Compound A Increases Cell Infiltration in Target Organs of Acute Graft-versus-Host Disease (aGVHD) in a Mouse Model
Abstract
:1. Introduction
2. Results
2.1. Influence of CpdA on Proliferation and Inflammation In Vitro
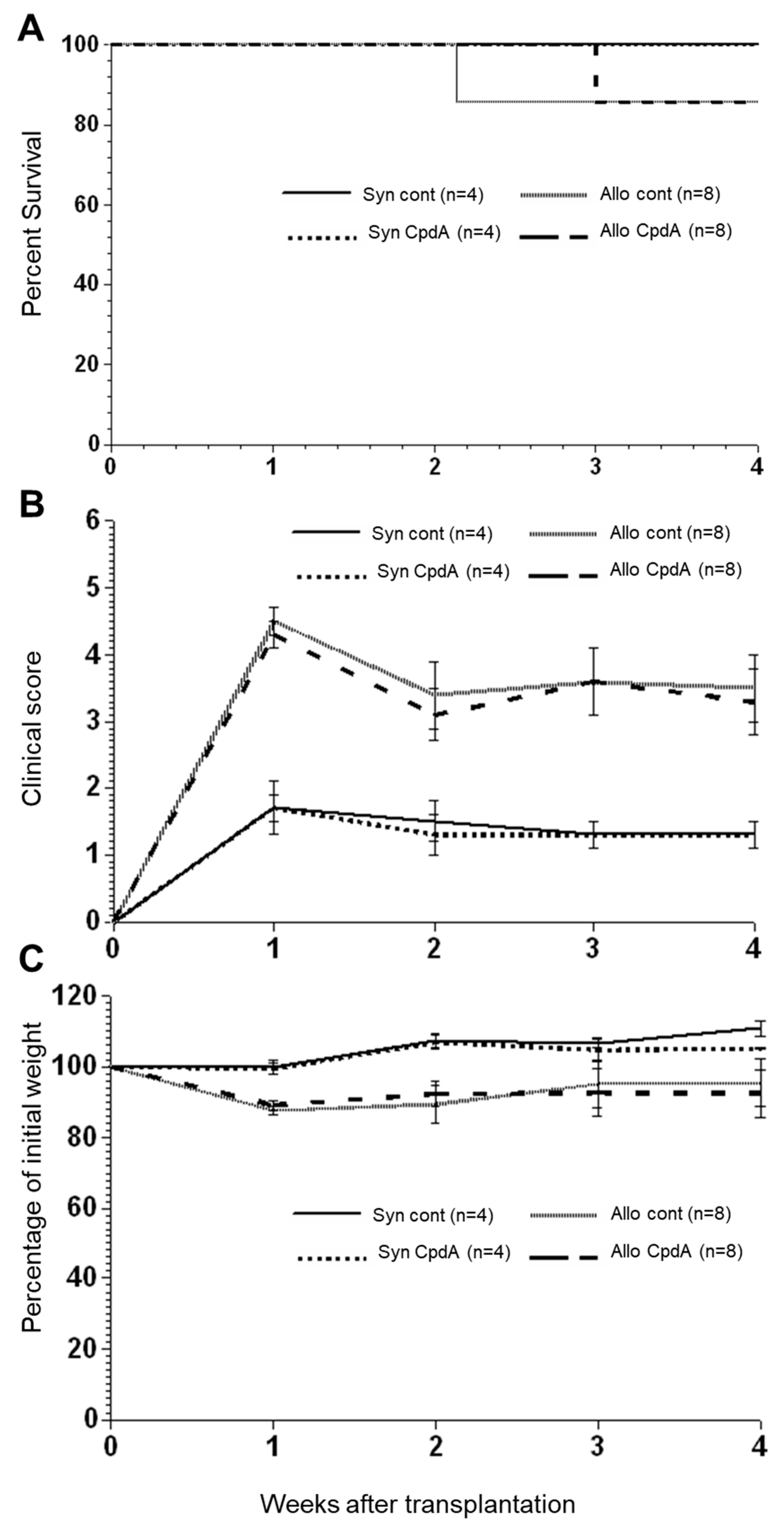
2.2. Effect of CpdA on Allo-BMT In Vivo
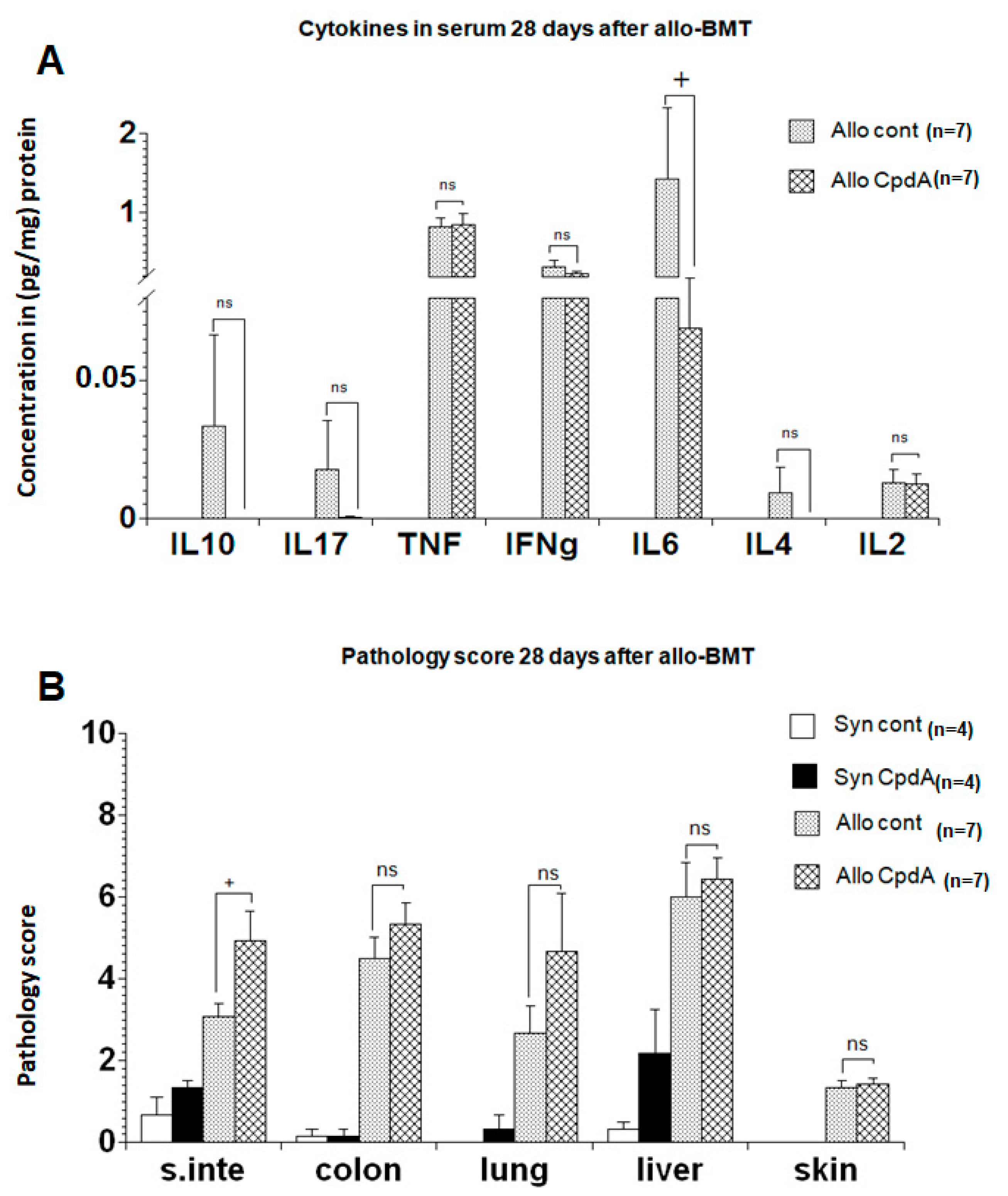
2.3. Cytokine Expression and Pathology Score
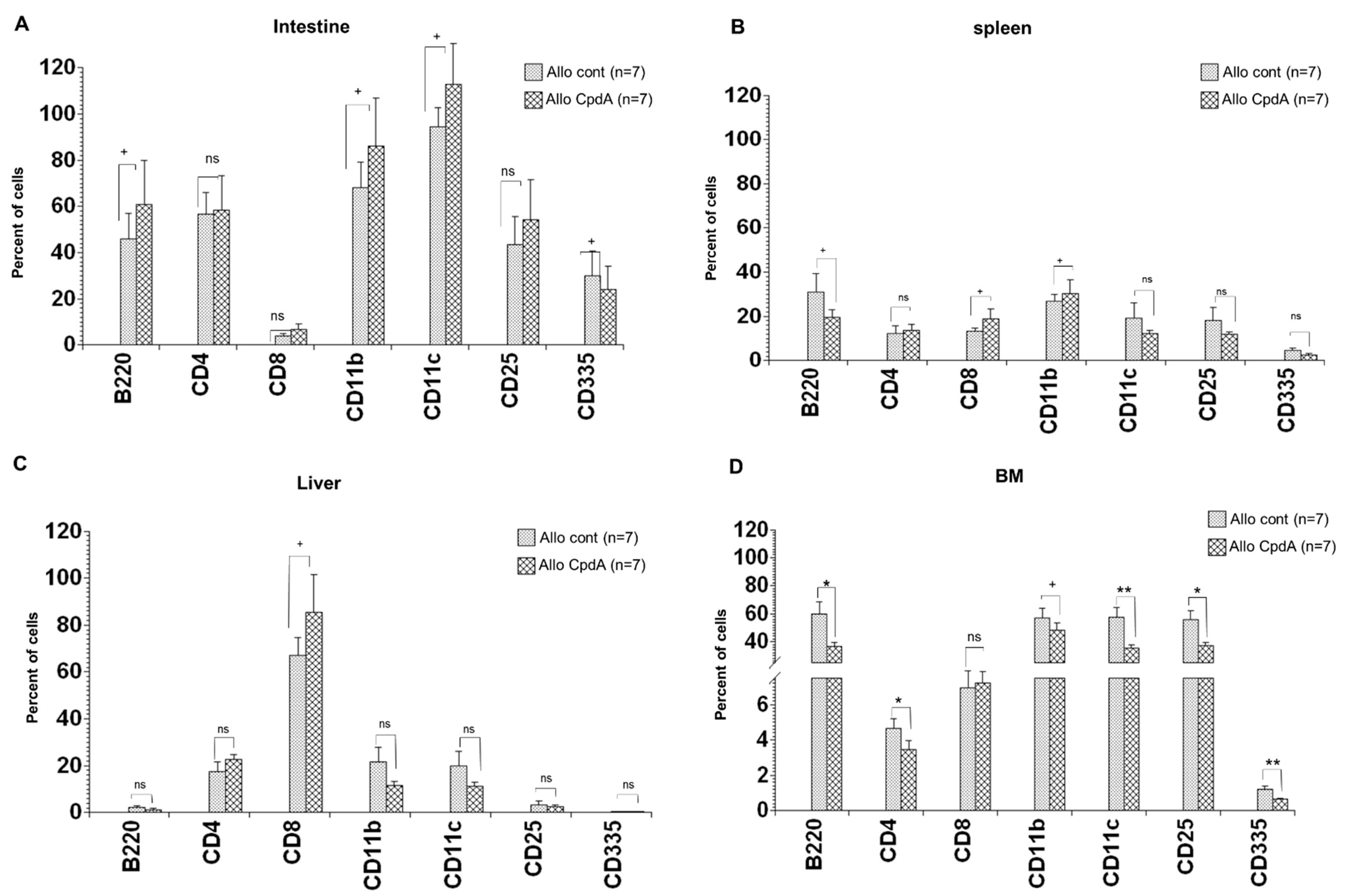
2.4. Analysis of Cell Suspensions of Target Organs
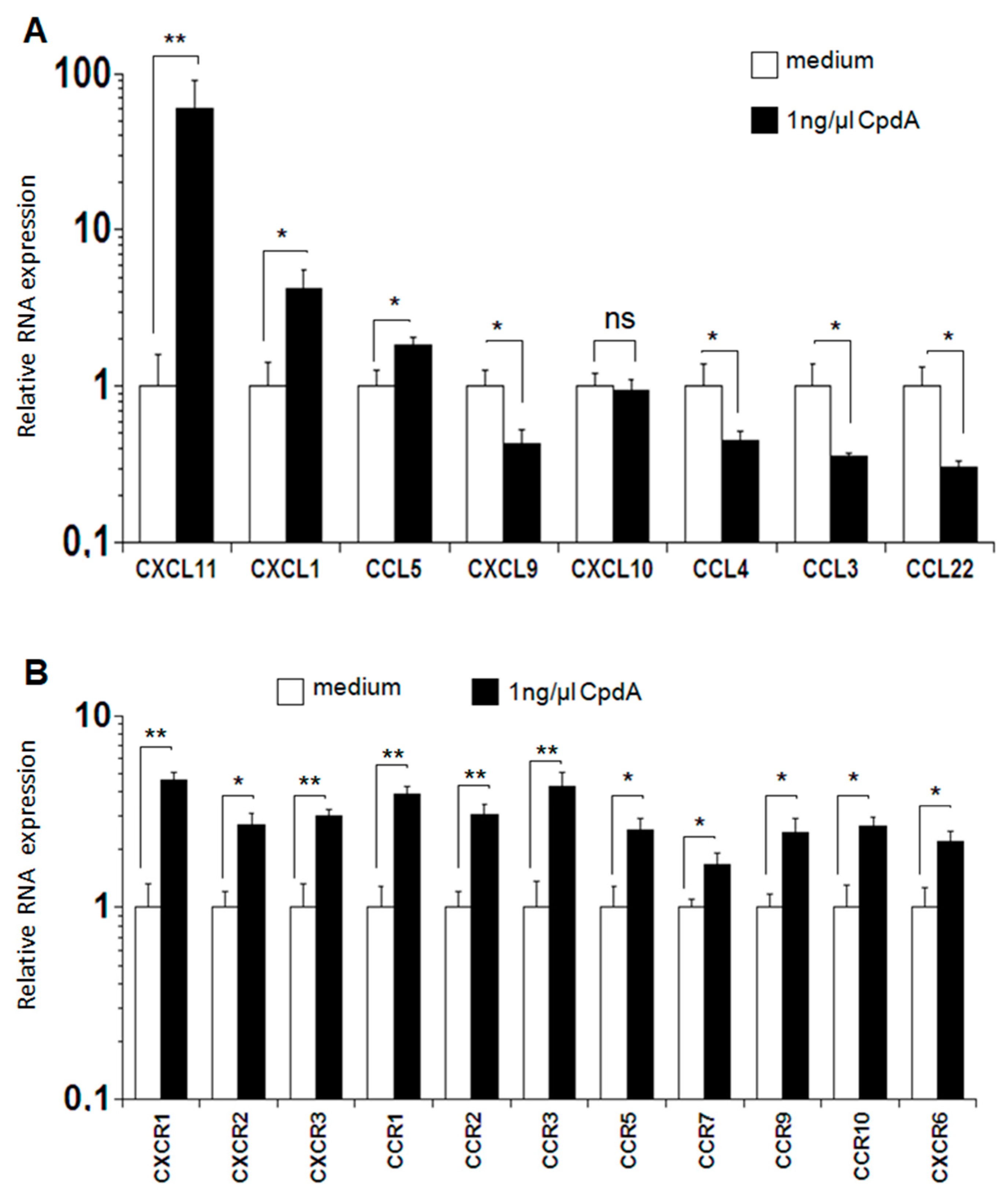
2.5. Expression of Chemokines and Chemokine Receptors after MLR
3. Discussion
4. Materials and Methods
4.1. Transplantation Procedure
4.2. Semiquantitative Histopathology of the Liver, Lung, Gastrointestinal Tract, and Skin
4.3. Serum Cytokine Analysis after BMT
4.4. Single-Cell Suspensions and Flow Cytometry Analysis
4.5. T Cell Proliferation and In Vitro Mixed Lymphocyte Reaction (MLR)
4.6. Determination of mRNA Expression Using Real-Time PCR
4.7. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ferrara, J.L.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef]
- Paczesny, S.; Choi, S.W.; Ferrara, J.L. Acute graft-versus-host disease: New treatment strategies. Curr. Opin. Hematol. 2009, 16, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armitage, J.O. Bone marrow transplantation. N. Engl. J. Med. 1994, 330, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Boumpas, D.T.; Paliogianni, F.; Anastassiou, E.D.; Balow, J.E. Glucocorticosteroid action on the immune system: Molecular and cellular aspects. Clin. Exp. Rheumatol. 1991, 9, 413–423. [Google Scholar] [PubMed]
- De Bosscher, K.; Vanden Berghe, W.; Beck, I.M.; Van Molle, W.; Hennuyer, N.; Hapgood, J.; Libert, C.; Staels, B.; Louw, A.; Haegeman, G. A fully dissociated compound of plant origin for inflammatory gene repression. Proc. Natl. Acad. Sci. USA 2005, 102, 15827–15832. [Google Scholar] [CrossRef] [Green Version]
- Wüst, S.; Tischner, D.; John, M.; Tuckermann, J.P.; Menzfeld, C.; Hanisch, U.-K.; van den Brandt, J.; Lühder, F.; Reichardt, H.M.J.P.o. Therapeutic and adverse effects of a non-steroidal glucocorticoid receptor ligand in a mouse model of multiple sclerosis. PLoS ONE 2009, 4, e8202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Loo, G.; Sze, M.; Bougarne, N.; Praet, J.; Mc Guire, C.; Ullrich, A.; Haegeman, G.; Prinz, M.; Beyaert, R.; De Bosscher, K. Antiinflammatory properties of a plant-derived nonsteroidal, dissociated glucocorticoid receptor modulator in experimental autoimmune encephalomyelitis. Mol. Endocrinol. 2010, 24, 310–322. [Google Scholar] [CrossRef] [Green Version]
