Expanding the Chemical Space of Benzimidazole Dicationic Ionic Liquids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Benzimidazole Dicationic Ionic Liquids
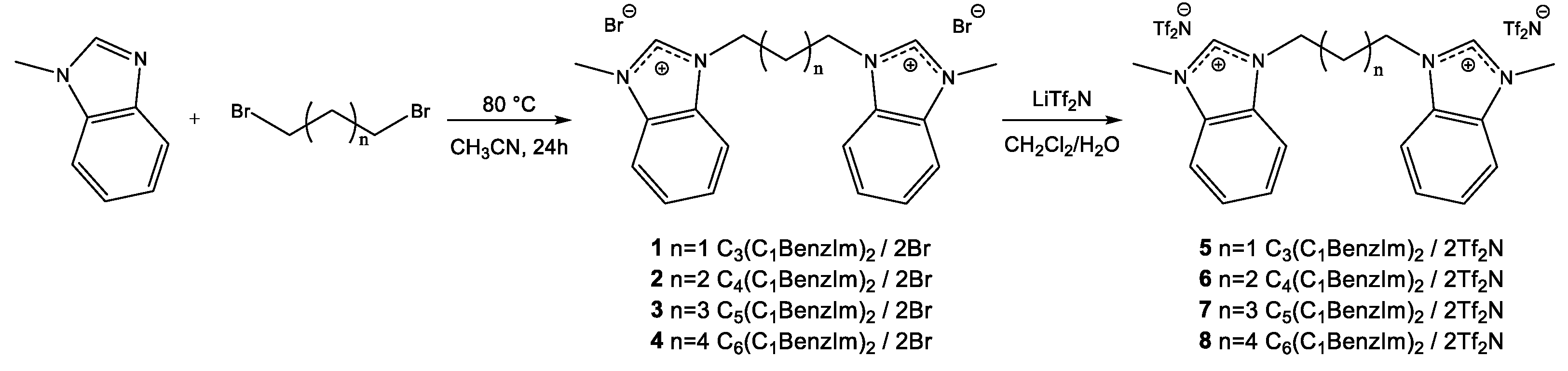
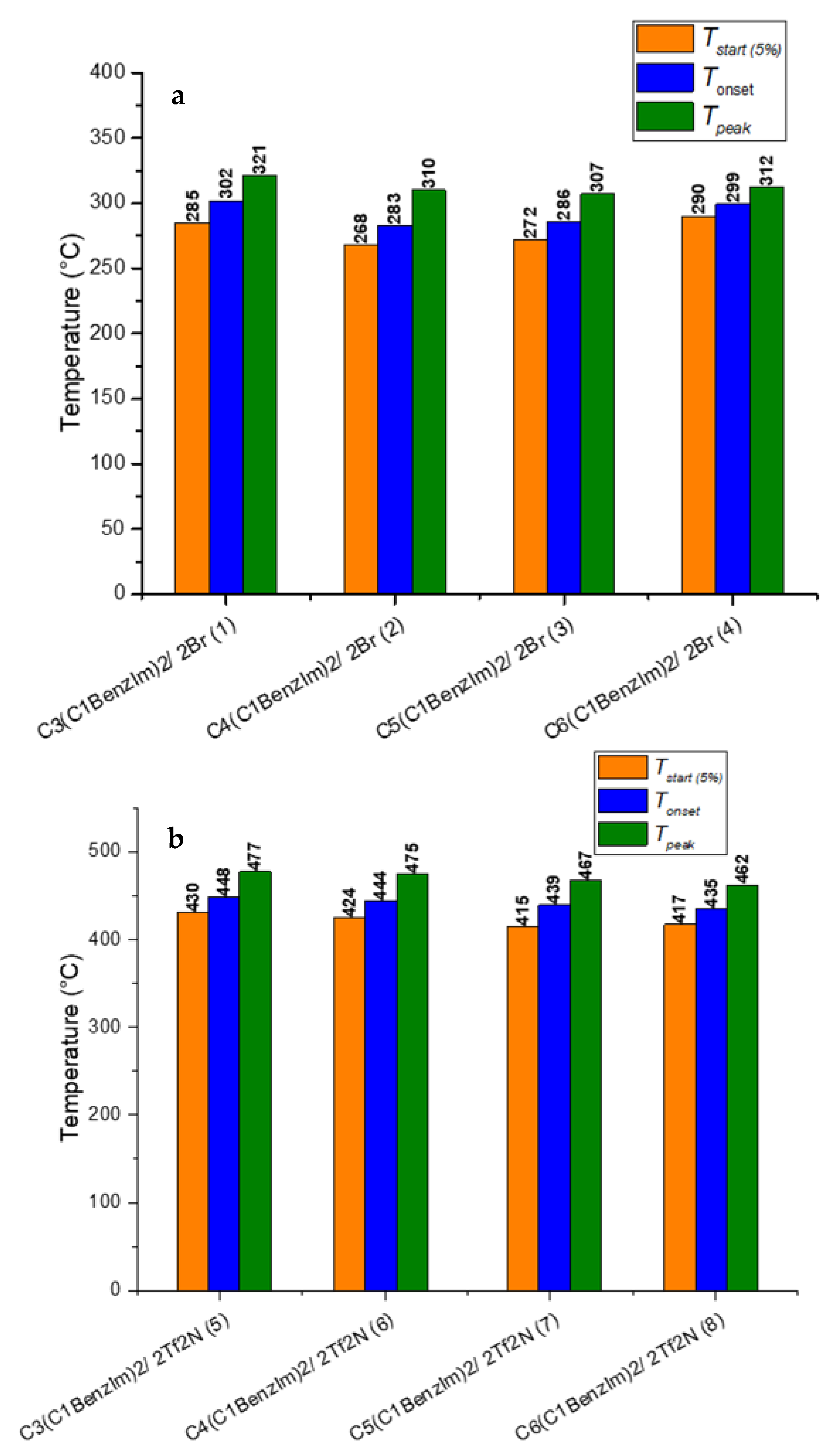
2.2. Thermal Gravimetric Analysis (TGA)
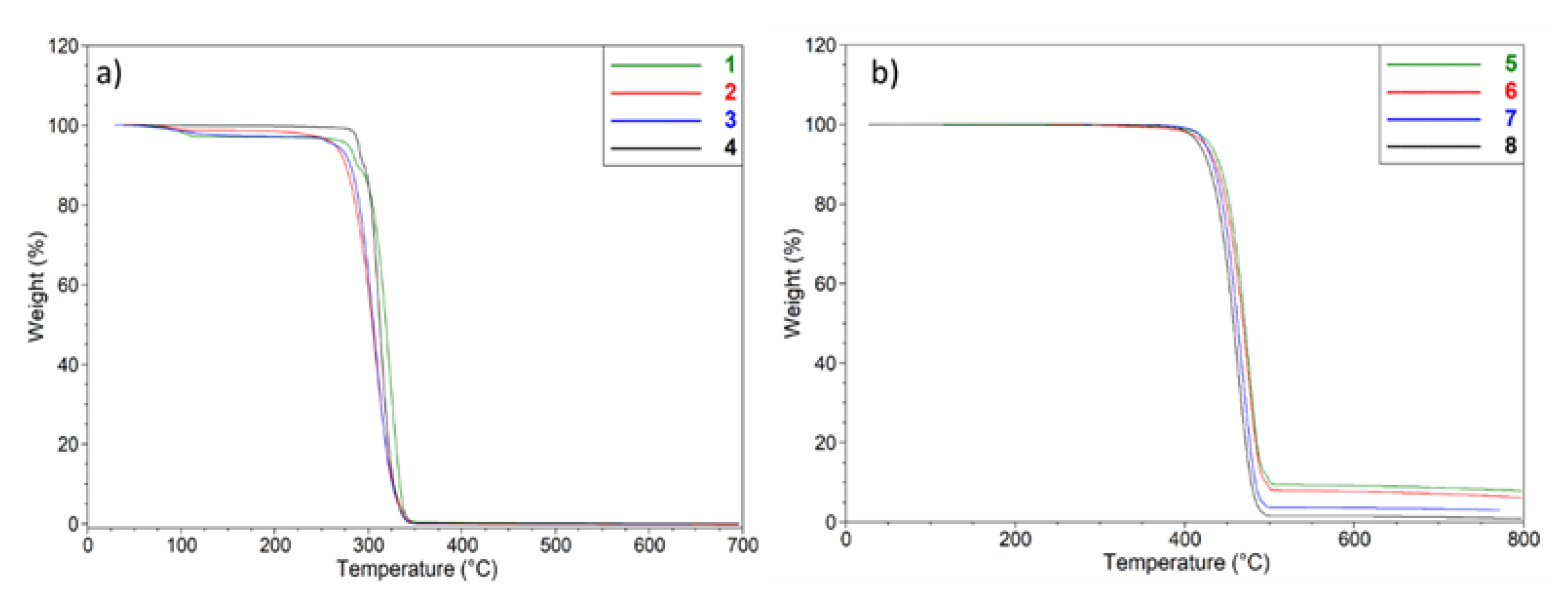
2.3. Differential Scanning Calorimetry (DSC)
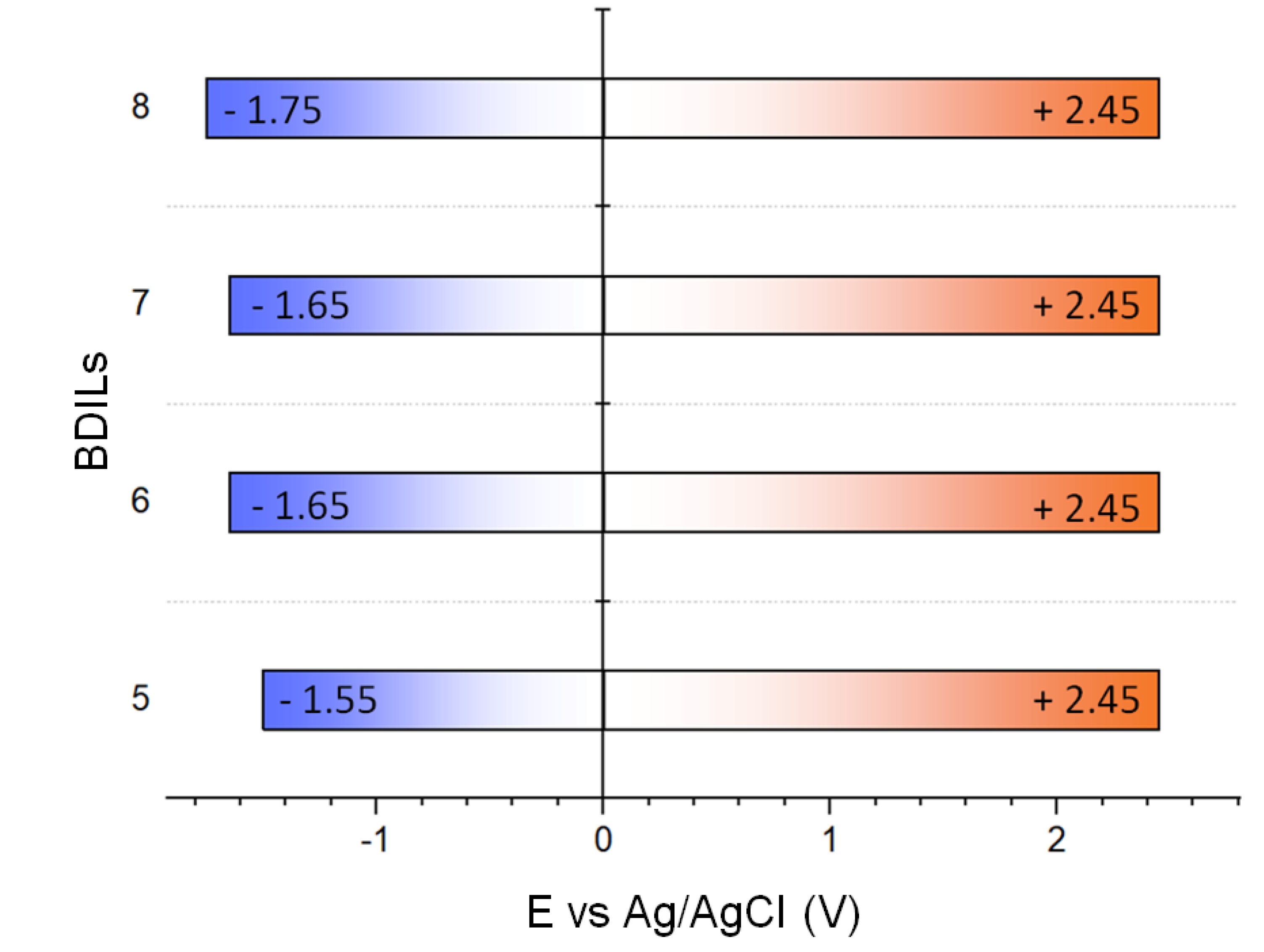
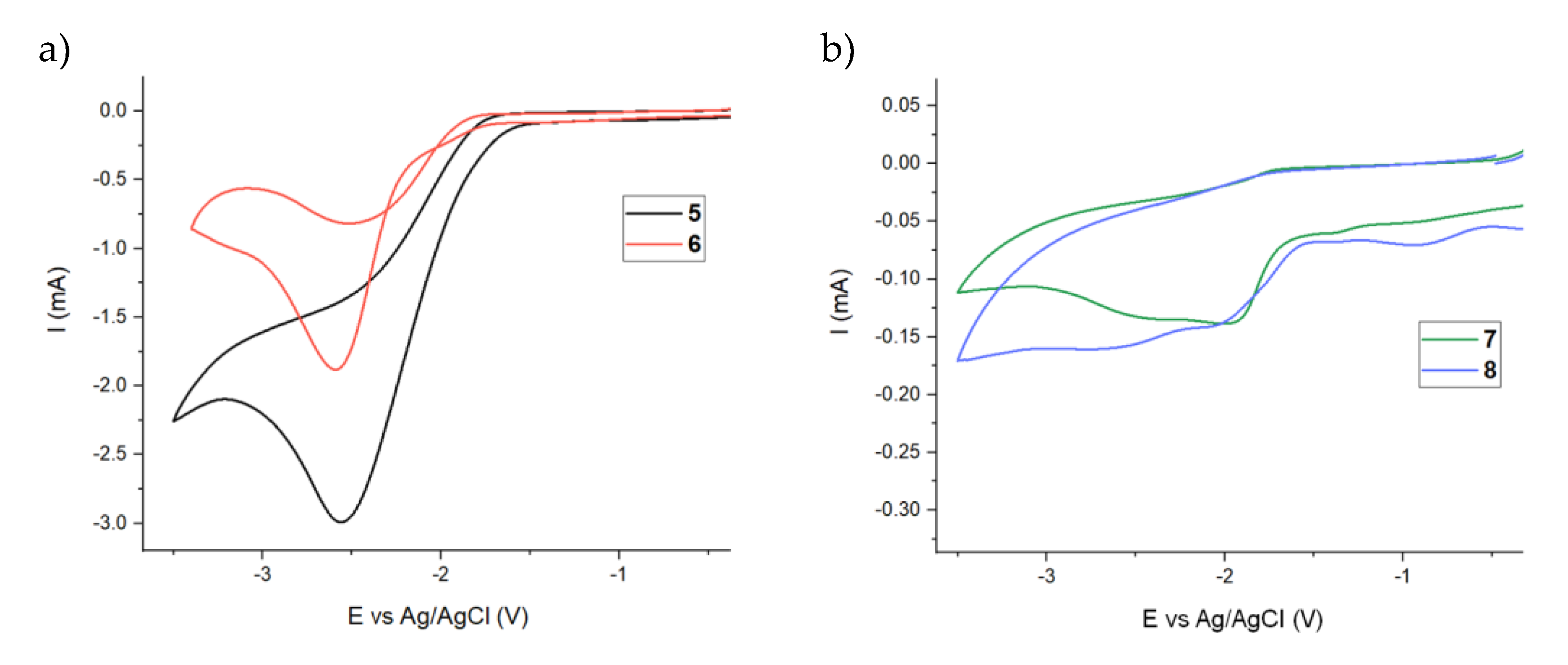
2.4. Electrochemical Window Determination
3. Materials and Methods
3.1. Material and Instruments
3.2. Synthesis of DILs
3.2.1. General Procedure for Synthesis of Bromide Benzimidazole Dicationic Ionic Liquids (Bdils)
3.2.2. General Procedure of the Metathesis Reaction
3.2.3. 3,3’-(Propane-1,3-diyl)bis(1-methyl-1H-benzimidazolium) Dibromide C3(C1BenzIm)2/2Br
3.2.4. 3,3’-(Butane-1,4-diyl)bis(1-methyl-1H-benzimidazolium) Dibromide C4(C1BenzIm)2/2Br (2)
3.2.5. 3,3’-(Pentane-1,5-diyl)bis(1-methyl-1H-benzimidazolium) Dibromide C5(C1BenzIm)2/2Br (3)
3.2.6. 3,3’-(Hexane-1,6-diyl)bis(1-methyl-1H-benzimidazolium) Dibromide C6(C1BenzIm)2/2Br (4)
3.2.7. 3,3’-(Propane-1,3-diyl)bis(1-methyl-1H-benzimidazolium) diBis[(Trifluoromethyl)Sulfonyl]Imide C3(C1BenzIm)2/2Tf2N (5)
3.2.8. 3,3’-(Butane-1,4-diyl)bis(1-methyl-1H-benzimidazolium) diBis[(Trifluoromethyl)Sulfonyl]Imide C4(C1BenzIm)2/2Tf2N (6)
3.2.9. 3,3’-(Pentane-1,5-diyl)bis(1-methyl-1H-benzimidazolium) diBis[(Trifluoromethyl)Sulfonyl]Imide C5(C1BenzIm)2/2Tf2N (7)
3.2.10. 3,3’-(Hexane-1,5-diyl)bis(1-methyl-1H-benzimidazolium) diBis[(Trifluoromethyl)Sulfonyl]Imide C6(C1BenzIm)2/2Tf2N (8)
3.3. Thermal Analysis
3.3.1. Thermal Gravimetric Analysis
3.3.2. Differential Scanning Calorimetry
3.3.3. Cyclic Voltammetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Singh, S.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, H. Tullo the time is now for ionic liquids. Chem. Eng. News 2020, 98, 3. [Google Scholar]
- Egorova, K.S.; Gordeev, E.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Renier, O.; Bousrez, G.; Yang, M.; Hoelter, M.; Mallick, B.; Smetana, V.; Mudring, A.-V. Developing design tools for introducing and tuning structural order in ionic liquids. CrystEngComm 2021, 23, 1785–1795. [Google Scholar] [CrossRef]
- He, F.; Chen, J.; Gong, Z.; Xu, Q.; Yue, W.; Xie, H. Dissolution pretreatment of cellulose by using levulinic acid-based protic ionic liquids towards enhanced enzymatic hydrolysis. Carbohydr. Polym. 2021, 269, 118271. [Google Scholar] [CrossRef]
- Mezzetta, A.; Becherini, S.; Pretti, C.; Monni, G.; Casu, V.; Chiappe, C.; Guazzelli, L. Insights into the levulinate-based ionic liquid class: Synthesis, cellulose dissolution evaluation and ecotoxicity assessment. New J. Chem. 2019, 43, 13010–13019. [Google Scholar] [CrossRef]
- Xu, C.; Cheng, Z. Thermal Stability of Ionic Liquids: Current Status and Prospects for Future Development. Processes 2021, 9, 337. [Google Scholar] [CrossRef]
- Clarke, C.J.; Bui-Le, L.; Hallett, J.P.; Licence, P. Thermally-Stable Imidazolium Dicationic Ionic Liquids with Pyridine Functional Groups. ACS Sustain. Chem. Eng. 2020, 8, 8762–8772. [Google Scholar] [CrossRef]
- Avila, J.; Lepre, L.F.; Santini, C.C.; Tiano, M.; Denis-Quanquin, S.; Szeto, K.C.; Padua, A.A.H.; Gomes, M.C. High-Performance Porous Ionic Liquids for Low-Pressure CO 2 Capture**. Angew. Chem. Int. Ed. 2021, 60, 12876–12882. [Google Scholar] [CrossRef]
- Arosa, Y.; Fernández, C.D.R.; Lago, E.L.; Amigo, A.; Varela, L.M.; Cabeza, O.; de la Fuente, R. Refractive index measurement of imidazolium based ionic liquids in the Vis-NIR. Opt. Mater. 2017, 73, 647–657. [Google Scholar] [CrossRef]
