Plants Secondary Metabolites as Blood Glucose-Lowering Molecules
Abstract
:1. Introduction
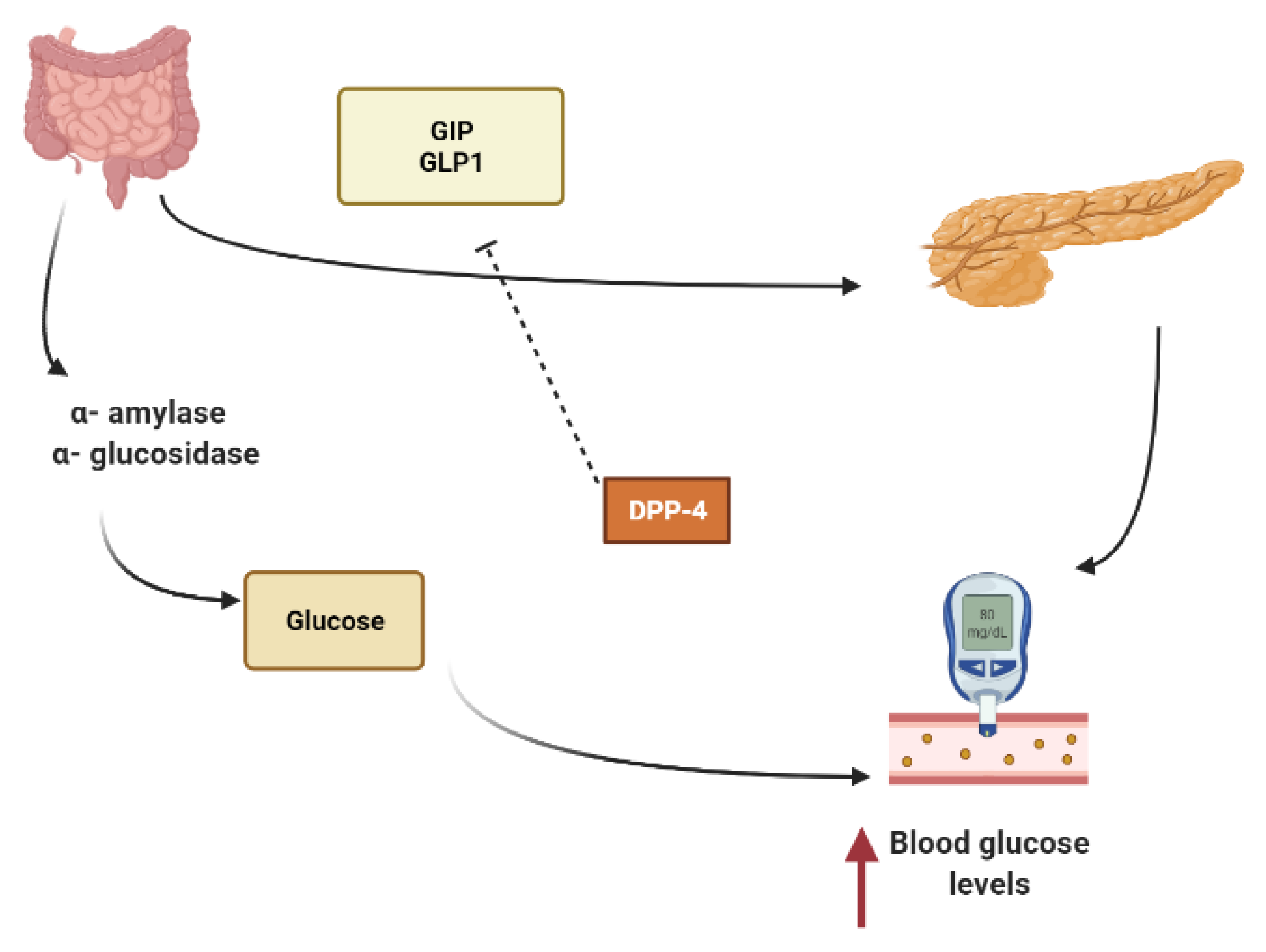
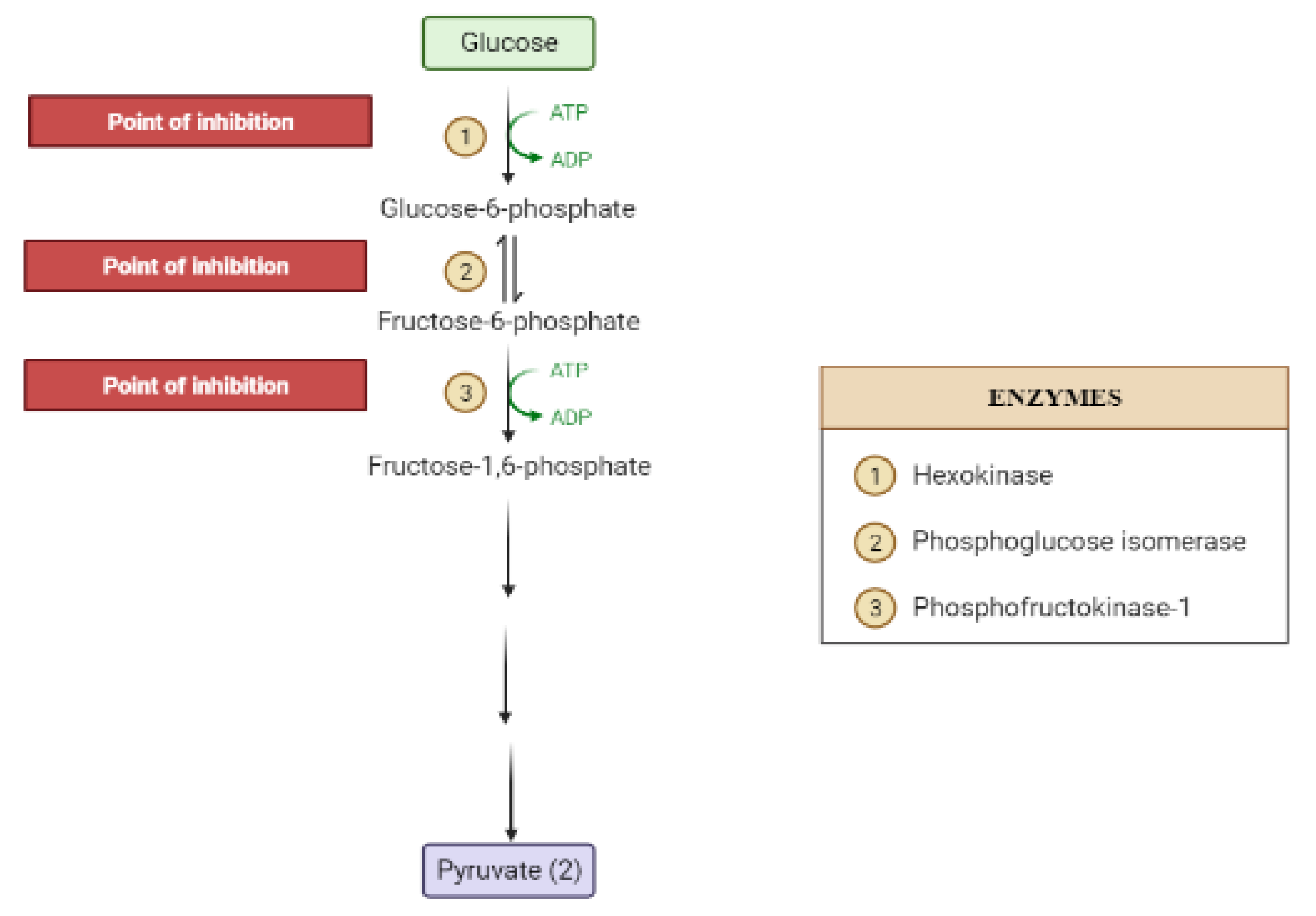
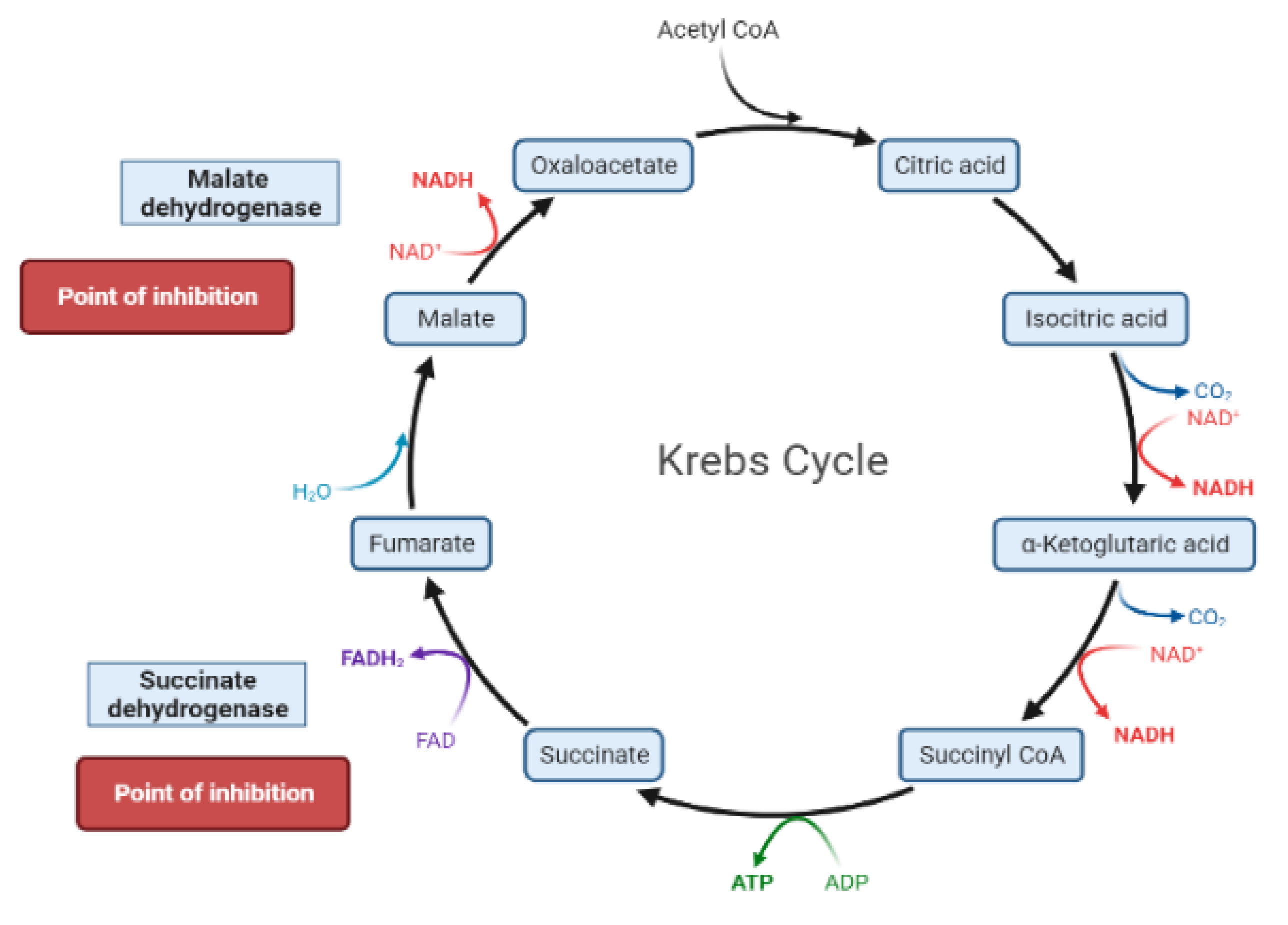
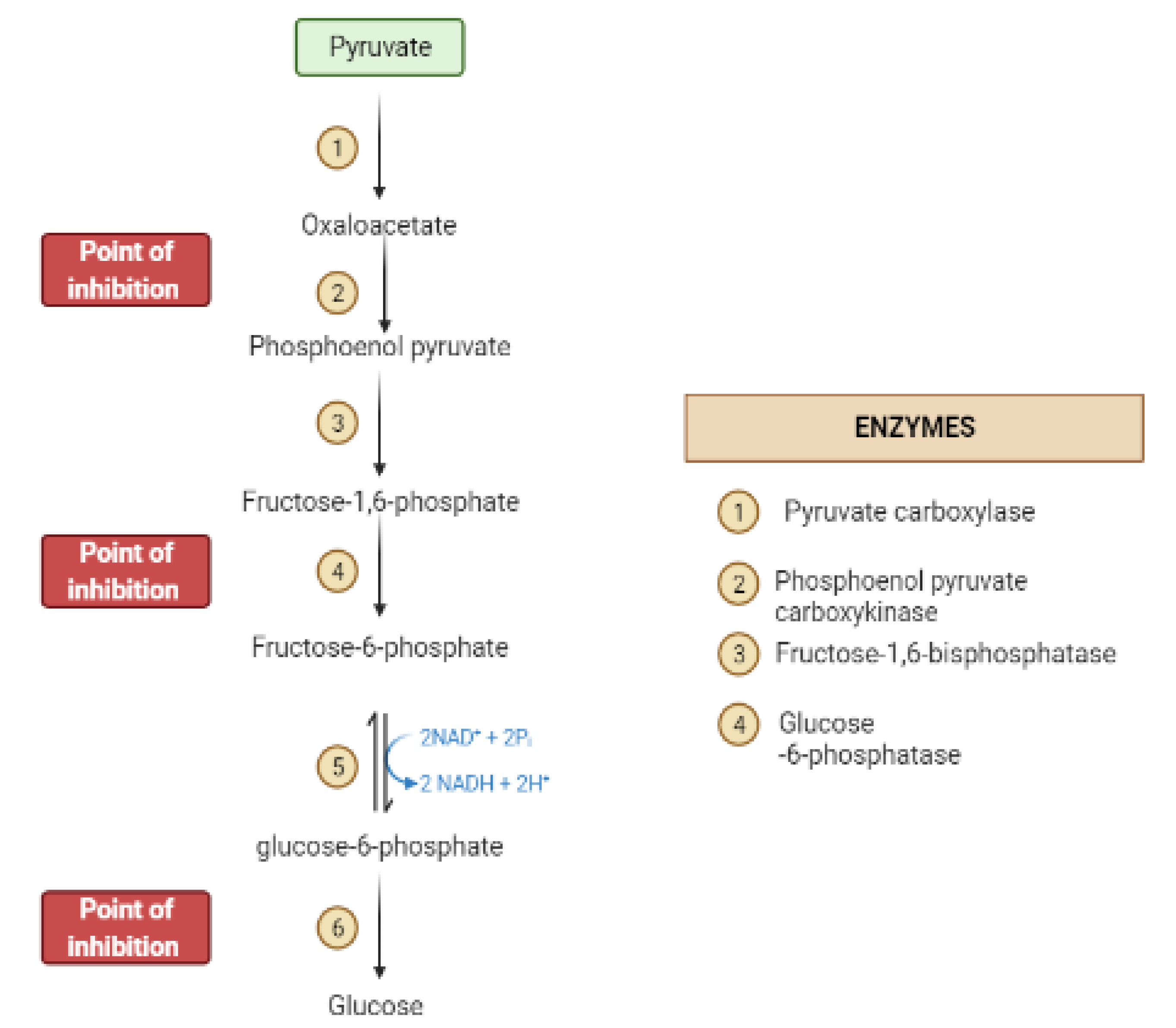
2. Mechanisms Involved in Glucose Metabolism and Homeostasis
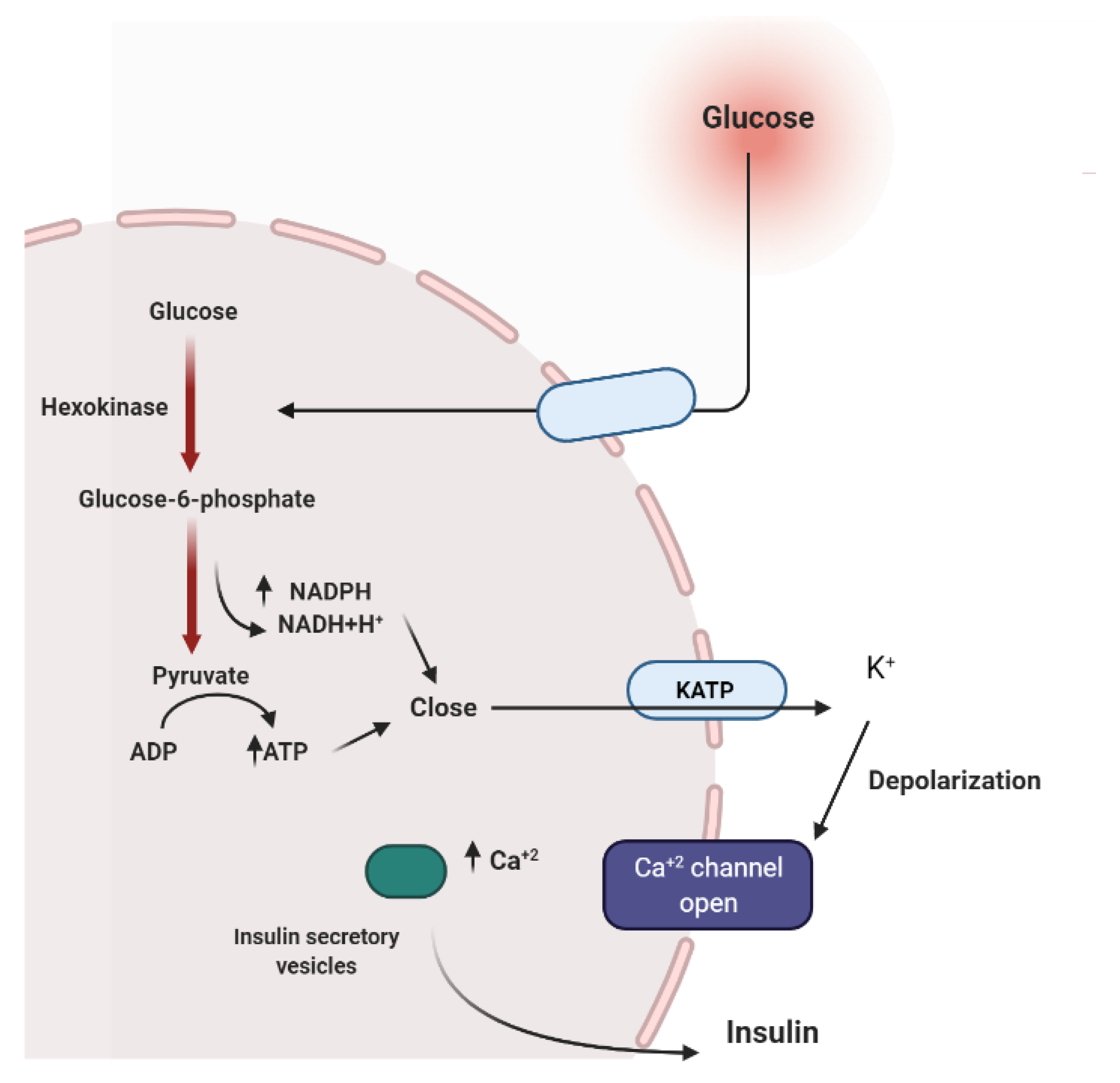
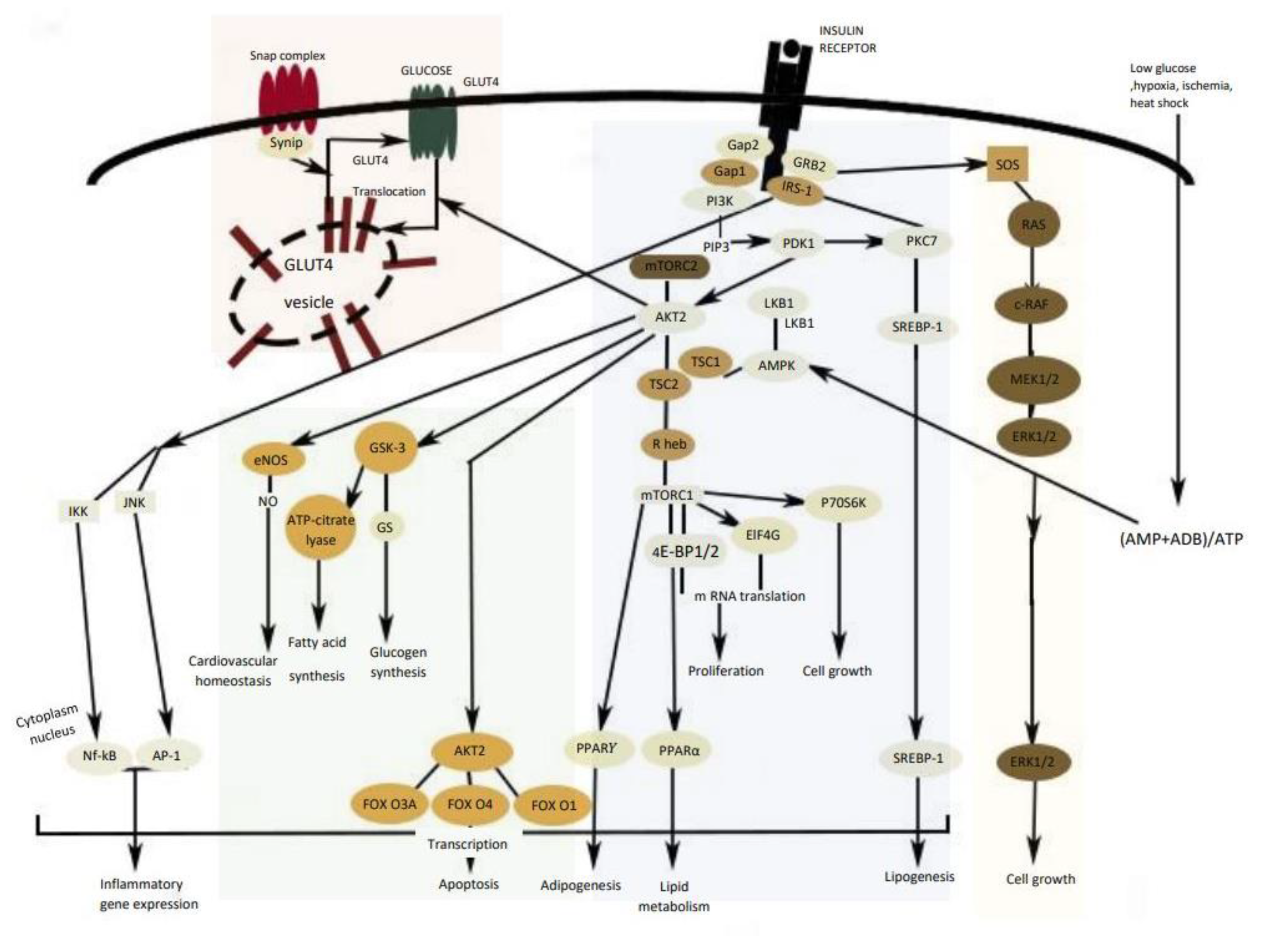
3. Insulin Signal Transduction
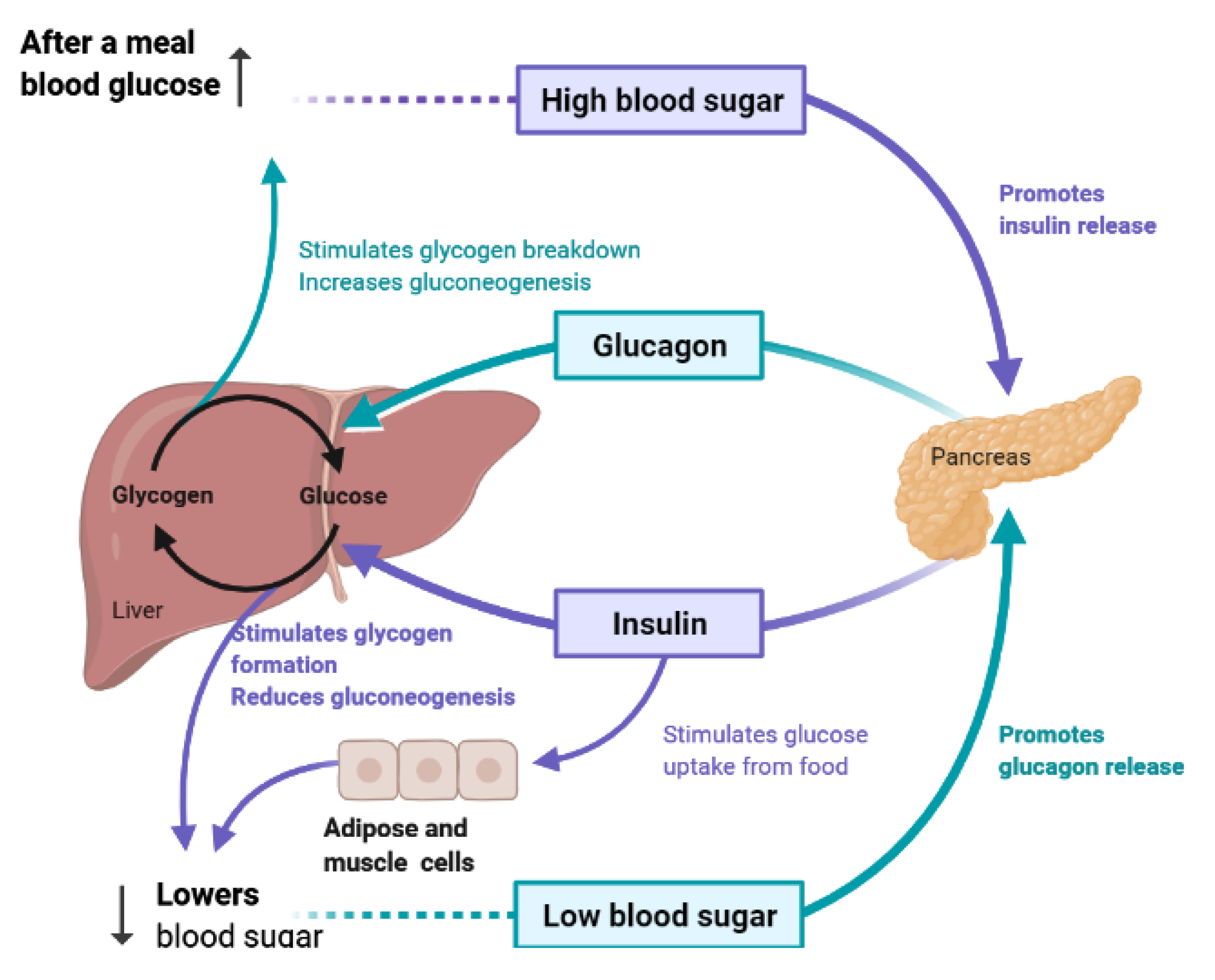
4. Liver and Glucose Homeostasis
5. Obstacles for Insulin Signal Transduction and Insulin Effects
6. Skeletal Muscle and Adipose Tissue
7. Methodology
8. Result and Discussion
8.1. Secondary Metabolites and Antidiabetic Activity
8.1.1. Alkaloids
| Name of Compound | Chemical Structure | Type of Alkaloid | Mechanism | References |
|---|---|---|---|---|
| Berberine | 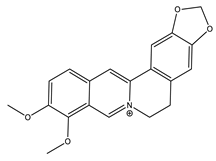 | Isoquinoline |
| [27,48,51,52] |
| Evodiamine |  | Quinolone |
| [27,46] |
| Glycosin |  | Quinazoline |
| [46] |
| Lupanine | 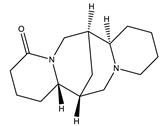 | Quinolizidine |
| [45,46] |
| Neferine |  | Isoquinoline |
| [27] |
| Piperine | 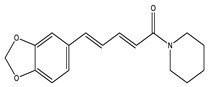 | Piperidine |
| [27] |
| Oxymatrine | 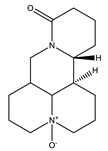 | Quinolizidine |
| [27,47] |
| Trigonelline |  | Pyridine |
| [27,48] |
| Vidoline | 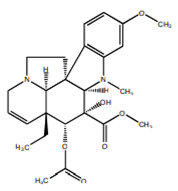 | Indole |
| [27,48] |
| Isolated Compound | Type of Alkaloids | Mechanism | Dose of the Tested Compound (Duration) | Model | References |
|---|---|---|---|---|---|
| Berberine | Isoquinoline | Increased mRNA and protein expressions of GLUT-4 and reduced activation of the hypothalamus-pituitary-adrenal axis. | 200 mg/kg (4 weeks) | High-fat diet and streptozocin-induced diabetic rats | [50] |
| Modulated gut microbiota. | 200 mg/kg (8 weeks) | High-fat diet obese rats | [51] | ||
| Inhibited expression of the gluconeogenic proteins (PEPCK and G-6-Pase) in the liver. | 156 mg/kg (12 weeks) | High-fat diet and streptozocin-induced diabetic rats | [52] | ||
| Increased expression of skeletal muscle GLUT- 4, mRNA had antioxidant activity. | 50, 100 mg/kg (6 weeks) | High- fat and glucose diet hamsters | [53] | ||
| Conophylline | Vinca | Pancreatic β-cells regenerator. | 0.1 µg/mL | In vitro: ICC cell line | [54] |
| Coptisine | Isoquinoline | PTP1B inhibition. | 6.25–50 µM | In vitro: enzymatic assay and in silico | [55] |
| Ephedrine | Phenylalanine derived | DDP-4 inhibition, IC50 was 124 µM | 10−5–10−3 M | In vitro: binding assay and in silico | [56] |
| Evodiamine | Quinolone | Activated AMPK phosphorylation. | 0.1, 1 mg/kg (6 months) | Ageing mice model | [57] |
| Koenidine | Carbazole | GLUT-4 translocation. | 25, 50 µM | In vitro: L6-GLUT4myc myotubes cell line | [58] |
| Lupanine | Quinolizidine | Potentiated insulin release by directly affecting KATP channels. | 0.5 mmol/L 20 mg/kg | In vitro: NS-1E cell line Streptozocin-induced diabetic mice | [59] |
| Magnoflorine | Aporphine | PTP1B inhibition. | 12.5 to 100 μM | In vitro: enzymatic assay and in silico | [55] |
| Neferine | Isoquinoline | Upregulated GLUT-4 expression and plasma membrane fusion. | 150 µM | In vitro: L6 cell line | [60] |
| Nigelladine | Norditerpene | Reduce PTP1B overexpression, promote glycogen synthesis and activated the PI3 K/Akt signaling pathway. | 50 µM | In vitro: L6 cell line | [61] |
| Nuciferine | Aporphine | Insulin secretion stimulator. | 10, 20 mM (24 h) | In vitro: INS1-E cell line | [62] |
| Picrasidine | Cathinone | PTP1B inhibition | IC50: 19.80 ± 0.62 µM | In vitro: Hepatocellular carcinoma (HepG2) cell line | [63] |
| Piperine | Piperidine | Down-regulation of mRNA levels of pro-inflammatory cytokines. | 40 mg/kg (10 weeks) | Monosodium glutamate diabetic mice | [64] |
| Ameliorated dysfunction of β-cell. | 15, 30 mm/kg (8 weeks) | High-fat-induced diet mice | [65] | ||
| Sanguinarine | Benzo-phenanthridine | Activation of AMPK. | 0.2, 1, 10 µM (1 h) | In vitro: cell based assay and in silico | [66] |
| Trigonelline | Pyridine | Reduction of insulin resistance through PAR-γ/GLUT4-leptin/TNF-α signaling pathway. | 40 mg/kg (8 weeks) | High-fat diet and Streptozocin-induced diabetic rats | [67] |
| Protection of β-cells Exhibited antioxidant activity. | 40 mg/kg (8 weeks) | High-fat and high fructose-induced diabetic rat | [68] | ||
| Vindolicine | Indole | PTP1B inhibition and induction of glucose uptake, IC50 was 73.5 ± 11.3 | 12.5, 50 µg/mL (24 h) | In vitro: β-TC6 and C2C12 cell lines | [69] |
| Vindolinine | Indole | PTP1B inhibition and induction of glucose uptake | 12.5, 50 µg/mL IC50: 57.6 ± 10.7 µM (24 h) | In vitro: β-TC6 and C2C12 cell lines | [69] |
| Vindolidine | Indole | PTP1B inhibition and induction of glucose uptake | 12.5, 50 µg/mL IC50: 180.1 ± 19.0 µM (24 h) | In vitro: β-TC6 and C2C12 cell lines | [69] |
| Vindogentianine | Indole alkaloid | PTP1B inhibition and induction of glucose uptake | 12.5, 25, 50, 100 and 200 μg/mL IC50: more than 50 μg/mL (24 h) | In vitro: β-TC6 and C2C12 cell lines | [70] |
8.1.2. Dietary Phenols
| Name of Compound | Chemical Structures | Mechanism | References |
|---|---|---|---|
| Caffeic acid |  |
| [10,75] |
| p-Coumaric acid | 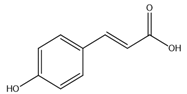 |
| [10,76] |
| Cinnamic acid | 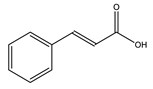 |
| [75,77] |
| Chlorogenic acid | 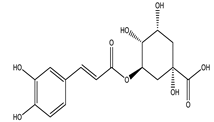 |
| [75,78] |
| Ellagic acid | 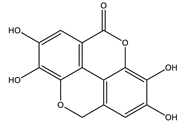 |
| [75,79] |
| Ferulic acid | 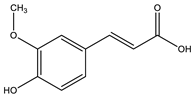 |
| [10,77] |
| Gallic acid | 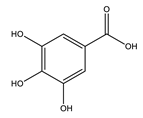 |
| [75,76] |
| Hydroxycinnamic acid | 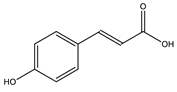 |
| [10,77] |
| Protocatechuic acid | 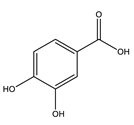 |
| [75,80] |
| Syringic acid | 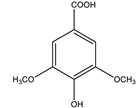 |
| [75,81] |
| Compound | Mechanism | Dose of the Tested Compound (Duration) | Model | References |
|---|---|---|---|---|
| Caffeic acid | Exhibited antioxidant effect. | 40 mg/kg (4 weeks) | Nicotinamide-streptozocin-induced diabetic mice | [82] |
| Exhibited antioxidant effect, increased insulin secretion and protected pancreatic cells. | 25, 35 mg/kg (5 weeks) | Streptozocin-induced diabetic rats | [83] | |
| Coumaric acid | Activation of pancreatic GLUT-2, increased level of insulin, decreased gluconeogenic enzymes (glucose-6-phosphatase and fructose-1, 6-bisphosphatase). | 100 mg/kg (4 weeks) | Streptozocin-induced diabetic rats | [84] |
| Decreased the level of TNF-α, increased the levels of PPARγ mRNA and adiponectin. | 40 mg/kg (6 weeks) | Streptozocin-induced diabetic rats | [85] | |
| Exhibited antioxidant effect, increased insulin level, protected pancreatic cells. | 100 mg/kg (4 weeks) | Streptozocin-induced diabetic rats | [86] | |
| Cinnamic acid | Stimulated glucose-induced insulin secretion | 5, 10 mg/kg | Streptozocin-induced diabetic rats | [87] |
| Chlorogenic acid | Increased expression of adiponectin receptors, increased phosphorylation of AMPK in the liver and muscle, increased mRNA and protein levels of PPAR-α in the liver. | 80 mg/kg (12 weeks) | Diabetic mice | [88] |
| Ellagic acid | Activated insulin signaling pathway in the liver by increasing phosphorylated Akt had an antioxidant effect | 50 mg/kg (28 days) | Insulin resistant diabetic rats | [89] |
| Ferulic acid | Improved hepatic glycogenesis by phosphorylating and inhibiting GSK3β, suppressed gluconeogenesis by phosphorylating FoxO1, Reduced IRS1, PKC-ε and PTP1B, which are known to inhibit the insulin signaling. | 50 mg/kg (4 weeks) | High-fat and fructose-induced diet diabetic rat | [90] |
| Reduced GLUT-2 expression. | 50 mg/kg (4 weeks) | High-fat and fructose-induced diet diabetic rat | [91] | |