- Gossye, V.; Elewaut, D.; Bougarne, N.; Bracke, D.; Van Calenbergh, S.; Haegeman, G.; De Bosscher, K. Differential mechanism of NF-kappaB inhibition by two glucocorticoid receptor modulators in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2009, 60, 3241–3250. [Google Scholar] [CrossRef]
- Reuter, K.C.; Grunwitz, C.R.; Kaminski, B.M.; Steinhilber, D.; Radeke, H.H.; Stein, J. Selective glucocorticoid receptor agonists for the treatment of inflammatory bowel disease: Studies in mice with acute trinitrobenzene sulfonic acid colitis. J. Pharmacol. Exp. Ther. 2012, 341, 68–80. [Google Scholar] [CrossRef] [Green Version]
- Reber, L.L.; Daubeuf, F.; Plantinga, M.; De Cauwer, L.; Gerlo, S.; Waelput, W.; Van Calenbergh, S.; Tavernier, J.; Haegeman, G.; Lambrecht, B.N.; et al. A dissociated glucocorticoid receptor modulator reduces airway hyperresponsiveness and inflammation in a mouse model of asthma. J. Immunol. 2012, 188, 3478–3487. [Google Scholar] [CrossRef] [Green Version]
- Rauner, M.; Thiele, S.; Sinningen, K.; Winzer, M.; Salbach-Hirsch, J.; Gloe, I.; Peschke, K.; Haegeman, G.; Tuckermann, J.P.; Hofbauer, L.C. Effects of the selective glucocorticoid receptor modulator compound A on bone metabolism and inflammation in male mice with collagen-induced arthritis. Endocrinology 2013, 154, 3719–3728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehouse, M.W. Anti-inflammatory glucocorticoid drugs: Reflections after 60 years. Inflammopharmacology 2011, 19, 1–19. [Google Scholar] [CrossRef]
- Lesovaya, E.; Yemelyanov, A.; Swart, A.C.; Swart, P.; Haegeman, G.; Budunova, I. Discovery of Compound A–a selective activator of the glucocorticoid receptor with anti-inflammatory and anti-cancer activity. Oncotarget 2015, 6, 30730–30744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groth, C.G.; Gahrton, G.; Lundgren, G.; Moller, E.; Pihlstedt, P.; Ringden, O.; Sundelin, P. Successful treatment with prednisone and graft-versus-host disease in an allogeneic bone-marrow transplant recipient. Scand. J. Haematol. 1979, 22, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Storb, R.; Kolb, H.J.; Graham, T.C.; Kolb, H.; Weiden, P.L.; Thomas, E.D. Treatment of established graft-versus-host disease in dogs by antithymocyte serum or prednisone. Blood 1973, 42, 601–609. [Google Scholar] [CrossRef]