- Barulli, L.; Mezzetta, A.; Brunetti, B.; Guazzelli, L.; Ciprioti, S.V.; Ciccioli, A. Evaporation thermodynamics of the tetraoctylphosphonium bis (trifluoromethansulfonyl) imide ([P8888] NTf2) and tetraoctylphosphonium nonafluorobutane-1-sulfonate ([P8888] NFBS) ionic liquids. J. Mol. Liq. 2021, 333, 115892. [Google Scholar] [CrossRef]
- Cimini, A.; Palumbo, O.; Simonetti, E.; De Francesco, M.; Appetecchi, G.B.; Fantini, S.; Lin, R.; Falgayrat, A.; Paolone, A. Decomposition temperatures and vapour pressures of selected ionic liquids for electrochemical applications. J. Therm. Anal. Calorim. 2020, 142, 1791–1797. [Google Scholar] [CrossRef]
- Wu, H.-B.; Zhang, B.; Liu, S.-H.; Chen, C.-C. Flammability estimation of 1-hexyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide. J. Loss Prev. Process. Ind. 2020, 66, 104196. [Google Scholar] [CrossRef]
- Hulsbosch, J.; De Vos, D.E.; Binnemans, K.; Ameloot, R. Biobased Ionic Liquids: Solvents for a Green Processing Industry? ACS Sustain. Chem. Eng. 2016, 4, 2917–2931. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic Liquids Toxicity—Benefits and Threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef] [PubMed]
- Florio, W.; Becherini, S.; D’Andrea, F.; Lupetti, A.; Chiappe, C.; Guazzelli, L. Comparative evaluation of antimicrobial activity of different types of ionic liquids. Mater. Sci. Eng. C 2019, 104, 109907. [Google Scholar] [CrossRef] [PubMed]
- Hijo, A.T.; Maximo, G.J.; Costa, M.C.; Batista, E.A.C.; Meirelles, A.J.A. Applications of Ionic Liquids in the Food and Bioproducts Industries. ACS Sustain. Chem. Eng. 2016, 4, 5347–5369. [Google Scholar] [CrossRef]
- Martins, P.; Braga, A.R.; De Rosso, V.V. Can ionic liquid solvents be applied in the food industry? Trends Food Sci. Technol. 2017, 66, 117–124. [Google Scholar] [CrossRef]
- Lu, B.; Yi, M.; Hu, S.; Wu, D.; Zhu, Z.; Wu, C.; Wang, Z.; Li, Y.; Zhang, J. Taurine-Based Ionic Liquids for Transdermal Protein Delivery and Enhanced Anticancer Activity. ACS Sustain. Chem. Eng. 2021, 9, 5991–6000. [Google Scholar] [CrossRef]
- Demurtas, M.; Onnis, V.; Zucca, P.; Rescigno, A.; Lachowicz, J.I.; Engelbrecht, L.d.V.; Nieddu, M.; Ennas, G.; Scano, A.; Mocci, F.; et al. Cholinium-Based Ionic Liquids from Hydroxycinnamic Acids as New Promising Bioactive Agents: A Combined Experimental and Theoretical Investigation. ACS Sustain. Chem. Eng. 2021, 9, 2975–2986. [Google Scholar] [CrossRef]
- Pedro, S.; Freire, C.R.; Silvestre, A.; Freire, M. The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. Int. J. Mol. Sci. 2020, 21, 8298. [Google Scholar] [CrossRef]
- Tampucci, S.; Guazzelli, L.; Burgalassi, S.; Carpi, S.; Chetoni, P.; Mezzetta, A.; Nieri, P.; Polini, B.; Pomelli, C.S.; Terreni, E.; et al. pH-Responsive Nanostructures Based on Surface Active Fatty Acid-Protic Ionic Liquids for Imiquimod Delivery in Skin Cancer Topical Therapy. Pharmaceutics 2020, 12, 1078. [Google Scholar] [CrossRef]
- Anderson, J.L.; Ding, R.; Ellern, A.; Armstrong, D.W. Structure and Properties of High Stability Geminal Dicationic Ionic Liquids. J. Am. Chem. Soc. 2005, 127, 593–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talebi, M.; Patil, R.A.; Armstrong, D.W. Physicochemical properties of branched-chain dicationic ionic liquids. J. Mol. Liq. 2018, 256, 247–255. [Google Scholar] [CrossRef]
