Bufadienolides from the Skin Secretions of the Neotropical Toad Rhinella alata (Anura: Bufonidae): Antiprotozoal Activity against Trypanosoma cruzi
Abstract
:1. Introduction
2. Results and Discussion
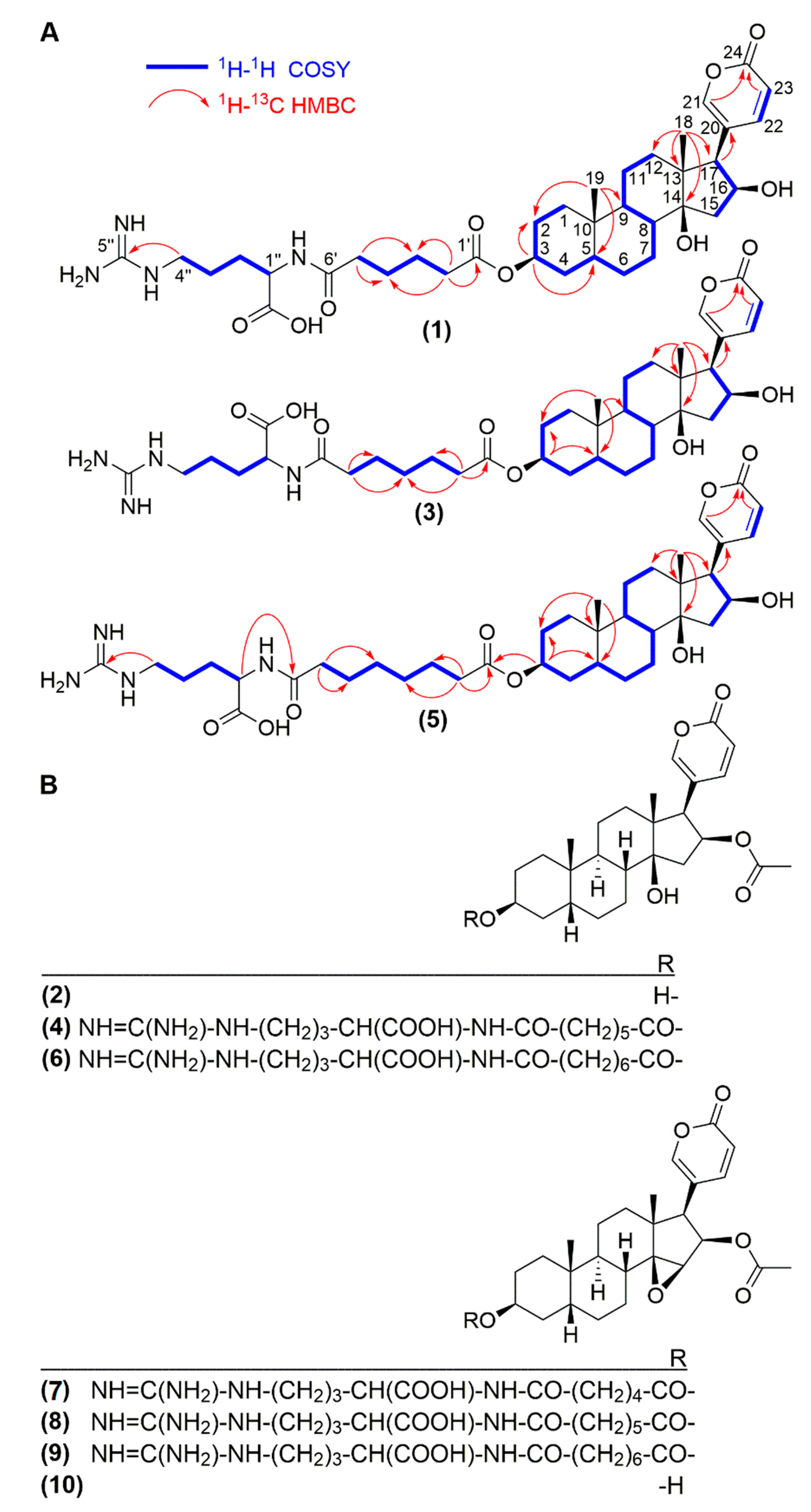
2.1. Isolation and Structural Elucidation
2.2. Antitrypanosomal Activity
2.3. Mammalian Cytotoxicity
2.4. Anticancer Assay
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Collection of Animals and Venom Extraction
3.3. Isolation of Bufadienolides
3.4. Antiprotozoal Activity against T. cruzi
3.5. Mammalian Cytotoxicity
3.6. Anticancer Assay
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sousa, L.Q.; Machado, K.C.; Oliveira, S.F.C.; Araújo, L.S.; Monção-Filho, E.S.; de Melo-Cavalcante, A.A.C.; Vieira-Júnior, G.M.; Ferreira, P.M.P. Bufadienolides from amphibians: A promising source of anticancer prototypes for radical innovation, apoptosis triggering and Na+/K+-ATPase inhibition. Toxicon 2017, 127, 63–76. [Google Scholar] [CrossRef]
- Ferreira, F.S.; Brito, S.V.; Ribeiro, S.C.; Almeida, W.O.; Alves, R.R.N. Zootherapeutics utilized by residents of the community Poço Dantas, Crato-CE, Brazil. J. Ethnobiol. Ethnomed. 2009, 5, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Barros, F.B.; Varela, S.A.M.; Pereira, H.M.; Vicente, L. Medicinal use of fauna by a traditional community in the Brazilian Amazonia. J. Ethnobiol. Ethnomed. 2012, 8. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Zehl, M.; Leitner, A.; Wu, X.; Wang, Z.; Kopp, B. Comparison of toad venoms from different Bufo species by HPLC and LC-DAD-MS/MS. J. Ethnopharmacol. 2010, 131, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Fontana, P.L.M.; Antoniazzi, M.M.; Toledo, L.F.; Verdade, V.K.; Sciani, J.M.; Barbaro, K.C.; Pimenta, D.C.; Rodrigues, M.T.; Jared, C. Passive and Active Defense in Toads: The Parotoid Macroglands in Rhinella marina and Rhaebo guttatus. J. Exp. Zool. 2013, 321, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, K.; Forman, M.E.; Umile, T.P.; Kueneman, J.; McKenzie, V.; Salinas, I.; Minbiole, K.P.C.; Woodhams, D.C. Identification of Bufadienolides from the Boreal Toad, Anaxyrus boreas, Active Against a Fungal Pathogen. Microb. Ecol. 2017, 74, 990–1000. [Google Scholar] [CrossRef]