- Kendra, J.; Barrett, A.J.; Lucas, C.; Joshi, R.; Joss, V.; Desai, M.; Halil, O.; Rogers, T.R.; Hobbs, J.R.; Hugh-Jones, K. Response of graft versus host disease to high doses of methyl prednisolone. Clin. Lab. Haematol. 1981, 3, 19–26. [Google Scholar] [CrossRef]
- Kanojia, M.D.; Anagnostou, A.A.; Zander, A.R.; Vellekoop, L.; Spitzer, G.; Verma, D.S.; Jagannath, S.; Dicke, K.A. High-dose methylprednisolone treatment for acute graft-versus-host disease after bone marrow transplantation in adults. Transplantation 1984, 37, 246–249. [Google Scholar] [CrossRef]
- Knop, S.; Hebart, H.; Gratwohl, A.; Kliem, C.; Faul, C.; Holler, E.; Apperley, J.; Kolb, H.J.; Schaefer, A.; Niederwieser, D.; et al. Treatment of steroid-resistant acute GVHD with OKT3 and high-dose steroids results in better disease control and lower incidence of infectious complications when compared to high-dose steroids alone: A randomized multicenter trial by the EBMT Chronic Leukemia Working Party. Leukemia 2007, 21, 1830–1833. [Google Scholar]
- Quellmann, S.; Schwarzer, G.; Hubel, K.; Engert, A.; Bohlius, J. Corticosteroids in the prevention of graft-vs-host disease after allogeneic myeloablative stem cell transplantation: A systematic review and meta-analysis. Leukemia 2008, 22, 1801–1803. [Google Scholar] [CrossRef] [Green Version]
- Van Lint, M.T.; Milone, G.; Leotta, S.; Uderzo, C.; Scime, R.; Dallorso, S.; Locasciulli, A.; Guidi, S.; Mordini, N.; Sica, S.; et al. Treatment of acute graft-versus-host disease with prednisolone: Significant survival advantage for day +5 responders and no advantage for nonresponders receiving anti-thymocyte globulin. Blood 2006, 107, 4177–4181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ancin, I.; Ferra, C.; Gallardo, D.; Peris, J.; Berlanga, J.; Gonzalez, J.R.; Virgili, N.; Granena, A. Do corticosteroids add any benefit to standard GVHD prophylaxis in allogeneic BMT? Bone Marrow Transpl. 2001, 28, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Storb, R.; Pepe, M.; Anasetti, C.; Appelbaum, F.R.; Beatty, P.; Doney, K.; Martin, P.; Stewart, P.; Sullivan, K.M.; Witherspoon, R.; et al. What role for prednisone in prevention of acute graft-versus-host disease in patients undergoing marrow transplants? Blood 1990, 76, 1037–1045. [Google Scholar] [CrossRef] [Green Version]
- D’Costa, S.; Hurwitz, J.L. Antibody and pre- plus post-transplant prednisone treatments support T cell-depleted stem cell engraftment without drug-induced morbidity. Bone Marrow Transpl. 2002, 29, 553–556. [Google Scholar] [CrossRef] [Green Version]