- Jin, C.-M.; Ye, C.; Phillips, B.S.; Zabinski, J.S.; Liu, X.; Liu, W.; Shreeve, J.M. Polyethylene glycol functionalized dicationic ionic liquids with alkyl or polyfluoroalkyl substituents as high temperature lubricants. J. Mater. Chem. 2006, 16, 1529–1535. [Google Scholar] [CrossRef]
- Rizzo, C.; Marullo, S.; Dintcheva, N.T.; D’Anna, F. Carbon Nanomaterial Doped Ionic Liquid Gels for the Removal of Pharmaceutically Active Compounds from Water. Molecues 2019, 24, 2788. [Google Scholar] [CrossRef] [Green Version]
- Clarke, C.J.; Morgan, P.J.; Hallett, J.P.; Licence, P. Linking the Thermal and Electronic Properties of Functional Dicationic Salts with Their Molecular Structures. ACS Sustain. Chem. Eng. 2021, 9, 6224–6234. [Google Scholar] [CrossRef]
- Boumediene, M.; Haddad, B.; Paolone, A.; Drai, M.; Villemin, D.; Rahmouni, M.; Bresson, S.; Abbas, O. Synthesis, thermal stability, vibrational spectra and conformational studies of novel dicationic meta-xylyl linked bis-1-methylimidazolium ionic liquids. J. Mol. Struct. 2019, 1186, 68–79. [Google Scholar] [CrossRef]
- Zullo, V.; Górecki, M.; Guazzelli, L.; Mezzetta, A.; Pescitelli, G.; Iuliano, A. Exploiting isohexide scaffolds for the preparation of chiral ionic liquids tweezers. J. Mol. Liq. 2021, 322, 114528. [Google Scholar] [CrossRef]
- Majhi, D.; Seth, S.; Sarkar, M. Differences in the behavior of dicationic and monocationic ionic liquids as revealed by time resolved-fluorescence, NMR and fluorescence correlation spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 7844–7856. [Google Scholar] [CrossRef]
- Ferdeghini, C.; Guazzelli, L.; Pomelli, C.S.; Ciccioli, A.; Brunetti, B.; Mezzetta, A.; Ciprioti, S.V. Synthesis, thermal behavior and kinetic study of N-morpholinium dicationic ionic liquids by thermogravimetry. J. Mol. Liq. 2021, 332, 115662. [Google Scholar] [CrossRef]
- Kuhn, B.L.; Osmari, B.F.; Heinen, T.M.; Bonacorso, H.G.; Zanatta, N.; Nielsen, S.O.; Ranathunga, D.T.; Villetti, M.A.; Frizzo, C.P. Dicationic imidazolium-based dicarboxylate ionic liquids: Thermophysical properties and solubility. J. Mol. Liq. 2020, 308, 112983. [Google Scholar] [CrossRef]
- Vieira, J.C.; Villetti, M.A.; Frizzo, C.P. Thermal stability and decomposition mechanism of dicationic imidazolium-based ionic liquids with carboxylate anions. J. Mol. Liq. 2021, 330, 115618. [Google Scholar] [CrossRef]
- Mezzetta, A.; Perillo, V.; Guazzelli, L.; Chiappe, C. Thermal behavior analysis as a valuable tool for comparing ionic liquids of different classes. J. Therm. Anal. Calorim. 2019, 138, 3335–3345. [Google Scholar] [CrossRef]
- Shirota, H.; Mandai, T.; Fukazawa, H.; Kato, T. Comparison between Dicationic and Monocationic Ionic Liquids: Liquid Density, Thermal Properties, Surface Tension, and Shear Viscosity. J. Chem. Eng. Data 2011, 56, 2453–2459. [Google Scholar] [CrossRef]
- Montalbán, M.G.; Víllora, G.; Licence, P. Ecotoxicity assessment of dicationic versus monocationic ionic liquids as a more environmentally friendly alternative. Ecotoxicol. Environ. Saf. 2018, 150, 129–135. [Google Scholar] [CrossRef]
- Guglielmero, L.; Mezzetta, A.; Pomelli, C.S.; Chiappe, C.; Guazzelli, L. Evaluation of the effect of the dicationic ionic liquid structure on the cycloaddition of CO2 to epoxides. J. CO2 Util. 2019, 34, 437–445. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Yang, Z.; Zhang, Z.; Fang, W.; Niu, D.; He, H.; Xu, F. Efficient synthesis of isosorbide-based polycarbonate with scalable dicationic ionic liquid catalysts by balancing the reactivity of the endo-OH and exo-OH. Green Chem. 2021, 23, 973–982. [Google Scholar] [CrossRef]