- Rodríguez, C.; Rollins-Smith, L.; Ibáñez, R.; Durant-Archibold, A.A.; Gutiérrez, M. Toxins and pharmacologically active compounds from species of the family Bufonidae (Amphibia, Anura). J. Ethnopharmacol. 2017, 198, 235–254. [Google Scholar] [CrossRef] [Green Version]
- Matsukawa, M.; Mukai, T.; Akizawa, T.; Miyatake, S.; Yoshioka, M.; Morris, J.F.; Butler, V.P. Isolation and Characterization of Novel Endogenous Digitalis-like Factors in the Ovary of the Giant Toad, Bufo marinus. J. Nat. Prod. 1998, 61, 1476–1481. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Wu, F.K.; Qiu, Y.K.; Wu, Z.; Jiang, Y.T.; Chen, J.Y. Studies on cytotoxic constituents from the skin of the toad Bufo bufo gargarizans. J. Asian Nat. Prod. Res. 2010, 12, 793–800. [Google Scholar] [CrossRef]
- Wu, F.K.; Qiu, Y.K.; Zhao, H.Y.; Wu, Z.; Li, F.M.; Jiang, Y.T.; Chen, J.Y. Cytotoxic constituents from the skin of the toad Bufo bufo gargarizans. J. Asian Nat. Prod. Res. 2011, 13, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Córdova, W.H.P.; Leitão, S.G.; Cunha-filho, G.; Bosch, R.A.; Alonso, I.P.; Pereda-miranda, R.; Touza, N.A.; Quintas, L.E.M.; Noël, F. Bufadienolides from the parotoid gland secretions of Cuban toad Peltophryne fustiger (Bufonidae): Inhibition of human (Na+/K+)-ATPase activity. Toxicon 2016, 110, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha-Filho, G.A.; Schwartz, C.A.; Resck, I.S.; Murta, M.M.; Lemos, S.S.; Castro, M.S.; Kyaw, C.; Pires, O.R.; Leite, J.R.S.; Bloch, C.; et al. Antimicrobial activity of the bufadienolides marinobufagin and telocinobufagin isolated as major components from skin secretion of the toad Bufo rubescens. Toxicon 2005, 45, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Tempone, A.G.; Pimenta, D.C.; Lebrun, I.; Sartorelli, P.; Taniwaki, N.N.; Andrade, H.F.; Antoniazzi, M.M.; Jared, C. Antileishmanial and antitrypanosomal activity of bufadienolides isolated from the toad Rhinella jimi parotoid macrogland secretion. Toxicon 2008, 52, 13–21. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/chagas/en/ (accessed on 6 November 2019).
