Chemopreventive Effect of Cratoxylum formosum (Jack) ssp. pruniflorum on Initial Stage Hepatocarcinogenesis in Rats
Abstract
:1. Introduction
2. Results
2.1. Effects of CP on Body/Internal Organ Weights and Food/Water Consumption
2.2. Effects of CP on ALT and AST Levels
2.3. Effect of CP on GST-P Positive Foci
2.4. Effect of CP on PCNA Expression
2.5. Effect of CP on Apoptosis Induction
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Materials
4.3. Animals and Exposures
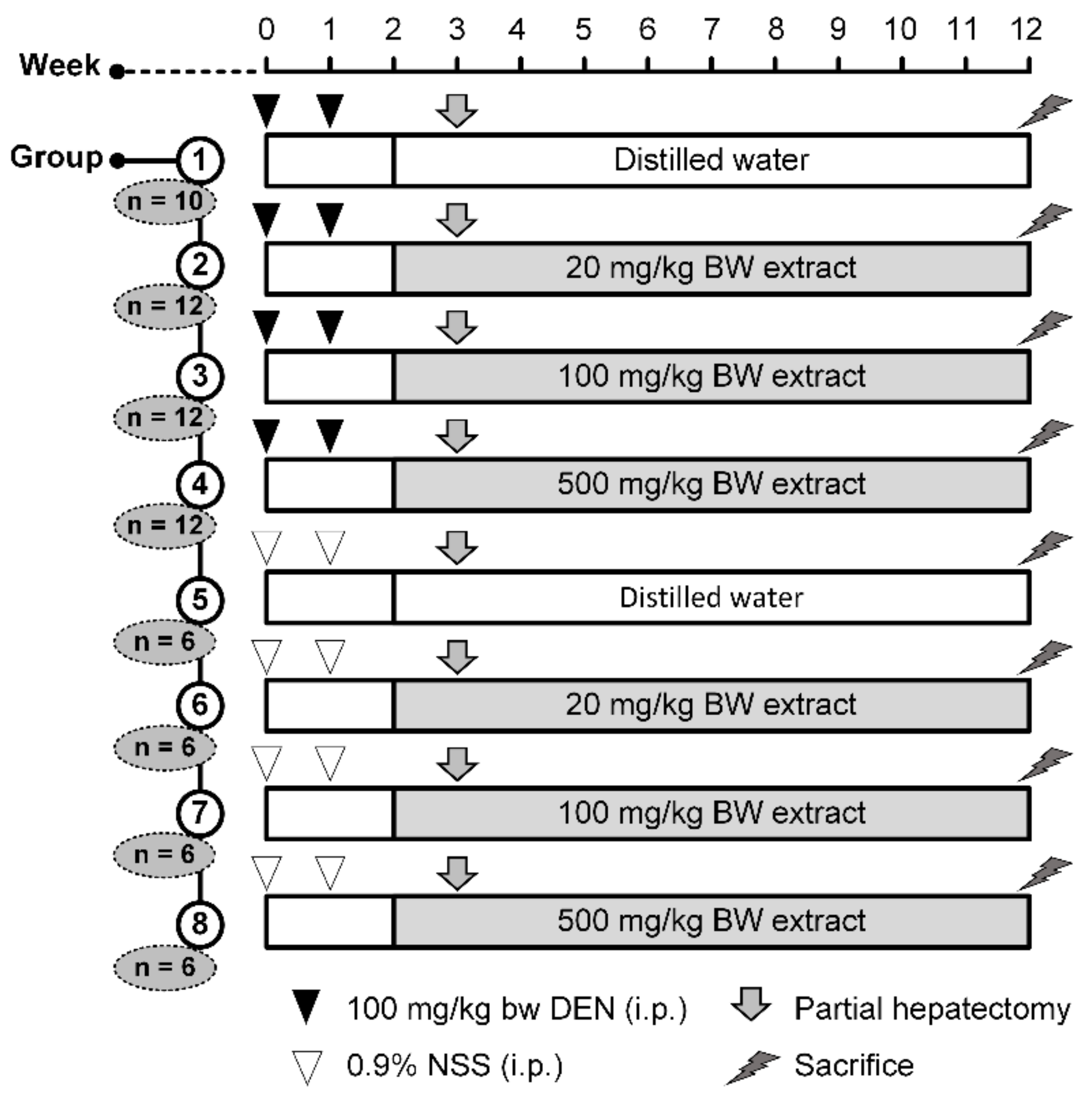
4.4. Experimental Design
4.5. Determination of GST-P Positive Foci
4.6. Determination of PCNA
4.7. Determination of Apoptotic Cells by TUNEL Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kuvatanasuchati, J.; Laphookhieo, S.; Rodanant, P. Antimicrobial activity against periodontopathic bacteria and cytotoxic study of Cratoxylum formosum and Clausena lansium. J. Med. Plants Res. 2011, 5, 5988–5992. [Google Scholar]
- Iinuma, M.; Tosa, H.; Ito, T.; Tanaka, T.; Madulid, D.A. Two xanthones from roots of Cratoxylum formosanum. Phytochemistry 1996, 42, 1195–1198. [Google Scholar] [CrossRef]
- Maisuthisakul, P.; Gordon, M.H.; Pongsawatmanit, R.; Suttajit, M. Enhancing the oxidative stability of rice crackers by addition of the ethanolic extract of phytochemicals from Cratoxylum formosum Dyer. Asia Pac. J. Clin. Nutr. 2007, 16, 37–42. [Google Scholar] [PubMed]
- Xiong, J.; Liu, X.H.; Bui, V.B.; Hong, Z.L.; Wang, L.J.; Zhao, Y.; Fan, H.; Yang, G.X.; Hu, J.F. Phenolic constituents from the leaves of Cratoxylum formosum ssp. pruniflorum. Fitoterapia 2014, 94, 114–119. [Google Scholar] [CrossRef]
- Fan, Q.; Na, Z.; Hu, H.; Xu, Y.K. Chemical compounds from the bark of Cratoxylum formosum ssp. pruniflorum. Chem. Nat. Compd. 2014, 50, 137–138. [Google Scholar] [CrossRef]
- Maisuthisakul, P.; Pongsawatmanit, R.; Gordon, M.H. Characterization of the phytochemicals and antioxidant properties of extracts from Teaw (Cratoxylum formosum Dyer). Food Chem. 2007, 100, 1620–1629. [Google Scholar] [CrossRef]
- Boonsri, S.; Karalai, C.; Ponglimanont, C.; Kanjana-opas, A.; Chantrapromma, K. Antibacterial and cytotoxic xanthones from the roots of Cratoxylum formosum. Phytochemistry 2006, 67, 723–727. [Google Scholar] [CrossRef]
- Choi, S.J.; Tai, B.; Cuong, N.; Kim, Y.H.; Jang, H.D. Antioxidative and anti-inflammatory effect of quercetin and its glycosides isolated from Mampat (Cratoxylum formosum). Food Sci. Biotechnol. 2012, 21, 587–595. [Google Scholar] [CrossRef]
- Sripanidkulchai, K.; Teepsawang, S.; Sripanidkulchai, B. Protective effect of Cratoxylum formosum extract against acid/alcohol-induced gastric mucosal damage in rats. J. Med. Food 2010, 13, 1097–1103. [Google Scholar] [CrossRef]