- Chatterjee, K.; Pathak, A.D.; Lakma, A.; Sharma, C.S.; Sahu, K.K.; Singh, A.K. Synthesis, characterization and application of a non-flammable dicationic ionic liquid in lithium-ion battery as electrolyte additive. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, C.; Dong, R.; Shi, Y.; Wang, Y.; Bai, Y.; Zhang, J.; Cai, M.; Zhou, F.; Liu, W. Physicochemical and tribological properties of gemini-type halogen-free dicationic ionic liquids. Friction 2021, 9, 344–355. [Google Scholar] [CrossRef]
- Heydar, K.T.; Azadeh, A.M.; Yaghoubnejad, S.; Ghonouei, N.; Sharifi, A.; Rahnama, M.A. Characterization of a dicationic imidazolium-based ionic liquid as a gas chromatography stationary phase. J. Chromatogr. A 2017, 1511, 92–100. [Google Scholar] [CrossRef]
- Zafer, C.; Ocakoglu, K.; Ozsoy, C.; Icli, S. Dicationic bis-imidazolium molten salts for efficient dye sensitized solar cells: Synthesis and photovoltaic properties. Electrochimica Acta 2009, 54, 5709–5714. [Google Scholar] [CrossRef]
- Scalfani, V.F.; Al Alshaikh, A.; Bara, J.E.; Al Alshaikh, A.A. Analysis of the Frequency and Diversity of 1,3-Dialkylimidazolium Ionic Liquids Appearing in the Literature. Ind. Eng. Chem. Res. 2018, 57, 15971–15981. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Jain, R.; Lomaka, A.; Petrukhin, R.; Karelson, M.; Visser, A.A.; Rogers, R.D. Correlation of the Melting Points of Potential Ionic Liquids (Imidazolium Bromides and Benzimidazolium Bromides) Using the CODESSA Program. J. Chem. Inf. Comput. Sci. 2002, 42, 225–231. [Google Scholar] [CrossRef]
- Shannon, M.S.; Hindman, M.S.; Danielsen, S.; Tedstone, J.M.; Gilmore, R.D.; Bara, J.E. Properties of alkylbenzimidazoles for CO2 and SO2 capture and comparisons to ionic liquids. Sci. China Ser. B Chem. 2012, 55, 1638–1647. [Google Scholar] [CrossRef]
- Sang, Y.; Huang, J. Benzimidazole-based hyper-cross-linked poly (ionic liquid) s for efficient CO2 capture and conversion. Chem. Eng. J. 2020, 385, 123973. [Google Scholar] [CrossRef]
- Gupta, S.; Chatterjee, A.; Das, S.; Basu, B.; Das, B. Electrical Conductances of 1-Butyl-3-propylimidazolium Bromide and 1-Butyl-3-propylbenzimidazolium Bromide in Water, Methanol, and Acetonitrile at (308, 313, and 318) K at 0.1 MPa. J. Chem. Eng. Data 2013, 58, 1–6. [Google Scholar] [CrossRef]
- Tricoli, V.; Orsini, G.; Anselmi, M. Ion transport in a class of imidazole-based liquid/solid protic ionics. Phys. Chem. Chem. Phys. 2012, 14, 10979–10986. [Google Scholar] [CrossRef]
- Carrillo, R.H.; Suarez-Guevara, J.; Torres-González, L.C.; Gomez-Romero, P.; Sánchez, E.M. Incorporation of benzimidazolium ionic liquid in proton exchange membranes ABPBI-H3PO4. J. Mol. Liq. 2013, 181, 115–120. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, F.; Qiu, L.; Zhang, Y.; Chen, X.; Sun, B. Benzimidazolyl functionalized ionic liquids as an additive for high performance dye-sensitized solar cells. Chem. Commun. 2011, 47, 11516–11518. [Google Scholar] [CrossRef]
- Bains, D.; Singh, G.; Bhinder, J.; Agnihotri, P.K.; Singh, N. Ionic Liquid-Functionalized Multiwalled Carbon Nanotube-Based Hydrophobic Coatings for Robust Antibacterial Applications. ACS Appl. Bio Mater. 2020, 3, 2092–2103. [Google Scholar] [CrossRef]
- Bansode, P.; Patil, P.; Choudhari, P.; Bhatia, M.; Birajdar, A.; Somasundaram, I.; Rashinkar, G. Anticancer activity and molecular docking studies of ferrocene tethered ionic liquids. J. Mol. Liq. 2019, 290, 111182. [Google Scholar] [CrossRef]