- Coura, J.R. The main sceneries of chagas disease transmission. The vectors, blood and oral transmissions—A comprehensive review. Mem. Inst. Oswaldo Cruz. 2015, 110, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Viotti, R.; Vigliano, C.; Lococo, B.; Alvarez, M.G.; Petti, M.; Bertocchi, G.; Armenti, A. Side effects of benznidazole as treatment in chronic Chagas disease: Fears and realities. Expert Rev. Anti. Infect. Ther. 2009, 7, 157–163. [Google Scholar] [CrossRef]
- Sueth-Santiago, V.; Decote-Ricardo, D.; Morrot, A.; Freire-de-Lima, C.G.; Lima, M.E.F. Challenges in the chemotherapy of Chagas disease: Looking for possibilities related to the differences and similarities between the parasite and host. World J. Biol. Chem. 2017, 8, 57–80. [Google Scholar] [CrossRef]
- Santos, S.P.; Ibáñez, R.; Ron, S.R. Systematics of the Rhinella margaritifera complex (Anura, Bufonidae) from western Ecuador and Panama with insights in the biogeography of Rhinella alata. Zookeys 2015, 145, 109–145. [Google Scholar] [CrossRef]
- Amphibian Species of the World. American Museum of Natural History. Available online: https://amphibiansoftheworld.amnh.org (accessed on 6 November 2019).
- Ibáñez, R.D.; Stanley-Rand, A.; Jaramillo, C.A. The Amphibians of Barro Colorado Nature Monument, Soberania National Park and Adjacent Areas, 1st ed.; Editorial Mizrachi & Pujol: Panama City, Panama, 1999. [Google Scholar]
- Cei, J.M.; Erspamer, V.; Roseghini, M. Taxonomic and Evolutionary Significance of Biogenic Amines and Polypeptides Occurring in Amphibian Skin. II. Toads of the Genera Bufo and Melanophryniscus. Syst. Zool. 1968, 17, 232–245. [Google Scholar] [CrossRef]
- Shimada, K.; Sato, Y.; Fujii, Y.; Nambara, T. Occurrence of Bufalitoxin, Cinobufotoxin and their Homologs in Japanese Toad. Chem. Pharm. Bull. 1976, 24, 1118–1120. [Google Scholar] [CrossRef] [Green Version]
- Flier, J.; Edwards, M.W.; Daly, J.W.; Myers, C.W. Widespread Occurrence in Frogs and Toads of Skin Compounds Interacting with the ouabain site of Na+, K+-ATPase. Science 1980, 208, 503–505. [Google Scholar] [CrossRef]
- Shimada, K.; Fujii, Y.; Yamashita, E.; NiIzaki, Y.; Sato, Y.; Nambara, T. Studies on Cardiotonic Steroids from the Skin of Japanese Toad. Chem. Pharm. Bull. 1977, 25, 714–730. [Google Scholar] [CrossRef] [Green Version]
- Verpoorte, R.; Svendsen, A.B. Chemical Constituents of Vietnamese Toad Venom, Collected From Bufo Melanostictus Schneider. Part II. The Bufadienolides. J. Nat. Prod. 1980, 43, 347–352. [Google Scholar] [CrossRef]
- Daly, J.W.; Noimai, N.; Kongkathip, B.; Kongkathip, N.; Wilham, J.M.; Garraffo, H.M.; Kaneko, T.; Spande, T.F.; Nimit, Y.; Nabhitabhata, J.; et al. Biologically active substances from amphibians: Preliminary studies on anurans from twenty-one genera of Thailand. Toxicon 2004, 44, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Sciani, J.M.; Angeli, C.B.; Antoniazzi, M.M.; Jared, C.; Pimenta, D.C. Differences and Similarities among Parotoid Macrogland Secretions in South American Toads: A Preliminary Biochemical Delineation. Sci. World J. 2013, 2013, 937407. [Google Scholar] [CrossRef] [Green Version]