- Keowkase, R.; Weerapreeyakul, N. Cratoxylum formosum extract protects against amyloid-beta toxicity in a Caenorhabditis elegans model of Alzheimer’s disease. Planta Med. 2016, 82, 516–523. [Google Scholar] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Muralidharan, P.; Raj, J.P. Update in global trends and aetiology of hepatocellular carcinoma. Contemp. Oncol. 2018, 22, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, G.; Karin, M. NF-κB and STAT3—Key players in liver inflammation and cancer. Cell Res. 2011, 21, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Z.; Ma, X.; Duan, Z.; Chen, X. Diagnosis, therapy, and prognosis for hepatocellular carcinoma. Anal. Cell. Pathol. 2020, 2020, 8157406. [Google Scholar] [CrossRef] [Green Version]
- Nonpunya, A.; Sethabouppha, B.; Rufini, S.; Weerapreeyakul, N. Cratoxylum formosum ssp. pruniflorum activates the TRAIL death receptor complex and inhibits topoisomerase I. S. Afr. J. Bot. 2018, 114, 150–162. [Google Scholar] [CrossRef]
- Nonpunya, A.; Weerapreeyakul, N.; Barusrux, S. Cratoxylum formosum (Jack) Dyer ssp. pruniflorum (Kurz) Gogel. (Hóng yá mù) extract induces apoptosis in human hepatocellular carcinoma HepG2 cells through caspase-dependent pathways. Chin. Med. 2014, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senggunprai, L.; Thammaniwit, W.; Kukongviriyapan, V.; Prawan, A.; Kaewseejan, N.; Siriamornpun, S. Cratoxylum formosum extracts inhibit growth and metastasis of cholangiocarcinoma cells by modulating the NF-kappaB and STAT3 pathways. Nutr. Cancer 2016, 68, 328–341. [Google Scholar] [CrossRef]
- Boonnak, N.; Karalai, C.; Chantrapromma, S.; Ponglimanont, C.; Fun, H.-K.; Kanjana-Opas, A.; Laphookhieo, S. Bioactive prenylated xanthones and anthraquinones from Cratoxylum formosum ssp. pruniflorum. Tetrahedron 2006, 62, 8850–8859. [Google Scholar] [CrossRef]
- Duan, Y.H.; Dai, Y.; Wang, G.H.; Zhang, X.; Chen, H.F.; Chen, J.B.; Yao, X.S.; Zhang, X.K. Bioactive xanthones from the stems of Cratoxylum formosum ssp. pruniflorum. J. Nat. Prod. 2010, 73, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Buranrat, B.; Mairuae, N.; Kanchanarach, W. Cytotoxic and antimigratory effects of Cratoxy formosum extract against HepG2 liver cancer cells. Biomed. Rep. 2017, 6, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Tolba, R.; Kraus, T.; Liedtke, C.; Schwarz, M.; Weiskirchen, R. Diethylnitrosamine (DEN)-induced carcinogenic liver injury in mice. Lab. Anim 2015, 49, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Muriel, P.; Ramos-Tovar, E.; Montes-Páez, G.; Buendía-Montaño, L.D. Chapter 40—Experimental models of liver damage mediated by oxidative stress. In Liver Pathophysiology; Muriel, P., Ed.; Academic Press: Boston, MA, USA, 2017. [Google Scholar]