- Naikwade, A.G.; Jagadale, M.B.; Kale, D.P.; Gophane, A.D.; Garadkar, K.M.; Rashinkar, G.S. Photocatalytic Degradation of Methyl Orange by Magnetically Retrievable Supported Ionic Liquid Phase Photocatalyst. ACS Omega 2020, 5, 131–144. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Raj, P.D.; Singh, A.; Dubowski, J.J.; Kaur, N.; Singh, N. Metal–Organocatalyst for Detoxification of Phosphorothioate Pesticides: Demonstration of Acetylcholine Esterase Activity. Inorg. Chem. 2019, 58, 9773–9784. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, S.; Li, H.; Zhang, G. Oxidative desulfurization of simulated gasoline catalyzed by acetic acid-based ionic liquids at room temperature. Fuel Process. Technol. 2013, 109, 27–31. [Google Scholar] [CrossRef]
- Yuan, K.; Chen, S.; Chen, X.; Yao, S.; Wang, X.; Song, H.; Zhu, M. High effective extraction of selected anthraquinones from Polygonum multiflorum using ionic liquids with ultrasonic assistance. J. Mol. Liq. 2020, 314, 113342. [Google Scholar] [CrossRef]
- Singh, S.K.; Dhepe, P.L. Lignin Conversion Using Catalytic Ionic Liquids: Understanding the Role of Cations, Anions, and Hammett Acidity Functions. Ind. Eng. Chem. Res. 2019, 58, 21273–21284. [Google Scholar] [CrossRef]
- Nakamoto, H.; Noda, A.; Hayamizu, K.; Hayashi, S.; Hamaguchi, H.-O.; Watanabe, M. Proton-Conducting Properties of a Brønsted Acid−Base Ionic Liquid and Ionic Melts Consisting of Bis (trifluoromethanesulfonyl) imide and Benzimidazole for Fuel Cell Electrolytes. J. Phys. Chem. C 2007, 111, 1541–1548. [Google Scholar] [CrossRef]
- Nakamoto, H.; Watanabe, M. Brønsted acid–base ionic liquids for fuel cell electrolytes. Chem. Commun. 2007, 2539–2541. [Google Scholar] [CrossRef]
- Khiratkar, A.G.; Muskawar, P.N.; Bhagat, P.R. Polymer-supported benzimidazolium based ionic liquid: An efficient and reusable Brønsted acid catalyst for Biginelli reaction. RSC Adv. 2016, 6, 105087–105093. [Google Scholar] [CrossRef]
- Kotadia, D.A.; Soni, S.S. Silica gel supported –SO3H functionalised benzimidazolium based ionic liquid as a mild and effective catalyst for rapid synthesis of 1-amidoalkyl naphthols. J. Mol. Catal. A: Chem. 2012, 353-354, 44–49. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, N.; Dai, L. Novel Brønsted acidic ionic liquids based on benzimidazolium cation: Synthesis and catalyzed acetalization of aromatic aldehydes with diols. Catal. Commun. 2008, 9, 2475–2480. [Google Scholar] [CrossRef]
- Mohammadi, R.; Esmati, S.; Gholamhosseini-Nazari, M.; Teimuri-Mofrad, R. Novel ferrocene substituted benzimidazolium based ionic liquid immobilized on magnetite as an efficient nano-catalyst for the synthesis of pyran derivatives. J. Mol. Liq. 2019, 275, 523–534. [Google Scholar] [CrossRef]
- Abbasi, M. Design, preparation and characterization of a new ionic liquid, 1,3-disulfonic acid benzimidazolium chloride, as an efficient and recyclable catalyst for the synthesis of tetrahydropyridine under solvent-free conditions. RSC Adv. 2015, 5, 67405–67411. [Google Scholar] [CrossRef]
- Gajare, S.; Patil, A.; Kale, D.; Bansode, P.; Patil, P.; Rashinkar, G. Graphene Oxide-Supported Ionic Liquid Phase Catalyzed Synthesis of 3,4-Dihydro-2H-naphtho[2,3-e] [1,3] oxazine-5,10-diones. Catal. Lett. 2019, 150, 243–255. [Google Scholar] [CrossRef]
- Abbasi, M. 1,3-Disulfonic acid benzimidazolium chloride as an efficient and recyclable ionic liquid catalyst for the synthesis of 3,4-dihydropyrimidine-2-(1H)-ones/thiones. Res. Chem. Intermed. 2016, 42, 3303–3314. [Google Scholar] [CrossRef]
- Kaloglu, N.; Özdemir, I.; Gürbüz, N.; Achard, M.; Bruneau, C. Benzimidazolium sulfonate ligand precursors and application in ruthenium-catalyzed aromatic amine alkylation with alcohols. Catal. Commun. 2016, 74, 33–38. [Google Scholar] [CrossRef]