- Deeg, H.J.; Lin, D.; Leisenring, W.; Boeckh, M.; Anasetti, C.; Appelbaum, F.R.; Chauncey, T.R.; Doney, K.; Flowers, M.; Martin, P.; et al. Cyclosporine or cyclosporine plus methylprednisolone for prophylaxis of graft-versus-host disease: A prospective, randomized trial. Blood 1997, 89, 3880–3887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schacke, H.; Docke, W.D.; Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002, 96, 23–43. [Google Scholar] [CrossRef]
- Psarra, A.M.; Solakidi, S.; Trougakos, I.P.; Margaritis, L.H.; Spyrou, G.; Sekeris, C.E. Glucocorticoid receptor isoforms in human hepatocarcinoma HepG2 and SaOS-2 osteosarcoma cells: Presence of glucocorticoid receptor alpha in mitochondria and of glucocorticoid receptor beta in nucleoli. Int. J. Biochem. Cell Biol. 2005, 37, 2544–2558. [Google Scholar] [CrossRef]
- Amsterdam, A.; Sasson, R. The anti-inflammatory action of glucocorticoids is mediated by cell type specific regulation of apoptosis. Mol. Cell. Endocrinol. 2002, 189, 1–9. [Google Scholar] [CrossRef]
- Liberman, A.C.; Druker, J.; Garcia, F.A.; Holsboer, F.; Arzt, E. Intracellular molecular signaling. Basis for specificity to glucocorticoid anti-inflammatory actions. Ann. N. Y. Acad. Sci. 2009, 1153, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Kleiman, A.; Tuckermann, J.P. Glucocorticoid receptor action in beneficial and side effects of steroid therapy: Lessons from conditional knockout mice. Mol. Cell. Endocrinol. 2007, 275, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Dewint, P.; Gossye, V.; De Bosscher, K.; Vanden Berghe, W.; Van Beneden, K.; Deforce, D.; Van Calenbergh, S.; Muller-Ladner, U.; Vander Cruyssen, B.; Verbruggen, G.; et al. A plant-derived ligand favoring monomeric glucocorticoid receptor conformation with impaired transactivation potential attenuates collagen-induced arthritis. J. Immunol. 2008, 180, 2608–2615. [Google Scholar] [CrossRef] [Green Version]
- De Bosscher, K. Selective Glucocorticoid Receptor modulators. J. Steroid Biochem. Mol. Biol. 2010, 120, 96–104. [Google Scholar] [CrossRef]
- De Lange, M. Prolonged gestation in karakul ewes in South West Africa. In Proceedings of the 4th Intemat Congress, Helsinki, Finland, 4–9 September 1961; Anim Reprod The Hague. Volume 3, pp. 590–592. [Google Scholar]