| Gallic acid | Decreased the level of TNF-α, increased the levels of PPARγ mRNA and adiponectin. | 20 mg/kg (6 weeks) | Streptozocin-induced diabetic rats | [85] |
| Protocatechuic acid | Exhibited antioxidant effect, improved hepatic insulin resistance by modulating IRS1/PI3K/AKT2 pathways. | 100 mg/kg | Streptozocin-induced diabetic rats | [92] |
| Stimulated insulin signaling pathway increasing GLUT4 translocation and glucose uptake. | 1–150 µmol/L | In vitro: human visceral adipocytes | [93] | |
| Attenuated the increase in the expression of gluconeogenic enzymes, restored AKT protein phosphorylation (restores GLUT-4 translocation). | 50, 100 mg/kg (14 days) | Dexamethasone diabetic rats | [94] | |
| Syringic acid | Ameliorated the functional and histological abnormalities and hepatic mitochondria biogenesis (fight insulin resistance). | 25, 50 and 100 mg/kg (6 weeks) | Streptozocin-induced diabetic rats | [95] |
8.1.3. Anthocyanins
| Name of Compound | Chemical Structure | Mechanism | References |
|---|---|---|---|
| Cyanidin | 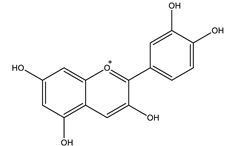 |
| [41,107] |
| Delphinidin | 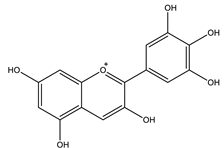 |
| [41,106] |
| Pelargonidin | 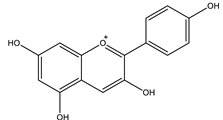 |
| [41,108] |
| Compound | Mechanism | Dose of the Tested Compound (Duration) | Model | References |
|---|---|---|---|---|
| Cyanidin | Increased intracellular Ca2+ stimulated insulin secretion and the expression of genes involved in this process. | 100 µM | In vitro: β-cells INS-1 | [109] |
| Delphinidin | Inhibited glucose absorption by free fatty acid receptor 1 (FFA1). | 100 µM | In vitro: Caco-2 and HT-29 cells | [110] |
| Inhibited α-amylase Inhibited α-glucosidase. | IC50: 601.56 nM IC50: 268.41 nM | In vitro: enzymatic assay | [111] | |
| Pelargonidin | Inhibited α-amylase Inhibited α-glucosidase. | IC50: 2067.78 nM IC50: 175.04 nM | In vitro: enzymatic assay, in silico | [111,112] |
8.1.4. Flavonoids
| Glucose Transporter | Hepatic Enzymes | Β-Cells Apoptosis | PPAR | AMPK | Tyrosine Kinase Inhibitor | NF-κB | |
|---|---|---|---|---|---|---|---|
| Target | IRS-1 | G6pase, FD pase PEPCK, G6PD Hexokinase | Bcl-2 family | Gene expression | AMPK | Tyrosine kinase inhibitors | |
 Flavonoid effects  | Activation of IRS-1 | Insulin signaling Liver glycogen | Apoptosis | -------- | -------- | Activity of tyrosine kinase | ------- |
| The synthesis and translocation of GLUT | Hexokinase activity in liver | --------- | Expression of PPARγ | AMPK activation | --------- | Activity of NF-kB |
| Name of Compound | Chemical Structure | Mechanism | References |
|---|---|---|---|
| Apigenin | 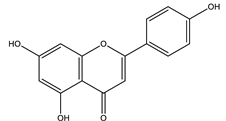 |
| [108,116] |
| Baicalein |  |
| [33,117] |
| Chrysin | 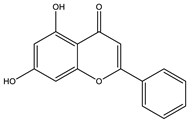 |
| [44,118,119] |
| Diosmin | 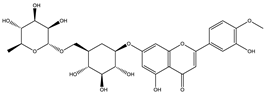 |
| [108,120] |
| Daidzein | 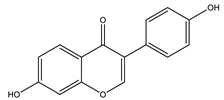 |
| [108,121] |
| Eriodictyol |  |
| [108,118] |
| Genistein |  |
| [41,121] |
| Fisetin | 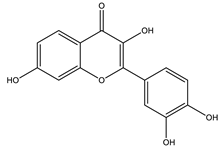 |
| [108,119] |
| Kaempferol | 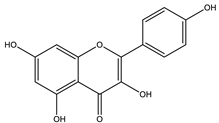 |
| [108,116] |
| Luteolin | 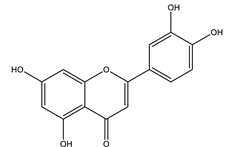 |
| [41,108] |
| Neohesperidin | 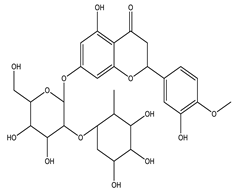 |
| [33,122] |
| Naringenin | 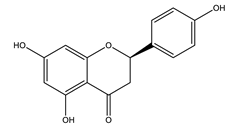 |
| [120,123] |
| Naringin |  |
| [110,120,124] |
| Morin | 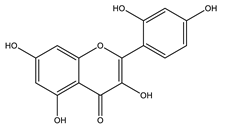 |
| [108,116] |
| Quercetin | 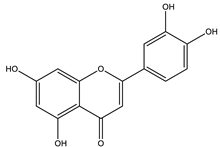 |
| [35,110,125] |
| Rutin | 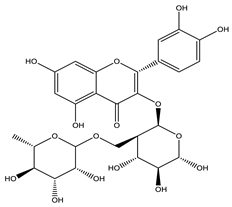 |
| [35,44,110] |
| Tangeretin | 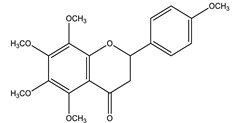 |
| [116,126] |
| Compound | Mechanism | Dose of the Tested Compound (Duration) | Model | References |
|---|---|---|---|---|
| Apigenin | Exhibited free radical scavenger and a regulator activity to antioxidant defenses of pancreatic cells. | * ND | In silico | [127] |
| Inhibited DPP-4 enzyme. | 1.5 mg/kg for every alternate (4 weeks) | High-fat and high fructose-induced diabetic rats, in silico | [124] | |
| Baicalein | Suppressed hepatic gluconeogenesis via activation of the AMPK and AKT signaling pathways. | 12.5 mM (24 h) | In vitro: HepG-2 cell line | [128] |
| Suppressed expression of PGC-1α (upregulate hepatic gluconeogenic gene expression) and gluconeogenic genes ameliorated hepatic insulin resistance and gluconeogenic activity by inhibiting the p38 MAPK/PGC-1α signal pathway. | 50 mg/kg (21 days) 100 µM (12 h) | High-fat diet-induced insulin-resistant mice Primary hepatocytes | [125] | |
| Promoted glucose uptake through enhancement of GLUT4, PGC-1α, MAPK, AKT and contents. | 100, 200, 400 μM (6,12, 24 h) | In vitro: L6 myoblast cell line, C2C12 cell line, animal model | [129,130] | |
| Chrysin | Modified oxidative stress. | 20, 40, 80 mg/kg | Streptozocin-induced diabetic rats | [131] |
| Activated insulin signal transduction such as IR, IRS-1, Akt | 100 mg/kg (4 weeks) | High fat-induced diet diabetic rats | [132] | |
| Eriodictyol | Exerted glucose-dependent insulinotropic effect through cAMP/PKA pathway. | 200 μM | In vitro: mice islets and MIN6 cell line | [133] |
| Activated glucose utilization, suppressed gluconeogenesis, decreased pro-inflammatory cytokines and increased anti-inflammatory cytokine. | 0.05% (w/w) (16 weeks) | Animal model | [134] | |
| Genistein | Increased insulin level, regenerated β-cells. | 10, 20 mg/kg (4 weeks) | Streptozocin-induced diabetic rats | [135] |
| Inhibited α-amylase Inhibited α-glucosidase. | IC50: 165.51 nM IC50: 1394.36 nM | In vitro: enzymatic assay | [111] | |
| Fisetin | Inhibited high glucose-induced reactive oxygen radical production through the activation of SIRTs and FOXO3a. | 3, 5 and 10 μM (48 h) | In vitro: THP-1 cell line | [136] |
| Hesperidin | Had antioxidant effect, protective effect for β-cells. | 100 mg/kg (15 days) | Streptozocin-induced diabetic rats | [137] |
| Decreased oxidative stress and NF-kB levels and increased while SIRT1 level. | 100 mg/kg (15 days) | Streptozocin-induced diabetic rats | [138] | |
| Improved glycogen content by reinstating the activities of glycogen synthase and glycogen phosphorylase. | 25, 50, 100 mg/kg (4 weeks) | Streptozocin-induced diabetic rats | [139] | |
| Luteolin | Inhibited high glucose-induced reactive oxygen radical production through the activation of SIRTs and FOXO3a. | 3, 5 and 10 μM (48 h) | In vitro: THP-1 cell line | [136] |
| Morin | Improved insulin signaling through inhibition of microRNA-29a (an essential regulator of insulin signaling and gluconeogenesis pathways). | 50 µM (24 h) | In vitro: HepG-2 cell line | [140] |
| Naringenin | Exhibited antioxidant and anti-inflammatory effects. | * ND | In silico | [127] |
| Decreased oxidative stress through promoting nuclear factor E2-related factor 2 (Nrf2), restored insulin expression, promoted glycolysis while inhibiting gluconeogenesis. | 50 mg/kg (45 days) | In vitro: MIN6 cell line, streptozocin-induced diabetic rats | [141] | |
| Improved mRNA expressions of insulin receptor b subunit, GLUT4 and adiponectin. | 100 mg/kg (4 weeks) | Streptozocin-induced diabetic rats | [142] | |
| Naringin | Improved mRNA expressions of insulin receptor b subunit, GLUT4 and adiponectin. | 100 mg/kg (4 weeks) | Streptozocin-induced diabetic rats | [142] |
| Inhibited both intrinsic and extrinsic pathways of β-cell apoptosis, possibly by interfering with DNA damage- and cytokine-induced apoptotic signaling by suppressing pancreatic reactive oxygen species accumulation and leukocyte infiltration. | 50, 100 mg/kg (2 weeks) | Streptozocin-induced diabetic rats | [143] | |
| Quercetin | Activated AMPK- MAPK pathway to induce glucose uptake. | 10, 100 µM (24 h) | In vitro: L6 myoblast cell line | [144] |