- Noguti, J.; Barbisan, L.F.; Cesar, A.; Dias Seabra, C.; Choueri, R.B.; Ribeiro, D.A. In vivo models for measuring placental glutathione S-transferase (GST-P 7-7) levels: A suitable biomarker for understanding cancer pathogenesis. In Vivo 2012, 26, 647–650. [Google Scholar] [PubMed]
- Hara, A.; Yamada, H.; Sakai, N.; Hirayama, H.; Tanaka, T.; Mori, H. Immunohistochemical demonstration of the placental form of glutathione S-transferase, a detoxifying enzyme in human gliomas. Cancer 1990, 66, 2563–2568. [Google Scholar] [CrossRef]
- Tew, K.D.; Townsend, D.M. Regulatory functions of glutathione S-transferase P1-1 unrelated to detoxification. Drug Met. Rev. 2011, 43, 179–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Jeong, M.S.; Han, C.W.; Yu, H.S.; Jang, S.B. Structural and functional insight into proliferating cell nuclear antigen. J. Microbiol. Biotechnol. 2016, 26, 637–647. [Google Scholar] [CrossRef]
- Cummings, J.; Ward, T.H.; Ranson, M.; Dive, C. Apoptosis pathway-targeted drugs—From the bench to the clinic. BBA Rev. Cancer 2004, 1705, 53–66. [Google Scholar] [CrossRef]
- Thumvijit, T.; Taya, S.; Punvittayagul, C.; Peerapornpisal, Y.; Wongpoomchai, R. Cancer chemopreventive effect of Spirogyra neglecta (Hassall) Kutzing on diethylnitrosamine-induced hepatocarcinogenesis in rats. APJCP 2014, 15, 1611–1616. [Google Scholar] [CrossRef] [Green Version]
- Boonnak, N.; Chantrapromma, S.; Fun, H.K.; Yuenyongsawad, S.; Patrick, B.O.; Maneerat, W.; Williams, D.E.; Andersen, R.J. Three types of cytotoxic natural caged-scaffolds: Pure enantiomers or partial racemates. J. Nat. Prod. 2014, 77, 1562–1571. [Google Scholar] [CrossRef]
- Boonnak, N.; Karalai, C.; Chantrapromma, S.; Ponglimanont, C.; Kanjana-Opas, A.; Chantrapromma, K.; Kato, S. Chromene and prenylated xanthones from the roots of Cratoxylum formosum ssp. pruniflorum. Chem. Pharm. Bull. 2010, 58, 386–389. [Google Scholar] [CrossRef] [Green Version]
- Chantrapromma, S.; Boonnak, N.; Fun, H.K.; Karalai, C.; Chantrapromma, K. Brasilixanthone. Acta Crystallogr. E 2010, 66, o2066–o2067. [Google Scholar] [CrossRef] [Green Version]
- Boonnak, N.; Chantrapromma, S.; Fun, H.-K.; Karalai, C. Vieillardiixanthone B. Acta Crystallogr. E 2010, 66, o817–o818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonnak, N.; Fun, H.-K.; Chantrapromma, S.; Karalai, C. Gerontoxanthone I methanol solvate. Acta Crystallogr. E 2007, 63, o3958–o3959. [Google Scholar] [CrossRef]
- Boonnak, N.; Chantrapromma, S.; Fun, H.K.; Karalai, C. 4,8-Dihydr-oxy-2,3-dimeth-oxy-1-(3-methyl-but-2-en-yl)-9H-xanthen-9-one. Acta Crystallogr. E 2007, 63, o4903–o4904. [Google Scholar] [CrossRef]