- De Bosscher, K.; Haegeman, G. Minireview: Latest perspectives on antiinflammatory actions of glucocorticoids. Mol. Endocrinol. 2009, 23, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Liberman, A.C.; Antunica-Noguerol, M.; Ferraz-de-Paula, V.; Palermo-Neto, J.; Castro, C.N.; Druker, J.; Holsboer, F.; Perone, M.J.; Gerlo, S.; De Bosscher, K.; et al. Compound A, a dissociated glucocorticoid receptor modulator, inhibits T-bet (Th1) and induces GATA-3 (Th2) activity in immune cells. PLoS ONE 2012, 7, e35155. [Google Scholar] [CrossRef]
- De Bosscher, K.; Beck, I.M.; Dejager, L.; Bougarne, N.; Gaigneaux, A.; Chateauvieux, S.; Ratman, D.; Bracke, M.; Tavernier, J.; Vanden Berghe, W.; et al. Selective modulation of the glucocorticoid receptor can distinguish between transrepression of NF-kappaB and AP-1. Cell. Mol. Life Sci. 2014, 71, 143–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhang, Z.Y.; Schluesener, H.J. Compound A, a plant origin ligand of glucocorticoid receptors, increases regulatory T cells and M2 macrophages to attenuate experimental autoimmune neuritis with reduced side effects. J. Immunol. 2009, 183, 3081–3091. [Google Scholar] [CrossRef]
- Serody, J.S.; Burkett, S.E.; Panoskaltsis-Mortari, A.; Ng-Cashin, J.; McMahon, E.; Matsushima, G.K.; Lira, S.A.; Cook, D.N.; Blazar, B.R. T-lymphocyte production of macrophage inflammatory protein-1alpha is critical to the recruitment of CD8(+) T cells to the liver, lung, and spleen during graft-versus-host disease. Blood 2000, 96, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, G.C.; Corrion, L.A.; Olkiewicz, K.M.; Lu, B.; Lowler, K.; Duffner, U.A.; Moore, B.B.; Kuziel, W.A.; Liu, C.; Cooke, K.R. Blockade of CXCR3 receptor:ligand interactions reduces leukocyte recruitment to the lung and the severity of experimental idiopathic pneumonia syndrome. J. Immunol. 2004, 173, 2050–2059. [Google Scholar] [CrossRef] [Green Version]
- Bouazzaoui, A.; Spacenko, E.; Mueller, G.; Miklos, S.; Huber, E.; Holler, E.; Andreesen, R.; Hildebrandt, G.C. Chemokine and chemokine receptor expression analysis in target organs of acute graft-versus-host disease. Genes Immun. 2009, 10, 687–701. [Google Scholar] [CrossRef] [Green Version]
- Duffner, U.; Lu, B.; Hildebrandt, G.C.; Teshima, T.; Williams, D.L.; Reddy, P.; Ordemann, R.; Clouthier, S.G.; Lowler, K.; Liu, C.; et al. Role of CXCR3-induced donor T-cell migration in acute GVHD. Exp. Hematol. 2003, 31, 897–902. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [Green Version]
- Murai, M.; Yoneyama, H.; Harada, A.; Yi, Z.; Vestergaard, C.; Guo, B.; Suzuki, K.; Asakura, H.; Matsushima, K. Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J. Clin. Investig. 1999, 104, 49–57. [Google Scholar] [CrossRef]