| Promoted hepatic glycogen synthesis and reduced blood glucose by increasing Akt phosphorylation, GSK-3 phosphorylation, and GCK protein expression levels. | 10, 50 mg/kg (12 weeks) | Streptozocin-induced diabetic rats | [145] | |
| Exhibited antioxidant effect, protective effect for β-cells. | 100 mg/kg (15 days) | Streptozocin-induced diabetic rats | [137] | |
| Decreased oxidative stress and NF-kB levels and increased while SIRT1 level. | 100 mg/kg (15 days) | Streptozocin-induced diabetic rats | [138] | |
| Tangeretin | Exhibited antiapoptotic property due to its inhibitory effect on oxidative stress. | 0, 10, 20, and 40 μM (12 h) | In vitro: INS-1 cell line | [146] |
| Exhibited a potential insulin action enhancer that functions by inhibiting the MEK-ERK1/2 pathway in hepatocytes. | 10,20 mM (48 h) 25, 50 mg/kg (1 month) | Animal model | [147] | |
| Inhibited α-glucosidase Inhibited α-amylase. | IC50 285.88 nM IC50 682.75 nM | In vitro: enzymatic assay | [111] |
8.1.5. Stilbenoids
8.1.6. Saponins
| Name of Compound | Type of Saponin | Chemical Structure | Mechanism | References |
|---|---|---|---|---|
| Arjunolic acid | Triterpene saponins | 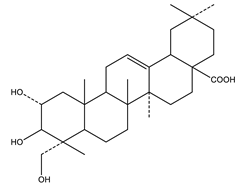 |
| [153,156] |
| Astragaloside IV | Steroidal saponins |  |
| [33,153] |
| Diosgenin | Steroidal saponins | 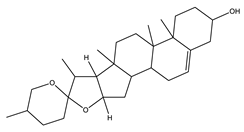 |
| [157,158] |
| Platyconic acid | Triterpene saponin |  |
| [153,157] |
| Ginsenoside K | Steroidal saponin |  |
| [33,159] |
| Ginsenoside Rg3 | Steroidal saponin |  |
| [33,160] |
| Ginsenoside Rg1 | Steroidal saponin |  |
| [33,161] |
8.1.7. Tannins
8.1.8. Polysaccharides
8.1.9. Coumarins
| Name of Compound | Chemical Structure | Mechanism | References |
|---|---|---|---|
| Esculetin | 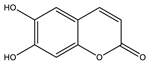 |
| [157,176] |
| Fraxetin |  |
| [157,176] |
| Umbelliferone | 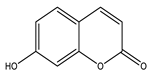 |
| [177] |
| Compound | Mechanism | Dose of the Tested Compound (Duration) | Model | References |
|---|---|---|---|---|
| Esculetin | Improved insulin resistance by increasing hepatic GLUT2 and glucokinase mRNA levels and decreased glucose-6-phosphatase mRNA level. | 0.02%, w/w (12 weeks) | C57BL/6J mice high-fat diet diabetic mice, liver histological model | [178] |
| Boosted Akt activation and promoted glucose uptake. | 40 mg/kg (14 days) | Dexamethasone-induced insulin resistance mice, C2C12 cell line | [179] | |
| Fraxetin | Boosted Akt activation and promoted glucose uptake. | 40 mg/kg (14 days) | Dexamethasone-induced insulin resistance mice, C2C12 cell line | [179] |
| Osthole | Increased GLUT4 mRNA expression in skeletal muscle. | 5–10 mg/kg (6 weeks) | High-fat and high-sucrose induced fatty liver with IR rats | [180] |
| Boosted Akt activation and promoted glucose uptake. | 20 mg/kg (14 days) | Dexamethasone-induced insulin resistance mice, C2C12 cell line | [179] | |
| Scopoletin | Stimulated GLUT-4 translocation through activation PI3K and AMPK pathway. | 1, 2.5, 5, 10, 15, 20 (24 h) | 3T3-L1 adipocyte cell lines | [181] |
| Inhibited carbohydrate digestive enzymes. Inhibited α-amylase Inhibited α- glucosidase | IC 50: 37.36 µM IC 50: 85.12 µM | In vitro study | [182] | |
| Umbelliferone | Stimulated muscle glucose uptake and stalled gluconeogenesis and oxidative stress. | 30–240 µg/mL (2 h) | Ex vivo: isolated psoas muscles | [177] |
| Shunted gluconeogenic enzymes, regeneration of the β-cells. | 100 mg/kg (4 weeks) | Alloxan-induced diabetic rat | [183] |
8.1.10. Terpenes
| Name of Compound | Class of Compound | Chemical Structure | Mechanism | References |
|---|---|---|---|---|
| Bassic acid | Triterpene | 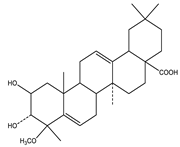 |
| [185] |
| Limonene | Monoterpene | 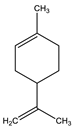 |
| [185,186] |
| Stevioside | Diterpene | 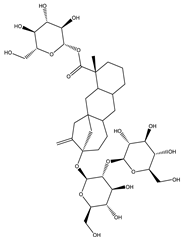 |
| [185,187] |
| Rebaudioside | Diterpene | 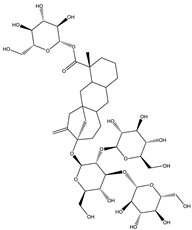 |
| [185,188] |
| Lupeol | Triterpene | 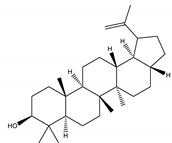 |
| [185,189] |
| Palbinone | Triterpene | 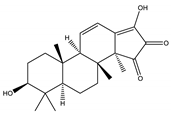 |
| [185,190] |
| Betulin | Triterpene |  |
| [50,190,191] |
| Compound | Mechanism | Dose of the Tested Compound (Duration) | Model | References |
|---|---|---|---|---|
| Asiatic acid | Exhibited antioxidant activity Increased insulin secretion in rats with sufficient insulin-secreting function Enhanced glucose uptake into skeletal muscle via PI3K-Akt signaling pathway. | 20 mg/kg (45 days) | Streptozocin-induced diabetic rats | [192] |
| Carvacrol | Increased activity of hexokinase, citrate synthase and 6-phosphofructokinase. | 10, 20 mg/kg (4, 6 weeks) | Streptozocin-induced diabetic mice | [193] |
| Limonene | Exhibited antioxidant activity. | 100 mg/kg (8 weeks) | Alloxan-induced diabetic rats | [194] |
| Stevioside | Increased level of GLUT-4. | 1–100 µM (24 h) | Rat L6 myoblast and mouse 3T3-L1 fibroblast cell lines | [195] |
| Increased leptin level Exhibited antioxidant activity, Restored normal pancreatic cell function Increased pyruvate kinase expression and insulin receptor substrate-1. | 300 mg/kg (4 weeks) | Streptozocin-induced diabetic rats | [196] | |
| Lupeol | Shunted gluconeogenic enzymes regenerated o β-cells. | 200 mg/kg (4 weeks) | Alloxan-induced diabetic rat | [183] |
| Inhibited carbohydrate digestive enzyme. | 10 mg/kg | Streptozocin-induced diabetic mice | [191] | |
| Regulated insulin receptor and GLUT-4 protein expression in muscular tissue. | 25 mg/kg (30 days) | High-fat diet and sucrose-induced diabetic mice | [197] | |
| Lupeol | Controlled insulin signaling molecules such as IR and GLUT2 protein expression in hepatocytes. | 25 mg/kg (One month) | High-fat diet diabetic rats | [198] |
| Betulinic acid | Exhibited pancreatic islet regenerative effects. | 10,20, 40 mg/kg (2 weeks) | Streptozocin-nicotinamide-induced diabetic mice | [199] |
| Improved the level of leptin and adiponectin. | 10, 20, 40 mg/kg | Streptozocin-nicotinamide-induced diabetic mice | [200] | |
| Enhanced AMPK phosphorylation, stimulated mRNA expression of glucose transporter 4. | 200 mg/kg | Alloxan- induced diabetic rats | [49] | |
| Inhibited α-glucosidase. | IC50: (1.06 ± 0.02) ×10−5 mol/L | In vitro: enzymatic assay | [201] | |
| Increased basal glucose uptake. | 5, 10 µM (4 days) | HepG2 and 3T3-L1 cell line | [202] | |
| Oleanolic acid | Inhibited α-glucosidase. | IC50: 10.11 ± 0.30 µM | In vitro: enzymatic assay | [203] |
| Ursolic acid | Increased GLUT-4 translocation, increased muscle glycogen content, increased insulin secretion | 0.1, 1 and 10 mg/kg | Hyperglycemic rats | [204] |
| Thymol | Exhibited Antioxidant activity. | 40 mg/kg (28 days) | Streptozocin-induced diabetic rats | [205] |
9. Summary
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Li, F.-S.; Weng, J.-K. Demystifying traditional herbal medicine with modern approach. Nat. Plants 2017, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dar, R.A.; Shahnawaz, M.; Rasool, S.; Qazi, P.H. Natural product medicines: A literature update. J. Phytopharmacol. 2017, 6, 340–342. [Google Scholar]
- Lahlou, M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef]
- Suaifan, G.A.R.Y.; Shehadeh, M.B.; Darwish, R.M.; Al-Ijel, H.; Abbate, V. Design, synthesis and in vivo evaluation of novel glycosylated sulfonylureas as antihyperglycemic agents. Molecules 2015, 20, 20063–20078. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Gupta, M.; Popli, H.; Aggarwal, G. Diabetes mellitus treatment using herbal drugs. Int. J. Phytomed. 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Verma, R.; Gupta, J. Challenges and future prospects of herbal medicine. Int. Res. Med. Health Sci. 2018, 1, 12–15. [Google Scholar] [CrossRef]
