Evaluation of the Health-Promoting Properties of Selected Fruits
Abstract
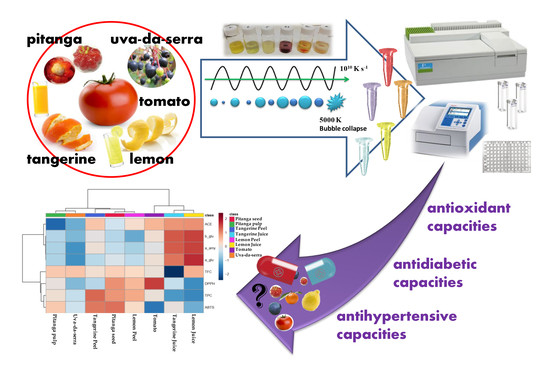
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Samples
2.3. Extraction
2.4. Evaluation of Bioactive Potential
2.4.1. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.4.2. Total Antioxidant Capacity (TAC)
2.4.3. Antihypertensive Capacity
2.4.4. Antidiabetic Capacity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Content and Antioxidant Capacity of the Selected Fruits Extracts
3.2. Enzymatic Inhibition Capacity
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Cheung, J.T.H.; Lok, J.; Gietel-Basten, S.; Koh, K. The Food Environments of Fruit and Vegetable Consumption in East and Southeast Asia: A Systematic Review. Nutrients 2021, 13, 148. [Google Scholar] [CrossRef]
- Mannucci, C.; Casciaro, M.; Sorbara, E.; Calapai, F.; Di Salvo, E.; Pioggia, G.; Navarra, M.; Calapai, G.; Gangemi, S. Nutraceuticals against Oxidative Stress in Autoimmune Disorders. Antioxidants 2021, 10, 261. [Google Scholar] [CrossRef]
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.G.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.M.; Barros, L.; Ferreira, I.C.F.R. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods 2020, 10, 37. [Google Scholar] [CrossRef]
- Figueira, J.A.; Pereira, J.; Câmara, J.S. Quantification of δ-, γ- and α-Tocopherol in Tomatoes Using an Improved Liquid-Dispersive Solid-Phase Extraction Combined with Ultrahigh Pressure Liquid Chromatography. Food Anal. Methods 2017, 10, 2507–2517. [Google Scholar] [CrossRef]
- Detopoulou, P.; Demopoulos, C.; Antonopoulou, S. Micronutrients, Phytochemicals and Mediterranean Diet: A Potential Protective Role against COVID-19 through Modulation of PAF Actions and Metabolism. Nutrients 2021, 13, 462. [Google Scholar] [CrossRef] [PubMed]
- El-Missiry, M.A.; Fekri, A.; Kesar, L.A.; Othman, A.I. Polyphenols are potential nutritional adjuvants for targeting COVID-19. Phytother. Res. 2021, 35, 2879–2889. [Google Scholar] [CrossRef]
- Ahmed, O.M.; AbouZid, S.F.; Ahmed, N.A.; Zaky, M.Y.; Liu, H. An Up-to-Date Review on Citrus Flavonoids: Chemistry and Benefits in Health and Diseases. Curr. Pharm. Des. 2021, 27, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Figueira, J.A.; Porto-Figueira, P.; Pereira, J.A.; Câmara, J.S. Tangerines Cultivated on Madeira Island—A High Throughput Natural Source of Bioactive Compounds. Foods 2020, 9, 1470. [Google Scholar] [CrossRef] [PubMed]
- Figueira, J.A.; Porto-Figueira, P.; Pereira, J.; Câmara, J.S. A comprehensive methodology based on NTME/GC-MS data and chemometric tools for lemons discrimination according to geographical origin. Microchem. J. 2020, 157, 104933. [Google Scholar] [CrossRef]
- TuTunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a flavanone with antiviral and anti-inflammatory effects: A promising treatment strategy against COVID-19. Phytother. Res. 2020, 34, 3137–3147. [Google Scholar] [CrossRef]
- Alberca, R.W.; Teixeira, F.M.E.; Beserra, D.R.; De Oliveira, E.A.; Andrade, M.M.D.S.; Pietrobon, A.J.; Sato, M.N. Perspective: The Potential Effects of Naringenin in COVID-19. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Carvalho, M.J.; Gouveia, C.S.; Vieira, A.C.; Pereira, A.C.; de Carvalho, M.A.A.P.; Marques, J.C. Nutritional and Phytochemical Composition of Vaccinium padifoliumSm Wild Berries and Radical Scavenging Activity. J. Food Sci. 2017, 82, 2554–2561. [Google Scholar] [CrossRef]
- Spínola, V.; Pinto, J.; Castilho, P.C. Hypoglycemic, anti-glycation and antioxidant in vitro properties of two Vaccinium species from Macaronesia: A relation to their phenolic composition. J. Funct. Foods 2018, 40, 595–605. [Google Scholar] [CrossRef]
- Figueira, J.A.; Porto-Figueira, P.; Pereira, J.A.M.; Câmara, J.S. Fingerprint of the free low molecular weight phenolics compo-sition and bioactivity of Vaccinium padifolium Sm. fruits. Food Res. Int. in press.