- Waldman, E.; Lu, S.X.; Hubbard, V.M.; Kochman, A.A.; Eng, J.M.; Terwey, T.H.; Muriglan, S.J.; Kim, T.D.; Heller, G.; Murphy, G.F.; et al. Absence of beta7 integrin results in less graft-versus-host disease because of decreased homing of alloreactive T cells to intestine. Blood 2006, 107, 1703–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrandt, G.C.; Olkiewicz, K.M.; Choi, S.; Corrion, L.A.; Clouthier, S.G.; Liu, C.; Serody, J.S.; Cooke, K.R. Donor T-cell production of RANTES significantly contributes to the development of idiopathic pneumonia syndrome after allogeneic stem cell transplantation. Blood 2005, 105, 2249–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, P.; Holmes, C.F.; Tsukitani, Y. Okadaic acid: A new probe for the study of cellular regulation. Trends Biochem. Sci. 1990, 15, 98–102. [Google Scholar] [CrossRef]
- Chang, J.; Voorhees, T.J.; Liu, Y.; Zhao, Y.; Chang, C.H. Interleukin-23 production in dendritic cells is negatively regulated by protein phosphatase 2A. Proc. Natl. Acad. Sci. USA 2010, 107, 8340–8345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jana, M.; Dasgupta, S.; Saha, R.N.; Liu, X.; Pahan, K. Induction of tumor necrosis factor-alpha (TNF-alpha) by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J. Neurochem. 2003, 86, 519–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khader, S.A.; Bell, G.K.; Pearl, J.E.; Fountain, J.J.; Rangel-Moreno, J.; Cilley, G.E.; Shen, F.; Eaton, S.M.; Gaffen, S.L.; Swain, S.L.; et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 2007, 8, 369–377. [Google Scholar] [CrossRef]
- Groom, J.R.; Luster, A.D. CXCR3 ligands: Redundant, collaborative and antagonistic functions. Immunol. Cell Biol. 2011, 89, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Bouazzaoui, A.; Huber, E.; Dan, A.; Al-Allaf, F.A.; Pfirstinger, J.; Sprotte, G.; Kostler, J.; Hiergeist, A.; Gessner, A.; Herr, W.; et al. Reduction of aGVHD using chicken antibodies directed against intestinal pathogens in a murine model. Blood 2017, 129, 1052–1055. [Google Scholar] [CrossRef] [Green Version]
- Cooke, K.R.; Kobzik, L.; Martin, T.R.; Brewer, J.; Delmonte, J.J.; Crawford, J.M.; Ferrara, J. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood 1996, 88, 3230–3239. [Google Scholar] [CrossRef] [Green Version]