- Rosado-de-Castro, P.H.; Pimentel-Coelho, P.M.; Barbosa da Fonseca, L.M.; de Freitas, G.R.; Mendez-Otero, R. The rise of cell therapy trials for stroke: Review of published and registered studies. Stem Cells Dev. 2013, 22, 2095–2111. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, D.S.; Gupta, P.; Pottoo, F.H.; Amir, M. Secondary metabolites in the treatment of diabetes mellitus: A paradigm shift. Curr. Drug Metab. 2020, 21, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef] [Green Version]
- Ehrenkranz, J.R.; Lewis, N.G.; Ronald Kahn, C.; Roth, J. Phlorizin: A review. Diabetes Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Cao, J.; Zhao, T.; Liu, Y.; Khan, A.; Cheng, G. The bioavailability, extraction, biosynthesis and distribution of natural dihydrochalcone: Phloridzin. Int. J. Mol. Sci. 2021, 22, 962. [Google Scholar] [CrossRef]
- Kovacich, N.; Chavez, B. Ertugliflozin (steglatro): A new option for SGLT2 inhibition. Pharm. Ther. 2018, 43, 736. [Google Scholar]
- Triantakonstanti, V.V.; Mountanea, O.G.; Papoulidou, K.-E.C.; Andreou, T.; Koftis, T.V.; Gallos, J.K. Studies towards the synthesis of ertugliflozin from l-Arabinose. Tetrahedron 2018, 74, 5700–5708. [Google Scholar] [CrossRef]
- Bharti, S.K.; Krishnan, S.; Kumar, A.; Kumar, A. Antidiabetic phytoconstituents and their mode of action on metabolic pathways. Ther. Adv. Endocrinol. Metab. 2018, 9, 81–100. [Google Scholar] [CrossRef]
- Alkhalidy, H.; Wang, Y.; Liu, D. Dietary flavonoids in the prevention of T2D: An overview. Nutrients 2018, 10, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fred-Jaiyesimi, A.; Kio, A.; Richard, W. α-Amylase inhibitory effect of 3β-olean-12-en-3-yl (9Z)-hexadec-9-enoate isolated from Spondias mombin leaf. Food Chem. 2009, 116, 285–288. [Google Scholar] [CrossRef]
- Dominguez Avila, J.A.; Rodrigo Garcia, J.; Gonzalez Aguilar, G.A.; De la Rosa, L.A. The antidiabetic mechanisms of polyphenols related to increased glucagon-like peptide-1 (GLP1) and insulin signaling. Molecules 2017, 22, 903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turdu, G.; Gao, H.; Jiang, Y.; Kabas, M. Plant dipeptidyl peptidase-IV inhibitors as antidiabetic agents: A brief review. Future Med. Chem. 2018, 10, 1229–1239. [Google Scholar] [CrossRef]
- Les, F.; Cásedas, G.; Gómez, C.; Moliner, C.; Valero, M.S.; López, V. The role of anthocyanins as antidiabetic agents: From molecular mechanisms to in vivo and human studies. J. Physiol. Biochem. 2020, 77, 1–23. [Google Scholar] [CrossRef]
- Gowd, V.; Jia, Z.; Chen, W. Anthocyanins as promising molecules and dietary bioactive components against diabetes–A review of recent advances. Trends Food Sci. Technol. 2017, 68, 1–13. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Doble, M. A target based therapeutic approach towards diabetes mellitus using medicinal plants. Curr. Diabetes Rev. 2008, 4, 291–308. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Eid, H.M.; Haddad, P.S. Mechanisms of action of indigenous antidiabetic plants from the boreal Forest of northeastern Canada. Adv. Endocrinol. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, K.; Xu, B. Anti-diabetic effects and mechanisms of dietary polysaccharides. Molecules 2019, 24, 2556. [Google Scholar] [CrossRef] [Green Version]
- Świderska, E.; Strycharz, J.; Wróblewski, A.; Szemraj, J.; Drzewoski, J.; Śliwińska, A. Role of PI3K/AKT pathway in insulin-mediated glucose uptake. Blood Glucose Lev. 2018, 1, 37–53. [Google Scholar]
- Christodoulou, M.-I.; Tchoumtchoua, J.; Skaltsounis, A.-L.; Scorilas, A.; Halabalaki, M. Natural alkaloids intervening the insulin pathway: New hopes for anti-diabetic agents? Curr. Med. Chem. 2019, 26, 5982–6015. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, K.-J.; Koh, E.-J.; Lee, B.-Y. Gelidium elegans extract ameliorates type 2 diabetes via regulation of MAPK and PI3K/Akt signaling. Nutrients 2018, 10, 51. [Google Scholar] [CrossRef] [Green Version]
- Han, H.-S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.-H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, T.; Amrein, H. Gluconeogenesis: An ancient biochemical pathway with a new twist. Fly 2017, 11, 218–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.Y.; Zaib, S.; Rahman, M.M.; Jannat, S.; Iqbal, J.; Park, S.K.; Chang, M.S. Didymin, a dietary citrus flavonoid exhibits anti-diabetic complications and promotes glucose uptake through the activation of PI3K/Akt signaling pathway in insulin-resistant HepG2 cells. Chem. Biol. Interact. 2019, 305, 180–194. [Google Scholar] [CrossRef] [PubMed]
- He, J.-H.; Chen, L.-X.; Li, H. Progress in the discovery of naturally occurring anti-diabetic drugs and in the identification of their molecular targets. Fitoterapia 2019, 134, 270–289. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Peiffer, C.; Biden, T.J. Protein kinase C function in muscle, liver, and β-cells and its therapeutic implications for type 2 diabetes. Diabetes 2008, 57, 1774–1783. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Gao, J.; Wei, F.; Zhao, J.; Wang, D.; Wei, J. Therapeutic potential of ginsenosides as an adjuvant treatment for diabetes. Front. Pharmacol. 2018, 9, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Kaikini, A.A.; Kanchan, D.M.; Nerurkar, U.N.; Sathaye, S. Targeting mitochondrial dysfunction for the treatment of diabetic complications: Pharmacological interventions through natural products. Pharmacogn. Rev. 2017, 11, 128. [Google Scholar]
- Sivitz, W.I.; Yorek, M.A. Mitochondrial dysfunction in diabetes: From molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 537–577. [Google Scholar] [CrossRef] [Green Version]
- Smith, U. Impaired (‘diabetic’) insulin signaling and action occur in fat cells long before glucose intolerance—Is insulin resistance initiated in the adipose tissue? Int. J. Obes. 2002, 26, 897–904. [Google Scholar] [CrossRef] [Green Version]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Galadari, A.; Thayyullathil, F. Role of ceramide in diabetes mellitus: Evidence and mechanisms. Lipids Health Dis. 2013, 12, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasouli, H.; Yarani, R.; Pociot, F.; Popović-Djordjević, J. Anti-diabetic potential of plant alkaloids: Revisiting current findings and future perspectives. Pharmacol. Res. 2020, 155, 104723. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Bribi, N. Pharmacological activity of alkaloids: A review. Asian J. Bot. 2018, 1, 1–6. [Google Scholar]
- Kumar, A.; Aswal, S.; Semwal, R.B.; Chauhan, A.; Joshi, S.K.; Semwal, D.K. Role of plant-derived alkaloids against diabetes and diabetes-related complications: A mechanism-based approach. Phytochem. Rev. 2019, 18, 1277–1298. [Google Scholar] [CrossRef]
- Ajebli, M.; Khan, H.; Eddouks, M. Natural alkaloids and diabetes mellitus: A Review. Endocr. Metab. Immune Disord. Drug Targets 2020, 21, 111–131. [Google Scholar] [CrossRef]
- Haq, I.U.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A review of its biological effects. Phytother. Res. 2020, 35, 680–700. [Google Scholar] [CrossRef]
- Dabur, R.; Sharma, B.; Mittal, A. Mechanistic approach of anti-diabetic compounds identified from natural sources. Chem. Biol. Lett. 2018, 5, 63–99. [Google Scholar]
- Song, T.-J.; Park, C.-H.; In, K.-R.; Kim, J.-B.; Kim, J.H.; Kim, M.; Chang, H.J. Antidiabetic effects of betulinic acid mediated by the activation of the AMP-activated protein kinase pathway. PLoS ONE. 2021, 16, e0249109. [Google Scholar] [CrossRef]