- Bicas, J.L.; Molina, G.; Dionísio, A.P.; Barros, F.F.C.; Wagner, R.; Maróstica, M.R., Jr.; Pastore, G.M. Volatile constituents of exotic fruits from Brazil. Food Res. International. 2011, 44, 1843–1855. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.C.; Zola, F.G.; Ávila Oliveira, B.D.; Sacramento, N.T.B.; Da Silva, E.R.; Bertoldi, M.C.; Taylor, J.G.; Pinto, U.M. Quorum Quenching and Microbial Control through Phenolic Extract of Eugenia Uniflora Fruits. J. Food Sci. 2016, 81, M2538–M2544. [Google Scholar] [CrossRef] [PubMed]
- Siebert, D.A.; De Mello, F.; Alberton, M.D.; Vitali, L.; Micke, G.A. Determination of acetylcholinesterase and α-glucosidase inhibition by electrophoretically-mediated microanalysis and phenolic profile by HPLC-ESI-MS/MS of fruit juices from Brazilian Myrtaceae Plinia cauliflora (Mart.) Kausel and Eugenia uniflora L. Nat. Prod. Res. 2019, 34, 2683–2688. [Google Scholar] [CrossRef]
- Biazotto, K.R.; Mesquita, L.M.D.S.; Neves, B.V.; Braga, A.; Tangerina, M.M.P.; Vilegas, W.; Mercadante, A.Z.; De Rosso, V.V. Brazilian Biodiversity Fruits: Discovering Bioactive Compounds from Underexplored Sources. J. Agric. Food Chem. 2019, 67, 1860–1876. [Google Scholar] [CrossRef]
- Sobeh, M.; El-Raey, M.; Rezq, S.; Abdelfattah, M.A.; Petruk, G.; Osman, S.; El-Shazly, A.M.; El-Beshbishy, H.A.; Mahmoud, M.; Wink, M. Chemical profiling of secondary metabolites of Eugenia uniflora and their antioxidant, anti-inflammatory, pain killing and anti-diabetic activities: A comprehensive approach. J. Ethnopharmacol. 2019, 240, 111939. [Google Scholar] [CrossRef]
- Aranha, E.S.P.; de Azevedo, S.G.; dos Reis, G.G.; Lima, E.S.; Machado, M.B.; de Vasconcellos, M.C. Essential oils from Eugenia spp.: In vitro antiproliferative potential with inhibitory action of metalloproteinases. Ind. Crop. Prod. 2019, 141, 111736. [Google Scholar] [CrossRef]
- Santos, D.N.; de Souza, L.L.; de Oliveira, C.A.F.; da Silva, E.R.; de Oliveira, A.L. Arginase inhibition, antibacterial and antioxidant activities of Pitanga seed (Eugenia uniflora L.) extracts from sustainable technologies of high pressure extraction. Food Biosci. 2015, 12, 93–99. [Google Scholar] [CrossRef]
- Soares, D.J.; Walker, J.; Pignitter, M.; Walker, J.M.; Imboeck, J.M.; Ehrnhoefer-Ressler, M.M.; Brasil, I.M.; Somoza, V. Pitanga (Eugenia uniflora L.) fruit juice and two major constituents thereof exhibit anti-inflammatory properties in human gingival and oral gum epithelial cells. Food Funct. 2014, 5, 2981–2988. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Destandau, E.; Fougère, L.; Lafosse, M. Isolation by pressurised fluid extraction (PFE) and identification using CPC and HPLC/ESI/MS of phenolic compounds from Brazilian cherry seeds (Eugenia uniflora L.). Food Chem. 2014, 145, 522–529. [Google Scholar] [CrossRef]
- Figueira, J.A.; Pereira, J.A.; Porto-Figueira, P.; Câmara, J.S. Ultrasound-assisted liquid-liquid extraction followed by ultrahigh pressure liquid chromatography for the quantification of major carotenoids in tomato. J. Food Compos. Anal. 2017, 57, 87–93. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Holmquist, B.; Bünning, P.; Riordan, J.F. A continuous spectrophotometric assay for angiotensin converting enzyme. Anal. Biochem. 1979, 95, 540–548. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldenberg, L.; Yaniv, Y.; Porat, R.; Carmi, N. Mandarin fruit quality: A review. J. Sci. Food Agric. 2017, 98, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-Y.; Chu, Y.-L.; Sridhar, K.; Tsai, P.-J. Effect of ultrasound, high-pressure processing, and enzymatic hydrolysis on car-bohydrate hydrolyzing enzymes and antioxidant activity of lemon (Citrus limon) flavedo. LWT 2021, 138, 110511. [Google Scholar] [CrossRef]