- Bouazzaoui, A.; Dickhofer, S.; Kreuz, M.; Huber, E.; Holler, E.; Wolff, D. Cytostatic conditioning in experimental allogeneic bone marrow transplantation: Busulfan causes less early gastrointestinal toxicity but Treosulfan results in improved immune reconstitution. Immunopharmacol. Immunotoxicol. 2014, 36, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Bouazzaoui, A.; Spacenko, E.; Mueller, G.; Huber, E.; Schubert, T.; Holler, E.; Andreesen, R.; Hildebrandt, G.C. Steroid treatment alters adhesion molecule and chemokine expression in experimental acute graft-vs.-host disease of the intestinal tract. Exp. Hematol. 2011, 39, 238–249.e1. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.R.; Crawford, J.M.; Cooke, K.R.; Brinson, Y.S.; Pan, L.; Ferrara, J.L. Total body irradiation and acute graft-versus-host disease: The role of gastrointestinal damage and inflammatory cytokines. Blood J. Am. Soc. Hematol. 1997, 90, 3204–3213. [Google Scholar] [CrossRef]
- Shulman, H.M.; Sharma, P.; Amos, D.; Fenster, L.F.; McDonald, G.B. A coded histologic study of hepatic graft-versus-host disease after human bone marrow transplantation. Hepatology 1988, 8, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Al-Allaf, F.A.; Abduljaleel, Z.; Taher, M.M.; Abdellatif, A.A.H.; Athar, M.; Bogari, N.M.; Al-Ahdal, M.N.; Al-Mohanna, F.; Al-Hassnan, Z.N.; Alzabeedi, K.H.Y.; et al. Molecular Dynamics Simulation Reveals Exposed Residues in the Ligand-Binding Domain of the Low-Density Lipoprotein Receptor that Interacts with Vesicular Stomatitis Virus-G Envelope. Viruses 2019, 11, 1063. [Google Scholar] [CrossRef] [Green Version]

| β-Act-sense | GCT GTC CCT GTA TGC CTC TG |
| β-Act-antisense | GTG GTG GTG AAG CTG TAG CC |
| CCL3(MIP-1α)-sense | TGC CTG CTG CTT CTC CTA CA |
| CCL3(MIP-1α)-antisense | TGG ACC CAG GTC TCT TTG GA |
| CCL4(MIP-1β)-sense | CCA GGG TTC TCA GCA CCA A |
| CCL4(MIP-1β)-antisense | GCT CAC TGG GGT TAG CAC AGA |
| CCL5 (RANTES) sense | CTCACCATATGGCTCGGACA |
| CCL5 (RANTES) antisense | CTTCTCTGGGTTGGCACACA |
| CXCL1(KC) sense | GCCTATCGCCAATGAGCTG |
| CXCL1(KC) antisense | CTGAACCAAGGGAGCTTCAGG |
| CXCL9(MIG)-sense | TGG GCA TCA TCT TCC TGG AG |
| CXCL9(MIG)-antisense | CCG GAT CTA GGC AGG TTT GA |
| CXCL10(IP-10)-sense | CCT CAT CCT GCT GGG TCT G |
| CXCL10(IP-10)-antisense | CTC AAC ACG TGG GCA GGA |
| CXCL11(I-TAC)-sense | CGG GAT GAA AGC CGT CAA |
| CXCL11(I-TAC)-antisense | TAT GAG GCG AGC TTG CTT GG |
| CCL22 (MDC) sense | TGGCTCTCGTCCTTCTTGCT |
| CCL22 (MDC) antisense | AGGCTTGCGGCAGGATTT |
| IFN γ-sens | CAG GCC ATC AGC AAC AAC AT |