- Boonnak, N.; Chantrapromma, S.; Tewtrakul, S.; Sudsai, T. Inhibition of nitric oxide production in lipopolysaccharide-activated RAW264.7 macrophages by isolated xanthones from the roots of Cratoxylum formosum ssp. pruniflorum. Arch. Pharm. Res. 2014, 37, 1329–1335. [Google Scholar] [CrossRef]
- Kaewpiboon, C.; Boonnak, N.; Yawut, N.; Kaowinn, S.; Chung, Y.H. Caged-xanthone from Cratoxylum formosum ssp. pruniflorum inhibits malignant cancer phenotypes in multidrug-resistant human A549 lung cancer cells through down-regulation of NF-κB. Bioorg. Med. Chem. 2019, 27, 2368–2375. [Google Scholar] [CrossRef]
- Boonnak, N.; Chantrapromma, S.; Fun, H.K. Molecular and crystal Structures of α,α,β-trimethylfuranylxanthone from Cratoxylum formosum ssp. pruniflorum: A partial racemate. Mol. Cryst. Liq. Cryst. 2015, 606, 165–175. [Google Scholar] [CrossRef]
- Fun, H.K.; Chantrapromma, S.; Boonnak, N.; Karalai, C.; Chantrapromma, K. Redetermination and absolute configuration of pruniflorone M monohydrate. Acta Crystallogr. E 2011, 67, o1916–o1917. [Google Scholar] [CrossRef] [Green Version]
- Boonnak, N.; Khamthip, A.; Karalai, C.; Chantrapromma, S.; Ponglimanont, C.; Kanjana-Opas, A.; Tewtrakul, S.; Chantrapromma, K.; Fun, H.K.; Kato, S. Nitric oxide inhibitory activity of xanthones from the green fruits of Cratoxylum formosum ssp. pruniflorum. Aust. J. Chem. 2010, 63, 1550–1556. [Google Scholar] [CrossRef]
- Kaewpiboon, C.; Boonnak, N.; Kaowinn, S.; Chung, Y.H. Formoxanthone C, isolated from Cratoxylum formosum ssp. pruniflorum, reverses anticancer drug resistance by inducing both apoptosis and autophagy in human A549 lung cancer cells. Bioorg. Med. Chem. Lett. 2018, 28, 820–825. [Google Scholar] [CrossRef]
- Palanuvej, C.; Ruangrungsi, N. Volatile constituents of Cratoxylum formosum ssp. pruniflorum. J. Health Res. 2020, 22, 53. [Google Scholar]
- Nzogong, R.T.; Ndjateu, F.S.T.; Ekom, S.E.; Fosso, J.A.M.; Awouafack, M.D.; Tene, M.; Tane, P.; Morita, H.; Choudhary, M.I.; Tamokou, J.D.D. Antimicrobial and antioxidant activities of triterpenoid and phenolic derivatives from two Cameroonian Melastomataceae plants: Dissotis senegambiensis and Amphiblemma monticola. BMC Complement. Altern. Med. 2018, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Rivas, A.C.; Lopes, P.M.; de Azevedo Barros, M.M.; Costa Machado, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar]