- Majumdar, S.; De, J.; Hossain, J.; Basak, A. Formylation of amines catalysed by protic ionic liquids under solvent-free condition. Tetrahedron Lett. 2013, 54, 262–266. [Google Scholar] [CrossRef]
- Gajare, S.; Patil, A.; Hangirgekar, S.; Dhanmane, S.; Rashinkar, G. Green synthesis of quinolines via A3-coupling by using graphene oxide-supported Brønsted acidic ionic liquid. Res. Chem. Intermed. 2020, 46, 2417–2436. [Google Scholar] [CrossRef]
- Chen, S.-H.; Zhao, Q.; Xu, X.-W. Preparation and characterization of a novel benzimidazolium brønsted acidic ionic liquid and its application in esterifications. J. Chem. Sci. 2008, 120, 481–483. [Google Scholar] [CrossRef]
- Khiratkar, A.G.; Balinge, K.R.; Patle, D.S.; Krishnamurthy, M.; Cheralathan, K.; Bhagat, P.R. Transesterification of castor oil using benzimidazolium based Brønsted acid ionic liquid catalyst. Fuel 2018, 231, 458–467. [Google Scholar] [CrossRef]
- Khiratkar, A.G.; Balinge, K.R.; Krishnamurthy, M.; Cheralathan, K.K.; Patle, D.S.; Singh, V.; Arora, S.; Bhagat, P.R. Sulphonic Acid-Functionalized Benzimidazolium Based Poly Ionic Liquid Catalyzed Esterification of Levulinic Acid. Catal. Lett. 2018, 148, 680–690. [Google Scholar] [CrossRef]
- Zhang, M.; Zang, H.; Ma, B.; Zhang, X.; Xie, R.; Cheng, B. Green Synthesis of 5-Hydroxymethylfurfural from Chitosan Biomass Catalyzed by Benzimidazole-Based Ionic Liquids. ChemistrySelect 2017, 2, 10323–10328. [Google Scholar] [CrossRef]
- Orazbayeva, D.; Koziel, J.A.; Trujillo-Rodríguez, M.J.; Anderson, J.L.; Kenessov, B. Polymeric ionic liquid sorbent coatings in headspace solid-phase microextraction: A green sample preparation technique for the determination of pesticides in soil. Microchem. J. 2020, 157, 104996. [Google Scholar] [CrossRef]
- Su, H.; Wang, Q.; Wang, N.; Yang, Y. Behavior, mechanism and equilibrium studies of Au(iii) extraction with an ionic liquid [C4-6-C4BIm] Br2. Dalton Trans. 2020, 49, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Tubaro, C.; Baron, M.; Costante, M.; Basato, M.; Biffis, A.; Gennaro, A.; Isse, A.A.; Graiff, C.; Accorsi, G. Dinuclear gold(i) complexes with propylene bridged N-heterocyclic dicarbene ligands: Synthesis, structures, and trends in reactivities and properties. Dalton Trans. 2013, 42, 10952–10963. [Google Scholar] [CrossRef]
- Hussaini, S.Y.; Haque, R.A.; Asekunowo, P.O.; Majid, A.M.S.A.; Agha, M.T.; Razali, M.R. Synthesis, characterization and anti-proliferative activity of propylene linked bis-benzimidazolium salts and their respective dinuclear Silver(I)- N -heterocyclic carbene complexes. J. Organomet. Chem. 2017, 840, 56–62. [Google Scholar] [CrossRef]
- Al-Mohammed, N.N.; Hussen, R.S.D.; Alias, Y.; Abdullah, Z. Tris-imidazolium and benzimidazolium ionic liquids: A new class of biodegradable surfactants. RSC Adv. 2014, 5, 2869–2881. [Google Scholar] [CrossRef]
- Al-Mohammed, N.N.; Hussen, R.S.D.; Ali, T.H.; Alias, Y.; Abdullah, Z. Tetrakis-imidazolium and benzimidazolium ionic liquids: A new class of biodegradable surfactants. RSC Adv. 2015, 5, 21865–21876. [Google Scholar] [CrossRef]
- Küçükbay, H.; Cetinkaya, E.; Durmaz, R. Synthesis and antimicrobial activity of substituted benzimidazole, benzothiazole and imidazole derivatives. Arzneimittelforschung 1995, 45, 1131–1134. [Google Scholar]
- Al-Mohammed, N.N.; Alias, Y.; Abdullah, Z. Bis-imidazolium and benzimidazolium based gemini-type ionic liquids structure: Synthesis and antibacterial evaluation. RSC Adv. 2015, 5, 92602–92617. [Google Scholar] [CrossRef]
