects of berberine on glucose metabolism by regulating intestinal microbiota and short-chain fatty acids in spontaneous type 2 diabetic KKAy mice. Front. Pharmacol. 2020, 11, 578943. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Kim, Y.G.; Pyo, Y.K.; Yi, R.; Zhao, X.; Park, K.Y. Inhibitory effect of Jangkanghwan (Korean traditional food) on experimental ulcerative colitis in mice. J. Food Biochem. 2020, 44, e13488. [Google Scholar] [CrossRef]
- Elinav, E.; Strowig, T.; Kau, A.L.; Henao-Mejia, J.; Thaiss, C.A.; Booth, C.J.; Peaper, D.R.; Bertin, J.; Eisenbarth, S.C.; Gordon, J.I.; et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 2011, 145, 745–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, D.P.; Rosendale, D.I.; Robertson, A.M. Prevotella enzymes involved in mucin oligosaccharide degradation and evidence for a small operon of genes expressed during growth on mucin. FEMS Microbiol. Lett. 2000, 190, 73–79. [Google Scholar] [CrossRef]
- Seo, S.H.; Unno, T.; Park, S.E.; Kim, E.J.; Lee, Y.M.; Na, C.S.; Son, H.S. Korean Traditional Medicine (Jakyakgamcho-tang) ameliorates colitis by regulating gut microbiota. Metabolites 2019, 9, 226. [Google Scholar] [CrossRef] [Green Version]
- Stephens, M.; von der Weid, P.Y. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes 2020, 11, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Zhang, J.; Ren, G.; Ding, L.; Sun, A.; Deng, C.; Wu, X.; Wei, X.; Mani, S.; Wang, Z. Mangiferin attenuates the symptoms of dextran sulfate sodium-induced colitis in mice via NF-κB and MAPK signaling inactivation. Int. Immunopharmacol. 2014, 23, 170–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, R.; Shen, Y.; Shu, W.; Jin, W.; Bai, F.; Wang, J.; Zhang, Y.; El-Seedi, H.; Sun, Q.; Yuan, L. Sturgeon hydrolysates alleviate DSS-induced colon colitis in mice by modulating NF-ĸB, MAPK and microbiota composition. Food Funct. 2020, 11, 6987–6999. [Google Scholar] [CrossRef]
- Jing, M.; Wang, Y.; Xu, L. Andrographolide derivativeAL-1 ameliorates dextran sodium sulfate-induced murine colitis by inhibiting NF-κB and MAPK signaling pathways. Oxid. Med. Cell Longev. 2019, 2019, 6138723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Ma, B.; Wang, J.; Peng, H.; Zheng, M.; Dai, W.; Liu, J. Novel pentapeptide derived from chicken by-product ameliorates DSS-induced colitis by enhancing intestinal barrier function via AhR-induced Src inactivation. J. Agric. Food Chem. 2020. [Google Scholar] [CrossRef]
- Wang, G.; Xu, B.; Shi, F.; Du, M.; Li, Y.; Yu, T.; Chen, L. Protective effect of methane-rich saline on acetic acid-induced ulcerative colitis via blocking the TLR4/NF-κB/MAPK pathway and promoting IL-10/JAK1/STAT3-mediated anti-inflammatory response. Oxid. Med. Cell Longev. 2019, 2019, 7850324. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Wang, C.; Yu, L.; Sheng, T.; Wu, Z.; Wang, X.; Zhang, D.; Lin, Y.; Gong, Y. Chlorogenic acid attenuates dextran sodium sulfate-induced ulcerative colitis in mice through MAPK/ERK/JNK pathway. Biomed. Res. Int. 2019, 2019, 6769789. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Cao, L.; Sun, Y.; Qing, D.-G.; Xu, X.-Q.; Wang, J.-C.; Si, J.-Y.; Li, N. The Regulatory Effects of Licochalcone A on the Intestinal Epithelium and Gut Microbiota in Murine Colitis. Molecules 2021, 26, 4149. https://doi.org/10.3390/molecules26144149
Zhang J, Cao L, Sun Y, Qing D-G, Xu X-Q, Wang J-C, Si J-Y, Li N. The Regulatory Effects of Licochalcone A on the Intestinal Epithelium and Gut Microbiota in Murine Colitis. Molecules. 2021; 26(14):4149. https://doi.org/10.3390/molecules26144149
Chicago/Turabian StyleZhang, Juan, Li Cao, Yu Sun, De-Gang Qing, Xiao-Qin Xu, Jun-Chi Wang, Jian-Yong Si, and Ning Li. 2021. "The Regulatory Effects of Licochalcone A on the Intestinal Epithelium and Gut Microbiota in Murine Colitis" Molecules 26, no. 14: 4149. https://doi.org/10.3390/molecules26144149
APA StyleZhang, J., Cao, L., Sun, Y., Qing, D.-G., Xu, X.-Q., Wang, J.-C., Si, J.-Y., & Li, N. (2021). The Regulatory Effects of Licochalcone A on the Intestinal Epithelium and Gut Microbiota in Murine Colitis. Molecules, 26(14), 4149. https://doi.org/10.3390/molecules26144149