- Wang, G.H.; Jiang, F.Q.; Duan, Y.H.; Zeng, Z.P.; Chen, F.; Dai, Y.; Chen, J.B.; Liu, J.X.; Liu, J.; Zhou, H.; et al. Targeting truncated retinoid X receptor-α by CF31 induces TNF-α-dependent apoptosis. Cancer Res. 2013, 73, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Manosroi, J.; Wilairat, R.; Kijjoa, A.; Manosroi, A. Free radical scavenging activity of extracts from Thai plants in Guttiferae and Schisandraceae families. Pharm. Biol. 2008, 43, 324–329. [Google Scholar] [CrossRef]
- Weerapreeyakul, N.; Machana, S.; Barusrux, S. Synergistic effects of melphalan and Pinus kesiya Royle ex Gordon (Simaosong) extracts on apoptosis induction in human cancer cells. Chin. Med. 2016, 11, 29. [Google Scholar] [CrossRef] [Green Version]
- Rizeq, B.; Gupta, I.; Ilesanmi, J.; Al Safran, M.; Rahman, M.; Ouhtit, A. The power of phytochemicals combination in cancer chemoprevention. J. Cancer 2020, 11, 4521–4533. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Cheng, P.; Chu, P.Y.; Li, C.J. Molecular targets in hepatocarcinogenesis and implications for therapy. J. Clin. Med. 2018, 7, 213. [Google Scholar] [CrossRef] [Green Version]
- Issara-Amphorn, J.; Thienprasert, N. T-Thienprasert NP. Preliminary In vitro pro-apoptotic effects of Cratoxylum formosum crude leaf extracts. Int. J. Appl. Res. Nat. Prod. 2014, 7, 26–30. [Google Scholar]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Bisteau, X.; Caldez, M.J.; Kaldis, P. The complex relationship between liver cancer and the cell cycle: A story of multiple regulations. Cancers 2014, 6, 79–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundarrajan, M.; Gupta, S.; Rao, K.V. Overexpression of cyclin D1 is associated with the decondensation of chromatin during den-induced sequential hepatocarcinogenesis. Cell Biol. Int. 2002, 26, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Parekh, P.; Rao, K.V. Overexpression of cyclin D1 is associated with elevated levels of MAP kinases, Akt and Pak1 during diethylnitrosamine-induced progressive liver carcinogenesis. Cell Biol. Int. 2007, 31, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Buranrat, B.; Mairuae, N.; Konsue, A. Cratoxy formosum leaf extract inhibits proliferation and migration of human breast cancer MCF-7 cells. Biomed. Pharmacother. 2017, 90, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Van de Waterbeemd, H.; Smith, D.A.; Jones, B.C. Lipophilicity in PK design: Methyl, ethyl, futile. J. Comput. Aided Mol. Des. 2001, 15, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Praphasawat, R.; Munkong, N. Anti-genotoxicity evaluation of Cratoxylum formosum Dyer leaves by comet assay and micronucleus test. APJCP 2017, 18, 1475–1478. [Google Scholar]