- Van Der Heyden, N.; Docampo, R. Proton and sodium pumps regulate the plasma membrane potential of different stages of Trypanosome cruzi. Mol. Biochem. Parasitol. 2002, 120, 127–139. [Google Scholar] [CrossRef]
- Kyoichi, I.; Yuko, M.; Muneaki, H.; Takeshi, N.; Yukichi, H.; Takashi, A. Molecular cloning and characterization of ouabain-insensitive Na+-ATPase in the parasitic protist, Trypanosoma cruzi. Biochim. Biophys. Acta 2006, 1758, 738–746. [Google Scholar] [CrossRef] [Green Version]
- Densmore, C.L.; Green, D.E. Diseases of Amphibians. ILAR J. 2007, 48, 235–254. [Google Scholar] [CrossRef] [Green Version]
- Garraffo, H.M.; Gras, E.G. Biosynthesis of Bufadienolides in Toads VI. Experiments with [1,2-3H] Cholesterol, [21-14C] Coprostanol, and 5-beta-[21-14C] Pregnonolone in the Toad Bufo arenarum. Steroids 1986, 48, 251–257. [Google Scholar] [CrossRef]
- Lichtstein, D.; Kachalsky, S.; Deutsch, J. Identification of a Ouabain-like Compound in Toad Skin and Plasma as a Bufadienolide Derivative. Life Sci. 1986, 38, 1261–1270. [Google Scholar] [CrossRef]
- Lee, S.S.; Derguini, F.; Bruening, R.C.; Nakanishi, K.; Wallick, E.T.; Akisawa, T.; Rosenbaum, C.S.; Vincent, B.J. Digitalis-like Compounds of Toad Bile: Sulfation and Reduction of Bufadienolides Decrease Potency of Na+, K+-ATPase Inhibition. Heterocycles 1994, 39, 669–686. [Google Scholar]
- Lu, J.; Deng, S.; Chen, H.; Hou, J.; Zhang, B.; Tian, Y.; Wang, C.; Ma, X. Microbial transformation of cinobufotalin by Alternaria alternate AS 3.4578 and Aspergillus niger AS 3.739. J. Mol. Catal. B Enzym. 2013, 89, 102–107. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, D.; Li, Y.; Chen, W.; Ruan, Z.; Deng, L.; Wang, L.; Tian, H.; Yiu, A.; Fan, C.; et al. Discovery of Bufadienolides as a Novel Class of ClC-3 Chloride Channel Activators with Antitumor Activities. J. Med. Chem. 2013, 56, 5734–5743. [Google Scholar] [CrossRef]
- Tian, H.Y.; Luo, S.L.; Liu, J.S.; Wang, L.; Wang, Y.; Zhang, D.M.; Zhang, X.Q.; Jiang, R.W.; Ye, W.C. C23 Steroids from the Venom of Bufo bufo gargarizans. J. Nat. Prod. 2013, 76, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.R.; Xu, Y.; Fang, J.F.; Zhou, F.; Deng, X.W.; Zhang, Y.Q. Bufotalin-induced apoptosis in osteoblastoma cells is associated with endoplasmic reticulum stress activation. Biochem. Biophys. Res. Commun. 2014, 451, 112–118. [Google Scholar] [CrossRef]
- Qi, F.; Inagaki, Y.; Gao, B.; Cui, X.; Xu, H.; Kokudo, N.; Li, A.; Tang, W. Bufalin and cinobufagin induce apoptosis of human hepatocellular carcinoma cells via Fas- and mitochondria-mediated pathways. Cancer Sci. 2011, 102, 951–958. [Google Scholar] [CrossRef]