- Mi, J.; He, W.; Lv, J.; Zhuang, K.; Huang, H.; Quan, S. Effect of berberine on the HPA-axis pathway and skeletal muscle GLUT4 in type 2 diabetes mellitus rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1717–1752. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zhang, Y.; Liu, Y.; Hou, L.; Li, S.; Tian, H.; Zhao, T. Berberine modulates gut microbiota and reduces insulin resistance via the TLR4 signaling pathway. Exp. Clin. Endocrinol. Diabetes 2018, 126, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-J.; Dong, H.; Li, J.-B.; Xu, L.-J.; Zou, X.; Wang, K.-F.; Lu, F.-E.; Yi, P. Berberine inhibits hepatic gluconeogenesis via the LKB1-AMPK-TORC2 signaling pathway in streptozotocin-induced diabetic rats. World J. Gastroenterol. 2015, 21, 7777–7785. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Song, Y.; Wu, D.; Zheng, X.; Li, P.; Jin, J.; Xu, N.; Li, L. Effects of berberine on amelioration of hyperglycemia and oxidative stress in high glucose and high fat diet-induced diabetic hamsters in vivo. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; Hirayama, A.; Tsuchiya, K.; Ohgawara, H.; Nakamura, M.; Umezawa, K. Promotion of β-cell differentiation by the alkaloid conophylline in porcine pancreatic endocrine cells. Biomed. Pharmacother. 2010, 64, 226–231. [Google Scholar] [CrossRef]
- Choi, J.S.; Ali, M.Y.; Jung, H.A.; Oh, S.H.; Choi, R.J.; Kim, E.J. Protein tyrosine phosphatase 1B inhibitory activity of alkaloids from Rhizoma Coptidis and their molecular docking studies. J. Ethnopharmacol. 2015, 171, 28–36. [Google Scholar] [CrossRef]
- Ojeda-Montes, M.J.; Ardid-Ruiz, A.; Tomás-Hernández, S.; Gimeno, A.; Cereto-Massagué, A.; Beltrán-Debón, R.; Mulero, M.; Garcia-Vallvé, S.; Pujadas, G.; Valls, C. Ephedrine as a lead compound for the development of new DPP-IV inhibitors. Future Med. Chem. 2017, 9, 2129–2146. [Google Scholar] [CrossRef]
- Yamashita, H.; Kusudo, T.; Takeuchi, T.; Qiao, S.; Tsutsumiuchi, K.; Wang, T.; Wang, Y. Dietary supplementation with evodiamine prevents obesity and improves insulin resistance in ageing mice. J. Funct. Foods 2015, 19, 320–329. [Google Scholar] [CrossRef]
- Patel, O.P.; Mishra, A.; Maurya, R.; Saini, D.; Pandey, J.; Taneja, I.; Raju, K.S.; Kanojiya, S.; Shukla, S.K.; Srivastava, M.N. Naturally occurring carbazole alkaloids from Murraya koenigii as potential antidiabetic agents. J. Nat. Prod. 2016, 79, 1276–1284. [Google Scholar] [CrossRef]
- Wiedemann, M.; Gurrola-Díaz, C.M.; Vargas-Guerrero, B.; Wink, M.; García-López, P.M.; Düfer, M. Lupanine improves glucose homeostasis by influencing KATP channels and insulin gene expression. Molecules 2015, 20, 19085–19100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Tian, D.; Song, G.; Ming, Q.; Liu, J.; Shen, J.; Liu, Q.-H.; Yang, X. Neferine promotes GLUT4 expression and fusion with the plasma membrane to induce glucose uptake in L6 cells. Front. Pharmacol. 2019, 10, 999–1015. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Chen, Q.-B.; Xin, X.-L.; Aisa, H.-A. Anti-diabetic effect of three new norditerpenoid alkaloids in vitro and potential mechanism via PI3K/Akt signaling pathway. Biomed. Pharmacother. 2017, 87, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.H.; Ta, T.N.; Pham, T.H.M.; Nguyen, Q.T.; Pham, H.D.; Mishra, S.; Nyomba, B.G. Nuciferine stimulates insulin secretion from beta cells—An in vitro comparison with glibenclamide. J. Ethnopharmacol. 2012, 142, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Li, W.; Higai, K.; Koike, K. Canthinone alkaloids are novel protein tyrosine phosphatase 1B inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 1979–1981. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, Y.; Zhou, J.; Hu, R.; Ji, L.; Jiang, G. Piperine ameliorates insulin resistance via inhibiting metabolic inflammation in monosodium glutamate-treated obese mice. BMC Endocr. Disord. 2020, 20, 1–15. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, J.; Hu, R.; Zou, L.; Ji, L.; Jiang, G. Piperine protects against pancreatic β-cell dysfunction by alleviating macrophage inflammation in obese mice. Life Sci. 2021, 274, 119312. [Google Scholar] [CrossRef]
- Choi, J.; He, N.; Sung, M.-K.; Yang, Y.; Yoon, S. Sanguinarine is an allosteric activator of AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2011, 413, 259–263. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, C.; Lou, Z.; Li, Q. Trigonelline reduced diabetic nephropathy and insulin resistance in type 2 diabetic rats through peroxisome proliferator-activated receptor-γ. Exp. Ther. Med. 2019, 18, 1331–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afifia, N.A.; Ramadana, A.; Erianb, E.Y.; Saleh, D.O.; Sedikb, A.A.; Badawic, M. Trigonelline attenuates hepatic complications and molecular alterations in high fat high fructose-induced insulin resistance in rats. Can. J. Physiol. Pharmacol. 2017, 95, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Tiong, S.H.; Looi, C.Y.; Hazni, H.; Arya, A.; Paydar, M.; Wong, W.F.; Cheah, S.-C.; Mustafa, M.R.; Awang, K. Antidiabetic and antioxidant properties of alkaloids from Catharanthus roseus (L.) G. Don. Molecules 2013, 18, 9770–9784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiong, S.H.; Looi, C.Y.; Arya, A.; Wong, W.F.; Hazni, H.; Mustafa, M.R.; Awang, K. Vindogentianine, a hypoglycemic alkaloid from Catharanthus roseu s (L.) G. Don (Apocynaceae). Fitoterapia 2015, 102, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Van Dijk, A.E.; Olthof, M.R.; Meeuse, J.C.; Seebus, E.; Heine, R.J.; Van Dam, R.M. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care 2009, 32, 1023–1025. [Google Scholar] [CrossRef] [Green Version]
- Olthof, M.R.; van Dijk, A.E.; Deacon, C.F.; Heine, R.J.; van Dam, R.M. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on incretin hormones. Nutr. Metab. 2011, 8, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic effects of simple phenolic acids: A comprehensive review. Phytother. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.; Abd El-Twab, S.M.; Yousef, A.I.; Ashour, M.B.; Reheim, E.S.A.; Hamed, M.A.A. New insights into the in vitro, in situ and in vivo antihyperglycemic mechanisms of gallic acid and p-coumaric acid. Arch. Physiol. Biochem. 2020, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S. Cinnamic acid and its derivatives: Mechanisms for prevention and management of diabetes and its complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Harakeh, S.; Almuhayawi, M.; Al Jaouni, S.; Almasaudi, S.; Hassan, S.; Al Amri, T.; Azhar, N.; Abd-Allah, E.; Ali, S.; El-Shitany, N.; et al. Antidiabetic effects of novel ellagic acid nanoformulation: Insulin-secreting and anti-apoptosis effects. Saudi J. Biol. Sci. 2020, 27, 3474–3480. [Google Scholar] [CrossRef]
- Alegbe, E.O.; Teralı, K.; Olofinsan, K.A.; Surgun, S.; Ogbaga, C.C.; Ajiboye, T.O. Antidiabetic activity-guided isolation of gallic and protocatechuic acids from Hibiscus sabdariffa calyxes. J. Food Biochem. 2019, 43, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.; Kumar, C.S. Syringic acid (SA)‒a review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Mohammed, F.Z.; El-Shehabi, M. Antidiabetic activity of caffeic acid and 18β-glycyrrhetinic acid and its relationship with the antioxidant property. Asian J. Pharm. Clin. Res. 2015, 8, 229–235. [Google Scholar]
- Xu, W.; Luo, Q.; Wen, X.; Xiao, M.; Mei, Q. Antioxidant and anti-diabetic effects of caffeic acid in a rat model of diabetes. Trop. J. Pharm. Res. 2020, 19, 1227–1232. [Google Scholar] [CrossRef]
- Amalan, V.; Vijayakumar, N.; Indumathi, D.; Ramakrishnan, A. Antidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats, role of pancreatic GLUT 2: In vivo approach. Biomed. Pharmacother. 2016, 84, 230–236. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.; Abd El-Twab, S.M.; Yousef, A.I.; Reheim, E.S.A.; Ashour, M.B. Modulation of hyperglycemia and dyslipidemia in experimental type 2 diabetes by gallic acid and p-coumaric acid: The role of adipocytokines and PPARγ. Biomed. Pharmacother. 2018, 105, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Amalan, V.; Vijayakumar, N.; Ramakrishnan, A. p-Coumaric acid regulates blood glucose and antioxidant levels in streptozotocin induced diabetic rats. J. Chem. Pharm. Res. 2015, 7, 831–839. [Google Scholar]