- Weerapreeyakul, N.; Nonpunya, A.; Barusrux, S.; Thitimetharoch, T.; Sripanidkulchai, B. Evaluation of the anticancer potential of six herbs against a hepatoma cell line. Chin. Med. 2012, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Shahrzad, S.; Aoyagi, K.; Winter, A.; Koyama, A.; Bitsch, I. Pharmacokinetics of gallic acid and its relative bioavailability from tea in healthy humans. J. Nutr. 2001, 131, 1207–1210. [Google Scholar] [CrossRef]
- Zanwar, A.A.; Badole, S.L.; Shende, P.S. Chapter 80—Role of gallic acid in cardiovascular disorders. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 1045–1047. [Google Scholar]
- Higgins, G.M.; Anderson, R.M. Experimental pathology of the liver. Restoration of the liver of the white rat following partial surgical removal. AMA Arch. Pathol. 1931, 12, 186–202. [Google Scholar]
- Dokkaew, A.; Punvittayagul, C.; Insuan, O.; Limtrakul Dejkriengkraikul, P.; Wongpoomchai, R. Protective effects of defatted sticky rice bran extracts on the early stages of hepatocarcinogenesis in rats. Molecules 2019, 24, 2142. [Google Scholar] [CrossRef] [Green Version]





| Group | Treatment * | Initial Weight (g) | Final Weight (g) | Food Consumption (g/rat/day) | Water Consumption (g/rat/day) | |
|---|---|---|---|---|---|---|
| Initiator | Test Compound | |||||
| 1 | DEN | DW | 206 ± 12 | 434 ± 52 | 22 ± 1 | 32 ± 4 |
| 2 | DEN | CP 20 mg/kg | 209 ± 7 | 438 ± 41 | 21 ± 2 | 27 ± 2 |
| 3 | DEN | CP 100 mg/kg | 205 ± 8 | 394 ± 43 | 22 ± 2 | 28 ± 3 |
| 4 | DEN | CP 500 mg/kg | 205 ± 9 | 393 ± 24 | 21 ± 2 | 26 ± 3 |
| 5 | NSS | DW | 211 ± 7 | 446 ± 21 | 23 ± 4 | 29 ± 9 |
| 6 | NSS | CP 20 mg/kg | 203 ± 8 | 447 ± 35 | 23 ± 4 | 31 ± 3 |
| 7 | NSS | CP 100 mg/kg | 208 ± 8 | 448 ± 33 | 21 ± 1 | 30 ± 3 |
| 8 | NSS | CP 500 mg/kg | 204 ± 10 | 465 ± 32 | 25 ± 3 | 30 ± 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pocasap, P.; Weerapreeyakul, N.; Wongpoomchai, R. Chemopreventive Effect of Cratoxylum formosum (Jack) ssp. pruniflorum on Initial Stage Hepatocarcinogenesis in Rats. Molecules 2021, 26, 4235. https://doi.org/10.3390/molecules26144235
Pocasap P, Weerapreeyakul N, Wongpoomchai R. Chemopreventive Effect of Cratoxylum formosum (Jack) ssp. pruniflorum on Initial Stage Hepatocarcinogenesis in Rats. Molecules. 2021; 26(14):4235. https://doi.org/10.3390/molecules26144235
Chicago/Turabian StylePocasap, Piman, Natthida Weerapreeyakul, and Rawiwan Wongpoomchai. 2021. "Chemopreventive Effect of Cratoxylum formosum (Jack) ssp. pruniflorum on Initial Stage Hepatocarcinogenesis in Rats" Molecules 26, no. 14: 4235. https://doi.org/10.3390/molecules26144235
APA StylePocasap, P., Weerapreeyakul, N., & Wongpoomchai, R. (2021). Chemopreventive Effect of Cratoxylum formosum (Jack) ssp. pruniflorum on Initial Stage Hepatocarcinogenesis in Rats. Molecules, 26(14), 4235. https://doi.org/10.3390/molecules26144235