- Grant, J.B.; Land, B. Transcutaneous Amphibian Stimulator (TAS): A device for the collection of amphibian skin secretions. Herpetol. Rev. 2002, 33, 38–41. [Google Scholar]
- Linde-Tempel, H.O. Konstitution der Bufotoxine. Helv. Chim. Acta 1970, 53, 2188–2196. [Google Scholar] [CrossRef]
- Buckner, F.S.; Verlinde, C.L.; La Flamme, A.C.; Van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid collorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Lopez, D.; Cherigo, L.; De Sedas, A.; Spadafora, C.; Martinez-Luis, S. Evaluation of antiparasitic, anticancer, antimicrobial and hypoglycemic properties of organic extracts from Panamanian mangrove plants. Asian Pac. J. Trop. Med. 2018, 11, 32–39. [Google Scholar] [CrossRef]


| No. | 1 | 3 | 5 | |||
|---|---|---|---|---|---|---|
| 1H, mult. (J) | 13C, Type | 1H, mult. (J) | 13C, Type | 1H, mult. (J) | 13C, Type | |
| 1a | 1.26 m | 27.6, CH2 | 1.25 m | 27.7, CH2 | 1.26 m | 27.7, CH2 |
| 1b | 1.93 m | 1.91 m | 1.93 m | |||
| 2a | 1.35 m | 31.6, CH2 | 1.35 m | 31.6, CH2 | 1.41 m | 31.6, CH2 |
| 2b | 1.58 m | 1.59 m | 1.58 m | |||
| 3 | 5.08 m | 72.2, CH | 5.07 m | 72.1, CH | 5.08 m | 72.1, CH |
| 4a | 1.42 m | 31.7, CH2 | 1.41 m | 31.7, CH2 | 1.41 m | 31.7, CH2 |
| 4b | 2.03 m | 2.02 m | 2.03 m | |||
| 5 | 1.66 m | 38.5, CH | 1.68 m | 38.6, CH | 1.68 m | 38.6, CH |
| 6a | 1.39 m | 22.3, CH2 | 1.38 m | 22.3, CH2 | 1.41 m | 22.3, CH2 |
| 6b | 1.34 m | 1.25 m | 1.26 m | |||
| 7a | 1.66 m | 26.0, CH2 | 1.64 m | 25.9, CH2 | 1.64 m | 26.0, CH2 |
| 7b | 1.34 m | 1.37 m | 1.36 m | |||
| 8 | 1.61 m | 42.9, CH | 1.64 m | 43.0, CH | 1.64 m | 43.0, CH |
| 9 | 1.66 m | 36.8, CH | 1.68 m | 36.8, CH | 1.68 m | 36.8, CH |
| 10 | 36.2, C | 36.3, C | 36.3, C | |||
| 11a | 1.26 m | 22.5, CH2 | 1.25 m | 22.5, CH2 | 1.23 m | 22.5, CH2 |
| 11b | 1.85 m | 1.85 m | 1.89 m | |||
| 12a | 1.39 m | 41.9, CH2 | 1.35 m | 42.0, CH2 | 1.38 m | 42.0, CH2 |
| 12b | 1.55 m | 1.58 m | 1.55 m | |||
| 13 | 50.4, C | 50.4, C | 50.4, C | |||
| 14 | 86.0, C | 86.0, C | 85.9, C | |||
| 15a | 1.78 d (14.6 Hz) | 43.1, CH2 | 1.78 d (14.1 Hz) | 43.1, CH2 | 1.78 d (14.7 Hz) | 43.1, CH2 |
| 15b | 2.56 dd (7.3, 14.6 Hz) | 2.56 dd (7.8, 15.1 Hz) | 2.56 dd (7.8, 14.7 Hz) | |||
| 16 | 4.51 t (7.3 Hz) | 73.4, CH | 4.51 t (7.8 Hz) | 73.5, CH | 4.51 t (7.8 Hz) | 73.5, CH |
| 17 | 2.76 d (7.6 Hz) | 59.5, CH | 2.76 d (7.8 Hz) | 59.6, CH | 2.76 d (7.8 Hz) | 59.5, CH |
| 18 | 0.78 s | 17.3, CH3 | 0.78 s | 17.3, CH3 | 0.78 s | 17.3, CH3 |
| 19 | 0.97 s | 24.3, CH3 | 0.97 s | 24.3, CH3 | 0.97 s | 24.3, CH3 |
| 20 | 120.5, C | 120.5, C | 120.5, C | |||
| 21 | 7.44 d (2.0 Hz) | 151.8, CH | 7.44 d (1.9 Hz) | 151.8, CH | 7.44 d (1.5 Hz) | 151.8, CH |
| 22 | 8.12 dd (2.5, 9.8 Hz) | 152.9, CH | 8.12 dd (2.4, 9.7 Hz) | 152.9, CH | 8.12 dd (2.4, 9.8 Hz) | 152.9, CH |
| 23 | 6.16 d (9.7 Hz) | 112.9, CH | 6.19 d (9.7 Hz) | 112.9, CH | 6.19 d (9.8 Hz) | 112.9, CH |