- Hafizur, R.M.; Hameed, A.; Shukrana, M.; Raza, S.A.; Chishti, S.; Kabir, N.; Siddiqui, R.A. Cinnamic acid exerts anti-diabetic activity by improving glucose tolerance in vivo and by stimulating insulin secretion in vitro. Phytomedicine 2015, 22, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Chang, C.; Zhang, L.; Liu, Y.; Huang, X.; Chen, Z. Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db mice. PLoS ONE 2015, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polce, S.A.; Burke, C.; França, L.M.; Kramer, B.; Paes, A.M.d.A.; Carrillo-Sepulveda, M.A. Ellagic acid alleviates hepatic oxidative stress and insulin resistance in diabetic female rats. Nutrients 2018, 10, 531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid exerts its antidiabetic effect by modulating insulin-signalling molecules in the liver of high-fat diet and fructose-induced type-2 diabetic adult male rat. Appl. Physiol. Nutr. Metab. 2015, 40, 769–781. [Google Scholar] [CrossRef]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid regulates hepatic GLUT2 gene expression in high fat and fructose-induced type-2 diabetic adult male rat. Eur. J. Pharmacol. 2015, 761, 391–397. [Google Scholar] [CrossRef]
- Abdelmageed, M.E.; Shehatou, G.S.; Suddek, G.M.; Salem, H.A. Protocatechuic acid improves hepatic insulin resistance and restores vascular oxidative status in type-2 diabetic rats. Environ. Toxicol. Pharmacol. 2021, 83, 103577. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Varì, R.; Filesi, C.; Del Gaudio, I.; D’Archivio, M.; Santangelo, C.; Iacovelli, A.; Galvano, F.; Pluchinotta, F.R.; Giovannini, C.; et al. Protocatechuic acid activates key components of insulin signaling pathway mimicking insulin activity. Mol. Nutr. Food Res. 2015, 59, 1472–1481. [Google Scholar] [CrossRef]
- El-Sonbaty, Y.A.; Suddek, G.M.; Megahed, N.; Gameil, N.M. Protocatechuic acid exhibits hepatoprotective, vasculoprotective, antioxidant and insulin-like effects in dexamethasone-induced insulin-resistant rats. Biochimie 2019, 167, 119–134. [Google Scholar] [CrossRef]
- Sabahi, Z.; Khoshnoud, M.J.; Khalvati, B.; Hashemi, S.-S.; Farsani, Z.G.; Gerashi, H.M.; Rashedinia, M. Syringic acid improves oxidative stress and mitochondrial biogenesis in the liver of streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 2020, 10, 111–119. [Google Scholar]
- Ghosh, D.; Konishi, T. Anthocyanins and anthocyanin-rich extracts: Role in diabetes and eye function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208. [Google Scholar] [PubMed]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Fernandes, A.; Brás, N.F.; Mateus, N.; de Freitas, V.; Fernandes, I. Anthocyanins as antidiabetic agents—In vitro and in silico approaches of preventive and therapeutic effects. Molecules 2020, 25, 3813. [Google Scholar] [CrossRef]
- Tsuda, T. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Nabavi, S.F.; Nabavi, S.M.; Habtemariam, S. Dietary anthocyanins and insulin resistance: When food becomes a medicine. Nutrients 2017, 9, 1111. [Google Scholar] [CrossRef]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human study and clinical trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Ž.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The therapeutic potential of anthocyanins: Current approaches based on their molecular mechanism of action. Front. Pharmacol. 2020, 11, 1300–1320. [Google Scholar] [CrossRef]
- Momtaz, S.; Salek-Maghsoudi, A.; Abdolghaffari, A.H.; Jasemi, E.; Rezazadeh, S.; Hassani, S.; Ziaee, M.; Abdollahi, M.; Behzad, S.; Nabavi, S.M. Polyphenols targeting diabetes via the AMP-activated protein kinase pathway; future approach to drug discovery. Crit. Rev. Clin. Lab. Sci. 2019, 56, 472–492. [Google Scholar] [CrossRef]
- Jennings, A.; Welch, A.A.; Spector, T.; Macgregor, A.; Cassidy, A. Intakes of anthocyanins and flavones are associated with biomarkers of insulin resistance and inflammation in women. J. Nutr. 2014, 144, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Soltani, R.; Gorji, A.; Asgary, S.; Sarrafzadegan, N.; Siavash, M. Evaluation of the effects of Cornus mas L. fruit extract on glycemic control and insulin level in type 2 diabetic adult patients: A randomized double-blind placebo-controlled clinical trial. Evid. Based Complement. Altern. Med. 2015, 2015, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Zhang, R.; Shi, W.; Li, L.; Liu, H.; Liu, Z.; Wu, L. The Multifunctional Benefits of Naturally Occurring Delphinidin and Its Glycosides. J. Agric. Food Chem. 2019, 67, 11288–11306. [Google Scholar] [CrossRef]
- Rupasinghe, H.V.; Arumuggam, N.; Amararathna, M.; De Silva, A. The potential health benefits of haskap (Lonicera caerulea L.): Role of cyanidin-3-O-glucoside. J. Funct. Foods 2018, 44, 24–39. [Google Scholar] [CrossRef]
- Aba, P.E.; Asuzu, I.U. Mechanisms of actions of some bioactive anti-diabetic principles from phytochemicals of medicinal plants: A review. Indian J. Nat. Prod. Resour. 2018, 9, 85–96. [Google Scholar]
- Suantawee, T.; Elazab, S.T.; Hsu, W.H.; Yao, S.; Cheng, H.; Adisakwattana, S. Cyanidin stimulates insulin secretion and pancreatic β-cell gene expression through activation of l-type voltage-dependent Ca2+ channels. Nutrients 2017, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, J.; Teuber, S.; Morera, F.J.; Ojeda, C.; Flores, C.A.; Hidalgo, M.A.; Núñez, L.; Villalobos, C.; Burgos, R.A. Delphinidin reduces glucose uptake in mice jejunal tissue and human intestinal cells lines through FFA1/GPR40. Int. J. Mol. Sci. 2017, 18, 750. [Google Scholar] [CrossRef] [Green Version]
- Demir, Y.; Durmaz, L.; Taslimi, P.; Gulçin, İ. Antidiabetic properties of dietary phenolic compounds: Inhibition effects on α-amylase, aldose reductase, and α-glycosidase. Biotechnol. Appl. Biochem. 2019, 66, 781–786. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini-Ghazvini, S.M.-B.; Adibi, H.; Khodarahmi, R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: A virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ma, Y.; Cheng, G.; Zhang, Y.; Cai, S. Comparative study of dietary flavonoids with different structures as α-glucosidase inhibitors and insulin sensitizers. J. Agric. Food Chem. 2019, 67, 10521–10533. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Murtaza, G.; Liu, G.; Rahu, N.; Kalhoro, M.S.; Kalhoro, D.H.; Adebowale, T.O.; Mazhar, M.U.; ur Rehman, Z. Flavonoids and type 2 diabetes: Evidence of efficacy in clinical and animal studies and delivery strategies to enhance their therapeutic efficacy. Pharmacol. Res. 2020, 152, 104629. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Luo, J.; Huang, J.; Wen, Q. Flavonoids intake and risk of type 2 diabetes mellitus: A meta-analysis of prospective cohort studies. Medicine 2018, 97, 1–7. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: An updated review. Oxid. Med. Cell. Longev. 2020, 2020, 893–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Luo, J.; Jia, Z.; Zhen, W.; Zhou, K.; Gilbert, E.; Liu, D. Baicalein protects against type 2 diabetes via promoting islet β-cell function in obese diabetic mice. Int. J. Endocrinol. 2014, 2014, 846742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, A.; Islam, M.S.; Rahman, M.K.; Uddin, M.N.; Akanda, M.R. The pharmacological and biological roles of eriodictyol. Arch. Pharm. Res. 2020, 43, 1–11. [Google Scholar] [CrossRef]
- Antika, L.D.; Dewi, R.M. Pharmacological aspects of fisetin. Asian Pac. J. Trop. Biomed. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Marella, S. Flavonoids—The most potent poly-phenols as antidiabetic agents: An overview. Mod. Approaches Drug Des. 2017, 1, 2–5. [Google Scholar] [CrossRef]