| IFN γ-antisens | CGC TTC CTG AGG CTG GAT T |
| TNF-sens | TAC GTG CTC CTC ACC CAC AC |
| TNF-antisens | AGT TGG TCC CCC TTC TCC AG |
| CCR1 sense | TTCCTCCTCTGGACCCCCTA |
| CCR1 antisense | TTGAAACAGCTGCCGAAGGT |
| CCR2 sense | CCACACCCTGTTTCGCTGTA |
| CCR2 antisense | TGCATGGCCTGGTCTAAGTG |
| CCR3 sense | TTTGGACCCCGTACAACCTG |
| CCR3 antisense | TTTCCGGAACCTCTCACCAA |
| CCR5 sense | CAGGGCTGTGAGGCTCATCT |
| CCR5 antisense | GGCAGCAGTGTGTCATTCCA |
| CCR7 sense | CCAATAGCAGCTGCGAAACC |
| CCR7 antisense | GCAGCCCAAGTCCTTGAAGA |
| CCR9 sense | GGTCACCTTGGGGTTTTTCC |
| CCR9 antisense | TAAGCGTCAACAGCCTGCAC |
| CCR10 sense | CCAGCAAGCGCAAGGATCTA |
| CCR10 antisense | AGCAGGAAGAAAGGCGGAGT |
| CXCR1 sense | TGGGGGTGATCTTTGCTGTT |
| CXCR1 antisense | TTTGGCCAACGAAGGCATAG |
| CXCR2 sense | CATCTTCGCTGTCGTCCTTGT |
| CXCR2 antisense | GCTGTGGAGGAAGCCAAGAA |
| CXCR3 sense | TAGTGGTGGTGGCAGCCTTT |
| CXCR3 antisense | AGGCATAGAGCAGCGGATTG |
| CXCR6 sense | CTTTCGGGCTTGCCTTAACC |
| CXCR6 antisense | CATTGTGGGAGGCAGAACAA |
| IL23a sense | GCCCCGTATCCAGTGTGAAG |
| IL23a antisense | CTGGGCATCTGTTGGGTCTC |
| IL17a sense | TCCACGTCACCCTGGACTCT |
| IL17a antisense | CCCACCAGCATCTTCTCGAC |
| IL2 sense | GGACCTCTGCGGCATGTT |
| IL2 antisense | TCTCCTCAGAAAGTCCACCACA |
| IL4 sense | GATGTGCCAAACGTCCTCAC |
| IL4 antisense | AAGCCCGAAAGAGTCTCTGC |
| IL10 sense | GGGTTGCCAAGCCTTATCG |
| IL10 antisense | TGCTCCACTGCCTTGCTCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouazzaoui, A.; Abdellatif, A.A.H.; Al-Allaf, F.A.; Bogari, N.M.; Taher, M.M.; Athar, M.; Schubert, T.; Habeebullah, T.M.; Qari, S.H. Compound A Increases Cell Infiltration in Target Organs of Acute Graft-versus-Host Disease (aGVHD) in a Mouse Model. Molecules 2021, 26, 4237. https://doi.org/10.3390/molecules26144237
Bouazzaoui A, Abdellatif AAH, Al-Allaf FA, Bogari NM, Taher MM, Athar M, Schubert T, Habeebullah TM, Qari SH. Compound A Increases Cell Infiltration in Target Organs of Acute Graft-versus-Host Disease (aGVHD) in a Mouse Model. Molecules. 2021; 26(14):4237. https://doi.org/10.3390/molecules26144237
Chicago/Turabian StyleBouazzaoui, Abdellatif, Ahmed A. H. Abdellatif, Faisal A. Al-Allaf, Neda M. Bogari, Mohiuddin M. Taher, Mohammad Athar, Thomas Schubert, Turki M. Habeebullah, and Sameer H. Qari. 2021. "Compound A Increases Cell Infiltration in Target Organs of Acute Graft-versus-Host Disease (aGVHD) in a Mouse Model" Molecules 26, no. 14: 4237. https://doi.org/10.3390/molecules26144237