- Clark, K.D.; Nacham, O.; Yu, H.; Li, T.; Yamsek, M.M.; Ronning, D.R.; Anderson, J.L. Extraction of DNA by Magnetic Ionic Liquids: Tunable Solvents for Rapid and Selective DNA Analysis. Anal. Chem. 2015, 87, 1552–1559. [Google Scholar] [CrossRef]
- Kar, B.; Bardhan, S.; Kundu, K.; Saha, S.K.; Paul, B.K.; Das, S. Physicochemical studies of water-in-oil nonionic microemulsion in presence of benzimidazole-based ionic liquid and probing of microenvironment using model C–C cross coupling (Heck) reaction. RSC Adv. 2014, 4, 21000. [Google Scholar] [CrossRef]
- Lin, Y.-R.; Chiu, C.-C.; Chiu, H.-T.; Lee, D.-S.; Lu, T.-J. Bis-benzimidazolium-palladium system catalyzed Suzuki-Miyaura coupling reaction of aryl bromides under mild conditions. Appl. Organomet. Chem. 2018, 32, e3896. [Google Scholar] [CrossRef]
- Iwamoto, K.-I.; Kimura, H.; Oike, M.; Sato, M. Methylene-bridged bis(benzimidazolium) salt as a highly efficient catalyst for the benzoin reaction in aqueous media. Org. Biomol. Chem. 2008, 6, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Berding, J.; Lutz, M.; Spek, A.L.; Bouwman, E. Synthesis of Novel Chelating Benzimidazole-Based Carbenes and Their Nickel (II) Complexes: Activity in the Kumada Coupling Reaction. Organometallics 2009, 28, 1845–1854. [Google Scholar] [CrossRef]
- Nozawa-Kumada, K.; Ito, S.; Noguchi, K.; Shigeno, M.; Kondo, Y. Super electron donor-mediated reductive desulfurization reactions. Chem. Commun. 2019, 55, 12968–12971. [Google Scholar] [CrossRef] [PubMed]
- Muskawar, P.N.; Thenmozhi, K.; Gajbhiye, J.M.; Bhagat, P.R. Facile esterification of carboxylic acid using amide functionalized benzimidazolium dicationic ionic liquids. Appl. Catal. A Gen. 2014, 482, 214–220. [Google Scholar] [CrossRef]
- Murphy, J.A.; Khan, T.A.; Zhou, S.-Z.; Thomson, D.W.; Mahesh, M. Highly Efficient Reduction of Unactivated Aryl and Alkyl Iodides by a Ground-State Neutral Organic Electron Donor. Angew. Chem. Int. Ed. 2005, 44, 1356–1360. [Google Scholar] [CrossRef]
- Murphy, J.A.; Zhou, S.-Z.; Thomson, D.W.; Schoenebeck, F.; Mahesh, M.; Park, S.R.; Tuttle, T.; Berlouis, L.E.A. The Generation of Aryl Anions by Double Electron Transfer to Aryl Iodides from a Neutral Ground-State Organic Super-Electron Donor. Angew. Chem. Int. Ed. 2007, 46, 5178–5183. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-Z.; Anderson, G.M.; Mondal, B.; Doni, E.; Ironmonger, V.; Kranz, M.; Tuttle, T.; Murphy, J.A. Organic super-electron-donors: Initiators in transition metal-free haloarene–arene coupling. Chem. Sci. 2013, 5, 476–482. [Google Scholar] [CrossRef] [Green Version]
- Sword, R.; Baldwin, L.A.; Murphy, J.A. Fragmentations observed in the reactions of α-methoxy-γ-alkoxyalkyl iodide substrates with super-electron-donors derived from 4-DMAP and N-methylbenzimidazole. Org. Biomol. Chem. 2011, 9, 3560–3570. [Google Scholar] [CrossRef] [PubMed]
- Broggi, J.; Rollet, M.; Clément, J.; Canard, G.; Terme, T.; Gigmes, D.; Vanelle, P. Polymerization Initiated by Organic Electron Donors. Angew. Chem. Int. Ed. 2016, 55, 5994–5999. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-T.; Ding, M.-F.; Chang, C.-W.; Lue, S.-S. Nuclear magnetic resonance spectroscopic study on ionic liquids of 1-alkyl-3-methylimidazolium salts. Tetrahedron 2004, 60, 9441–9446. [Google Scholar] [CrossRef]