- Sun, M.-Y.; Ye, Y.; Xiao, L.; Rahman, K.; Xia, W.; Zhang, H. Daidzein: A review of pharmacological effects. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Babu, P.V.A.; Liu, D.; Gilbert, E.R. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef] [Green Version]
- Den Hartogh, D.J.; Tsiani, E. Antidiabetic properties of naringenin: A citrus fruit polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagan, K.; Radika, M.K.; Priyadarshini, E.; Venkatraman, C. A study on the inhibitory potential of DPP-IV enzyme by apigenin through in silico and in vivo approaches. Res. J. Recent Sci. 2015, 2277, 22–29. [Google Scholar]
- Fang, P.; Sun, Y.; Gu, X.; Shi, M.; Bo, P.; Zhang, Z.; Bu, L. Baicalin ameliorates hepatic insulin resistance and gluconeogenic activity through inhibition of p38 MAPK/PGC-1α pathway. Phytomedicine 2019, 64, 153074. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Hur, H.J.; Kwon, D.Y.; Hwang, J.T. Tangeretin stimulates glucose uptake via regulation of AMPK signaling pathways in C2C12 myotubes and improves glucose tolerance in high-fat diet-induced obese mice. Mol. Cell. Endocrinol. 2012, 358, 127–134. [Google Scholar] [CrossRef]
- Wang, N.; Yi, W.J.; Tan, L.; Zhang, J.H.; Xu, J.; Chen, Y.; Qin, M.; Yu, S.; Guan, J.; Zhang, R. Apigenin attenuates streptozotocin-induced pancreatic β cell damage by its protective effects on cellular antioxidant defense. Vitr. Cell. Dev. Biol. Anim. 2017, 53, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiang, H.; Cao, S.; Chen, Q.; Cui, M.; Wang, Z.; Li, D.; Zhou, J.; Qiu, F.; Kang, N. Baicalin and its metabolites suppresses gluconeogenesis through activation of AMPK or AKT in insulin resistant HepG-2 cells. Eur. J. Med. Chem. 2017, 141, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Yu, M.; Min, W.; Wan, D.; Han, S.; Shan, Y.; Wang, R.; Shi, M.; Zhang, Z.; Bo, P. Effect of baicalin on GLUT4 expression and glucose uptake in myotubes of rats. Life Sci. 2018, 196, 156–161. [Google Scholar] [CrossRef]
- Fang, P.; Yu, M.; Zhang, L.; Wan, D.; Shi, M.; Zhu, Y.; Bo, P.; Zhang, Z. Baicalin against obesity and insulin resistance through activation of AKT/AS160/GLUT4 pathway. Mol. Cell. Endocrinol. 2017, 448, 77–86. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Samini, F.; Farkhondeh, T. Chrysin treatment improves diabetes and its complications in liver, brain, and pancreas in streptozotocin-induced diabetic rats. Can. J. Physiol. Pharmacol. 2016, 94, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, K.; Sravanthi, K.; Shaker, I.A.; Ponnulakshmi, R.; Selvaraj, J. Role of chrysin on expression of insulin signaling molecules. J. Ayurveda Integr. Med. 2015, 6, 248. [Google Scholar] [CrossRef] [Green Version]
- Hameed, A.; Hafizur, R.M.; Hussain, N.; Raza, S.A.; Rehman, M.; Ashraf, S.; Ul-Haq, Z.; Khan, F.; Abbas, G.; Choudhary, M.I. Eriodictyol stimulates insulin secretion through cAMP/PKA signaling pathway in mice islets. Eur. J. Pharmacol. 2018, 820, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.-Y.; Do, E.; Lee, D.; Kim, J.; Choi, M.-S. Elucidation of anti-obesity and anti-diabetic function of eriodictyol in diet-induced obese mice. Clin. Nutr. 2018, 37, 146. [Google Scholar] [CrossRef]
- Makena, W.; Hambolu, J.O.; Timbuak, J.A.; Umana, U.E.; Iliya, A.I.; Dibal, N.I. Mormodica charantia L. fruit and genistein ameliorates type 2 diabetes in rats by preventing lipid accumulation, insulin resistance and enhancing beta cell function. J. Diabetes Metab. Disord. 2020, 19, 1303–1310. [Google Scholar] [CrossRef]
- Kim, A.; Lee, W.; Yun, J.-M. Luteolin and fisetin suppress oxidative stress by modulating sirtuins and forkhead box O3a expression under in vitro diabetic conditions. Nutr. Res. Pract. 2017, 11, 430–434. [Google Scholar] [CrossRef] [Green Version]
- Dokumacioglu, E.; Iskender, H.; Sen, T.M.; Ince, I.; Dokumacioglu, A.; Kanbay, Y.; Erbas, E.; Saral, S. The effects of hesperidin and quercetin on serum tumor necrosis factor-alpha and interleukin-6 levels in streptozotocin-induced diabetes model. Pharmacogn. Mag. 2018, 14, 167–173. [Google Scholar]
- Iskender, H.; Dokumacioglu, E.; Sen, T.M.; Ince, I.; Kanbay, Y.; Saral, S. The effect of hesperidin and quercetin on oxidative stress, NF-κB and SIRT1 levels in a STZ-induced experimental diabetes model. Biomed. Pharmacother. 2017, 90, 500–508. [Google Scholar] [CrossRef]
- Sundaram, R.; Nandhakumar, E.; Haseena Banu, H. Hesperidin, a citrus flavonoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Toxicol. Mech. Methods 2019, 29, 644–653. [Google Scholar] [CrossRef]
- Razavi, T.; Kouhsari, S.M.; Abnous, K. Morin exerts anti-diabetic effects in human HepG2 cells via down-regulation of miR-29a. Exp. Clin. Endocrinol. Diabetes 2019, 127, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Rajappa, R.; Sireesh, D.; Salai, M.B.; Ramkumar, K.M.; Sarvajayakesavulu, S.; Madhunapantula, S.V. Treatment with naringenin elevates the activity of transcription factor Nrf2 to protect pancreatic β-cells from streptozotocin-induced diabetes in vitro and in vivo. Front. Pharmacol. 2019, 9, 1562–1582. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, O.M.; Hassan, M.A.; Abdel-Twab, S.M.; Azeem, M.N.A. Navel orange peel hydroethanolic extract, naringin and naringenin have anti-diabetic potentials in type 2 diabetic rats. Biomed. Pharmacother. 2017, 94, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Kim, J.H.; Pan, J.H.; Kim, J.K.; Park, T.S.; Kim, Y.J.; Lee, J.H.; Kim, J.H. Naringin protects pancreatic β-cells against oxidative stress-induced apoptosis by inhibiting both intrinsic and extrinsic pathways in insulin-deficient diabetic mice. Mol. Nutr. Food Res. 2018, 62, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, R.; Arya, A.; Nisha, P.; Jayamurthy, P. Quercetin, a lead compound against type 2 diabetes ameliorates glucose uptake via AMPK pathway in skeletal muscle cell line. Front. Pharmacol. 2017, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, Q.; Li, K.; Zhu, L.; Lin, X.; Lin, X.; Shen, Q.; Li, G.; Xie, X. Quercetin improves glucose and lipid metabolism of diabetic rats: Involvement of Akt signaling and SIRT1. J. Diabetes Res. 2017, 2017, 3417306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Han, J.; Zhou, Z.; Li, D. Tangeretin inhibits streptozotocin-induced cell apoptosis via regulating NF-κB pathway in INS-1 cells. J. Cell. Biochem. 2019, 120, 3286–3293. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, J.; Ren, W.; Zhu, Y.; Zhao, Q.; Zhang, K.; Su, D.; Qiu, C.; Zhang, W.; Li, K. Citrus flavone tangeretin is a potential insulin sensitizer targeting hepatocytes through suppressing MEK-ERK1/2 pathway. Biochem. Biophys. Res. Commun. 2020, 529, 277–282. [Google Scholar] [CrossRef]
- Akinwumi, B.C.; Bordun, K.-A.M.; Anderson, H.D. Biological activities of stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.-W.; Jiang, J.-L.; Zou, J.-J.; Yang, M.-Y.; Chen, F.-M.; Zhang, Y.-J.; Jia, L. Therapeutic potential of ginsenosides on diabetes: From hypoglycemic mechanism to clinical trials. J. Funct. Foods 2020, 64, 103630. [Google Scholar] [CrossRef]
- Sun, H.; Liu, X.; Long, S.R.; Ge, H.; Wang, Y.; Yu, S.; Xue, Y.; Zhang, Y.; Li, X.; Li, W. Antidiabetic effects of pterostilbene through PI3K/Akt signal pathway in high fat diet and STZ-induced diabetic rats. Eur. J. Pharmacol. 2019, 859, 172526. [Google Scholar] [CrossRef]