- Aguiar, J.; Gonçalves, J.L.; Alves, V.L.; Câmara, J.S. Chemical Fingerprint of Free Polyphenols and Antioxidant Activity in Dietary Fruits and Vegetables Using a Non-Targeted Approach Based on QuEChERS Ultrasound-Assisted Extraction Combined with UHPLC-PDA. Antioxidants 2020, 9, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raspo, M.A.; Vignola, M.B.; Andreatta, A.E.; Juliani, H.R. Antioxidant and antimicrobial activities of citrus essential oils from Argentina and the United States. Food Biosci. 2020, 36, 100651. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Moreno, D.A.; García-Viguera, C. Phytochemistry and biological activity of Spanish Citrus fruits. Food Funct. 2014, 5, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilyas, Z.; Shah, H.S.; Al-Oweini, R.; Kortz, U.; Iqbal, J. Antidiabetic potential of polyoxotungstates: In vitro and in vivo studies. Metallomics 2014, 6, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Błaszczak, W.; Jeż, M.; Szwengiel, A. Polyphenols and inhibitory effects of crude and purified extracts from tomato varieties on the formation of advanced glycation end products and the activity of angiotensin-converting and acetylcholinesterase enzymes. Food Chem. 2020, 314, 126181. [Google Scholar] [CrossRef]
- Rey, F.; Zacarías, L.; Rodrigo, M.J. Carotenoids, Vitamin C, and Antioxidant Capacity in the Peel of Mandarin Fruit in Relation to the Susceptibility to Chilling Injury during Postharvest Cold Storage. Antioxidants 2020, 9, 1296. [Google Scholar] [CrossRef]
- Alu’Datt, M.H.; Rababah, T.; Alhamad, M.N.; Al-Mahasneh, M.A.; Ereifej, K.; Al-Karaki, G.; Al-Duais, M.; Andrade, J.E.; Tranchant, C.C.; Kubow, S.; et al. Profiles of free and bound phenolics extracted from Citrus fruits and their roles in biological systems: Content, and antioxidant, anti-diabetic and anti-hypertensive properties. Food Funct. 2017, 8, 3187–3197. [Google Scholar] [CrossRef] [PubMed]





| DPPH | TFC | TPC | ABTS | β-glu | α-glu | α-amy | ACE |
|---|---|---|---|---|---|---|---|
| 1.00 | −0.80 | −0.65 | −0.83 | 0.22 | 0.13 | 0.02 | −0.11 |
| 1.00 | 0.27 | 0.57 | −0.21 | −0.27 | −0.33 | −0.12 | |
| 1.00 | 0.92 | 0.48 | 0.59 | 0.74 | 0.77 | ||
| 1.00 | 0.35 | 0.29 | 0.47 | 0.51 | |||
| 1.00 | 0.75 | 0.81 | 0.72 | ||||
| 1.00 | 0.92 | 0.96 | |||||
| 1.00 | 0.94 | ||||||
| 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueira, J.A.; Porto-Figueira, P.; Berenguer, C.; Pereira, J.A.M.; Câmara, J.S. Evaluation of the Health-Promoting Properties of Selected Fruits. Molecules 2021, 26, 4202. https://doi.org/10.3390/molecules26144202
Figueira JA, Porto-Figueira P, Berenguer C, Pereira JAM, Câmara JS. Evaluation of the Health-Promoting Properties of Selected Fruits. Molecules. 2021; 26(14):4202. https://doi.org/10.3390/molecules26144202
Chicago/Turabian StyleFigueira, José A., Priscilla Porto-Figueira, Cristina Berenguer, Jorge A. M. Pereira, and José S. Câmara. 2021. "Evaluation of the Health-Promoting Properties of Selected Fruits" Molecules 26, no. 14: 4202. https://doi.org/10.3390/molecules26144202
APA StyleFigueira, J. A., Porto-Figueira, P., Berenguer, C., Pereira, J. A. M., & Câmara, J. S. (2021). Evaluation of the Health-Promoting Properties of Selected Fruits. Molecules, 26(14), 4202. https://doi.org/10.3390/molecules26144202