| 24 | 165.1, C | 165.1, C | 165.0, C | |||
| 1′ | 174.9, C | 175.0, C | 175.1, C | |||
| 2′ | 2.36 m | 35.1, CH2 | 2.33 t (7.3Hz) | 35.4, CH2 | 2.32 t (7.3 Hz) | 35.5, CH2 |
| 3′ | 1.66 m | 25.7, CH2 | 1.64 m | 26.0, CH2 | 1.64 m | 26.1, CH2 |
| 4′ | 1.66 m | 26.3, CH2 | 1.35 m | 29.7, CH2 | 1.36 m | 30.0, CH2 |
| 5′ | 2.29 m | 36.4, CH2 | 1.64 m | 26.5, CH2 | 1.36 m | 30.0, CH2 |
| 6′ | 174.9, C | 2.26 t (7.3 Hz) | 36.4, CH2 | 1.64 m | 26.8, CH2 | |
| 7′ | 175.0, C | 2.26 t (6.4 Hz) | 36.7, CH2 | |||
| 8′ | 175.1, C | |||||
| 1″ | 4.43 m | 53.3, CH | 4.43 m | 53.2, CH | 4.42 dd (4.4, 8.3 Hz) | 53.1, CH |
| 2″a | 1.71 m | 29.9, CH2 | 1.74 m | 30.0, CH2 | 1.74 m | 30.0, CH2 |
| 2″b | 1.93 m | 1.93 m | 1.93 m | |||
| 3″ | 1.66 m | 26.2, CH2 | 1.64 m | 26.4, CH2 | 1.64 m | 26.4, CH2 |
| 4″ | 3.21 dd (6.3, 11.2 Hz) | 42.0, CH2 | 3.21 dd (6.3, 11.2 Hz) | 41.9, CH2 | 3.21 m | 41.9, CH2 |
| 5″ | 158.7, C | 158.7, C | 158.7, C | |||
| COOH | 176.0, C | 176.2, C | 176.4, C | |||
| Compounds | IC50 (µM) a |
|---|---|
| T. cruzib | |
| 1 | 8.0 ± 0.75 |
| 2 | 19.6 ± 3.38 |
| 3 | 8.7 ± 1.25 |
| 4 | 4.6 ± 1.75 |
| 5 | 6.9 ± 0.95 |
| 6 | 4.9 ± 1.00 |
| 7 | 16.7 ± 2.93 |
| 8 | 14.7 ± 1.80 |
| 9 | 19.0 ± 5.73 |
| 10 | 29.0 ± 2.57 |
| benznidazole | 2.73 ± 0.16 |
| Bufadienolides | IC50 (µM) a |
|---|---|
| Vero b | |
| 1 | 1.89 ± 0.63 |
| 2 | 46.03 ± 6.29 |
| 3 | 0.24 ± 0.20 |
| 4 | 0.17 ± 0.09 |
| 5 | 0.81 ± 0.35 |
| 6 | 0.34 ± 0.20 |
| 7 | 3.60 ± 1.64 |
| 8 | 0.72 ± 0.31 |
| 9 | 0.25 ± 0.08 |
| 10 | 54.11 ± 4.73 |
| doxorubicin | 0.23 ± 0.04 |
| Compounds | IC50 (nM) * | ||
|---|---|---|---|
| MCF-7 | NCI-H460 | SF-268 | |
| 2 | 94 ± 0.03 | 229 ± 0.08 | 20,530 ± 5.90 |
| 10 | 9 ± 0.01 | 14 ± 0.01 | 4,677 ± 1.55 |
| doxorubicin | 76 ± 3.01 | 59 ± 5.06 | 610 ± 57.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, C.; Ibáñez, R.; Mojica, L.; Ng, M.; Spadafora, C.; Durant-Archibold, A.A.; Gutiérrez, M. Bufadienolides from the Skin Secretions of the Neotropical Toad Rhinella alata (Anura: Bufonidae): Antiprotozoal Activity against Trypanosoma cruzi. Molecules 2021, 26, 4217. https://doi.org/10.3390/molecules26144217
Rodriguez C, Ibáñez R, Mojica L, Ng M, Spadafora C, Durant-Archibold AA, Gutiérrez M. Bufadienolides from the Skin Secretions of the Neotropical Toad Rhinella alata (Anura: Bufonidae): Antiprotozoal Activity against Trypanosoma cruzi. Molecules. 2021; 26(14):4217. https://doi.org/10.3390/molecules26144217
Chicago/Turabian StyleRodriguez, Candelario, Roberto Ibáñez, Luis Mojica, Michelle Ng, Carmenza Spadafora, Armando A. Durant-Archibold, and Marcelino Gutiérrez. 2021. "Bufadienolides from the Skin Secretions of the Neotropical Toad Rhinella alata (Anura: Bufonidae): Antiprotozoal Activity against Trypanosoma cruzi" Molecules 26, no. 14: 4217. https://doi.org/10.3390/molecules26144217