- Fahlbusch, T.; Frank, M.; Schatz, J.; Schühle, D.T. Kinetic Acidity of Supramolecular Imidazolium SaltsEffects of Substituent, Preorientation, and Counterions on H/D Exchange Rates. J. Org. Chem. 2006, 71, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Kermanioryani, M.; Mutalib, M.I.A.; Dong, Y.; Lethesh, K.C.; Ghanem, O.B.; Kurnia, K.A.; Aminuddin, N.F.; Leveque, J.-M. Physicochemical Properties of New Imidazolium-Based Ionic Liquids Containing Aromatic Group. J. Chem. Eng. Data 2016, 61, 2020–2026. [Google Scholar] [CrossRef]
- Guglielmero, L.; Mezzetta, A.; Guazzelli, L.; Pomelli, C.S.; D’Andrea, F.; Chiappe, C. Systematic Synthesis and Properties Evaluation of Dicationic Ionic Liquids, and a Glance into a Potential New Field. Front. Chem. 2018, 6, 612. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Mu, T. Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Gómez, E.; Calvar, N.; Domínguez, Á.; Macedo, E.A. Thermal behavior and heat capacities of pyrrolidinium-based ionic liquids by DSC. Fluid Phase Equilibria 2018, 470, 51–59. [Google Scholar] [CrossRef]
- Ramos, J.J.M.; Afonso, C.A.M.; Branco, L.C. Glass transition relaxation and fragility in two room temperature ionic liquids. J. Therm. Anal. Calorim. 2003, 71, 659–666. [Google Scholar] [CrossRef]
- Sippel, P.; Lunkenheimer, P.; Krohns, S.; Thoms, E.; Loidl, A. Importance of liquid fragility for energy applications of ionic liquids. Sci. Rep. 2015, 5, 13922. [Google Scholar] [CrossRef] [Green Version]
- Gómez, E.; Calvar, N.; Domínguez, Á.; Macedo, E.A. Thermal Analysis and Heat Capacities of 1-Alkyl-3-methylimidazolium Ionic Liquids with NTf2–, TFO–, and DCA– Anions. Ind. Eng. Chem. Res. 2013, 52, 2103–2110. [Google Scholar] [CrossRef]
- Ogawa, K.A.; Boydston, A.J. Electrochemical Characterization of Azolium Salts. Chem. Lett. 2014, 43, 907–909. [Google Scholar] [CrossRef] [Green Version]
- Rocco, D.; Chiarotto, I.; D’Anna, F.; Mattiello, L.; Pandolfi, F.; Rizzo, C.; Feroci, M. Cathodic Behaviour of Dicationic Imidazolium Bromides: The Role of the Spacer. ChemElectroChem 2019, 6, 4275–4283. [Google Scholar] [CrossRef] [Green Version]
- Kazemiabnavi, S.; Zhang, Z.; Thornton, K.; Banerjee, S. Electrochemical Stability Window of Imidazolium-Based Ionic Liquids as Electrolytes for Lithium Batteries. J. Phys. Chem. B 2016, 120, 5691–5702. [Google Scholar] [CrossRef] [PubMed]
- Riederer, S.K.U.; Gigler, P.; Hogerl, M.P.; Herdtweck, E.; Bechlars, B.; Herrmann, W.A.; Kuhn, F.E. Impact of Ligand Modification on Structures and Catalytic Activities of Chelating Bis-Carbene Rhodium(I) Complexes. Organometallics 2010, 29, 5681–5692. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezzetta, A.; Guglielmero, L.; Mero, A.; Tofani, G.; D’Andrea, F.; Pomelli, C.S.; Guazzelli, L. Expanding the Chemical Space of Benzimidazole Dicationic Ionic Liquids. Molecules 2021, 26, 4211. https://doi.org/10.3390/molecules26144211
Mezzetta A, Guglielmero L, Mero A, Tofani G, D’Andrea F, Pomelli CS, Guazzelli L. Expanding the Chemical Space of Benzimidazole Dicationic Ionic Liquids. Molecules. 2021; 26(14):4211. https://doi.org/10.3390/molecules26144211
Chicago/Turabian StyleMezzetta, Andrea, Luca Guglielmero, Angelica Mero, Giorgio Tofani, Felicia D’Andrea, Christian Silvio Pomelli, and Lorenzo Guazzelli. 2021. "Expanding the Chemical Space of Benzimidazole Dicationic Ionic Liquids" Molecules 26, no. 14: 4211. https://doi.org/10.3390/molecules26144211
APA StyleMezzetta, A., Guglielmero, L., Mero, A., Tofani, G., D’Andrea, F., Pomelli, C. S., & Guazzelli, L. (2021). Expanding the Chemical Space of Benzimidazole Dicationic Ionic Liquids. Molecules, 26(14), 4211. https://doi.org/10.3390/molecules26144211