- Gencoglu, H.; Tuzcu, M.; Hayirli, A.; Sahin, K. Protective effects of resveratrol against streptozotocin-induced diabetes in rats by modulation of visfatin/sirtuin-1 pathway and glucose transporters. Int. J. Food Sci. Nutr. 2015, 66, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Elango, B.; Dornadula, S.; Paulmurugan, R.; Ramkumar, K.M. Pterostilbene ameliorates streptozotocin-induced diabetes through enhancing antioxidant signaling pathways mediated by Nrf2. Chem. Res. Toxicol. 2016, 29, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Elekofehinti, O.O. Saponins: Anti-diabetic principles from medicinal plants—A review. Pathophysiology 2015, 22, 95–103. [Google Scholar] [CrossRef]
- Chen, W.; Balan, P.; Popovich, D.G. Review of ginseng anti-diabetic studies. Molecules 2019, 24, 4501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, K.; Takamura, Y.; Iwamoto, T.; Nomura, M.; Iwasaki, H.; Ohdera, M.; Murakoshi, M.; Sugiyama, K.; Matsuyama, K.; Manabe, Y.; et al. Dammarane-type triterpene extracts of Panax notoginseng root ameliorates hyperglycemia and insulin sensitivity by enhancing glucose uptake in skeletal muscle. Biosci. Biotechnol. Biochem. 2017, 81, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Hemalatha, T.; Pulavendran, S.; Balachandran, C.; Manohar, B.M.; Puvanakrishnan, R. Arjunolic acid: A novel phytomedicine with multifunctional therapeutic applications. Indian J. Exp. Boil. 2010, 48, 238–247. [Google Scholar]
- Li, H.; Yao, Y.; Li, L. Coumarins as potential antidiabetic agents. J. Pharm. Pharmacol. 2017, 69, 1253–1264. [Google Scholar] [CrossRef] [Green Version]
- Gan, Q.; Wang, J.; Hu, J.; Lou, G.; Xiong, H.; Peng, C.; Zheng, S.; Huang, Q. The role of diosgenin in diabetes and diabetic complications. J. Steroid Biochem. Mol. Biol. 2020, 198, 105575. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, H.-J. Ginsenoside compound k: Insights into recent studies on pharmacokinetics and health-promoting activities. Biomolecules 2020, 10, 1028. [Google Scholar] [CrossRef]
- Park, M.W.; Ha, J.; Chung, S.H. (S)-ginsenoside Rg3 enhances glucose-stimulated insulin secretion and activates AMPK. Biol. Pharm. Bull. 2008, 31, 748–751. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Yuan, H.D.; Chung, S.H. Ginsenoside Rg1 suppresses hepatic glucose production via AMP-activated protein kinase in HepG2 cells. Biol. Pharm. Bull. 2010, 33, 325–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, S.; Li, W.; Yu, Y.; Yao, F.; Lixiang, A.; Lan, X.; Guan, F.; Zhang, M.; Chen, L. Ginsenoside Compound K suppresses the hepatic gluconeogenesis via activating adenosine-5′ monophosphate kinase: A study in vitro and in vivo. Life Sci. 2015, 139, 8–15. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.; Luo, Y.; Wang, T.; Li, X.; Li, A.; Li, J.; Liu, K.; Liu, B. Ginsenoside Rb1 and compound K improve insulin signaling and inhibit ER stress-associated NLRP3 inflammasome activation in adipose tissue. J. Ginseng Res. 2016, 40, 351–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabandeh, M.R.; Jafari, H.; Hosseini, S.A.; Hashemitabar, M. Ginsenoside Rb1 stimulates adiponectin signaling in C2C12 muscle cells through up-regulation of AdipoR1 and AdipoR2 proteins. Pharma. Biol. 2015, 53, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Ye, L.; Zhang, H.; Zhao, J.; Wang, G.; Guo, C.; Shang, W. Ginsenoside Rb1 ameliorates liver fat accumulation by upregulating perilipin expression in adipose tissue of db/db obese mice. J. Ginseng Res. 2015, 39, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Ding, L.; Zhang, H.; Chu, Y.; Chang, Z.; Yu, Y.; Guo, D.; Zhang, S.; Liu, X. Ginsenoside Rb1 increases insulin sensitivity through suppressing 11β-hydroxysteroid dehydrogenase type I. Am. J. Transl. Res. 2017, 9, 1049–1057. [Google Scholar]
- Tian, W.; Chen, L.; Zhang, L.; Wang, B.; Li, X.; Fan, K.; Ai, C.; Xia, X.; Li, S.; Li, Y. Effects of ginsenoside Rg1 on glucose metabolism and liver injury in streptozotocin-induced type 2 diabetic rats. Genet. Mol. Res. 2017, 16, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Fan, D. Ginsenoside Rk3 ameliorates high-fat-diet/streptozocin induced type 2 diabetes mellitus in mice via the AMPK/Akt signaling pathway. Food Funct. 2019, 10, 2538–2551. [Google Scholar] [CrossRef]
- Kumari, M.; Jain, S. Tannins: An antinutrient with positive effect to manage diabetes. Res. J. Recent Sci. 2012, 1, 1–8. [Google Scholar]
- Ajebli, M.; Eddouks, M. The promising role of plant tannins as bioactive antidiabetic agents. Curr. Med. Chem. 2019, 26, 4852–4884. [Google Scholar] [CrossRef] [PubMed]
- Türkan, F.; Taslimi, P.; Saltan, F.Z. Tannic acid as a natural antioxidant compound: Discovery of a potent metabolic enzyme inhibitor for a new therapeutic approach in diabetes and Alzheimer’s disease. J. Biochem. Mol. Toxicol. 2019, 33, e22340. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Bueno, P.S.A.; Kato-Schwartz, C.G.; de Souza Lima, D.; Bracht, A.; Peralta, R.M.; Seixas, F.A.V. In silico evaluation of condensed and hydrolysable tannins as inhibitors of pancreatic α-amylase. J. Mol. Model. 2019, 25, 1–9. [Google Scholar] [CrossRef]
- Shoukat, M.; Sorrentino, A. Cereal β-glucan: A promising prebiotic polysaccharide and its impact on the gut health. Int. J. Food Sci. Technol. 2021, 56, 2088–2097. [Google Scholar] [CrossRef]
- Pan, G.; Zhao, L.; Xiao, N.; Yang, K.; Ma, Y.; Zhao, X.; Fan, Z.; Zhang, Y.; Yao, Q.; Lu, K.; et al. Total synthesis of 8-(6 ″-umbelliferyl)-apigenin and its analogs as anti-diabetic reagents. Eur. J. Med. Chem. 2016, 122, 674–683. [Google Scholar] [CrossRef]
- Murali, R.; Srinivasan, S.; Ashokkumar, N. Antihyperglycemic effect of fraxetin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Biochimie 2013, 95, 1848–1854. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Ibeji, C.U.; Koorbanally, N.A.; Islam, M.S. Umbelliferone stimulates glucose uptake; modulates gluconeogenic and nucleotide-hydrolyzing enzymes activities, and dysregulated lipid metabolic pathways in isolated psoas muscle. J. Funct. Foods 2020, 67, 103847. [Google Scholar] [CrossRef]
- Sim, M.-O.; Lee, H.-I.; Ham, J.R.; Seo, K.-I.; Lee, M.-K. Long-term supplementation of esculetin ameliorates hepatosteatosis and insulin resistance partly by activating AdipoR2–AMPK pathway in diet-induced obese mice. J. Funct. Foods 2015, 15, 160–171. [Google Scholar] [CrossRef]
- Mo, Z.; Li, L.; Yu, H.; Wu, Y.; Li, H. Coumarins ameliorate diabetogenic action of dexamethasone via Akt activation and AMPK signaling in skeletal muscle. J. Pharmacol. Sci. 2019, 139, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.-G.; Zhao, X.; Zhong, W.; Xie, M.-L. Osthole improves glucose and lipid metabolism via modulation of PPARα/γ-mediated target gene expression in liver, adipose tissue, and skeletal muscle in fatty liver rats. Pharm. Biol. 2016, 54, 882–888. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.H.; Park, J.E.; Han, J.S. Scopoletin increases glucose uptake through activation of PI3K and AMPK signaling pathway and improves insulin sensitivity in 3T3-L1 cells. Nutr. Res. 2020, 74, 52–61. [Google Scholar] [CrossRef]
- Jang, J.H.; Park, J.E.; Han, J.S. Scopoletin inhibits α-glucosidase in vitro and alleviates postprandial hyperglycemia in mice with diabetes. Eur. J. Pharmacol. 2018, 834, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Ramu, R.; Shirahatti, P.; Zameer, F.; Dhananjaya, B.L. Assessment of in vivo antidiabetic properties of umbelliferone and lupeol constituents of banana (Musa sp. var. Nanjangud Rasa Bale) flower in hyperglycaemic rodent model. PLoS ONE 2016, 11, e0151135. [Google Scholar]
- Kaur, K.K.; Allahbadia, G.; Singh, M. Monoterpenes—A class of terpenoid group of natural products as a source of natural antidiabetic agents in the future—A review. CPQ Nutr. 2019, 3, 1–21. [Google Scholar]
- Panigrahy, S.K.; Bhatt, R.; Kumar, A. Targeting type II diabetes with plant terpenes: The new and promising antidiabetic therapeutics. Biologia 2021, 76, 241–254. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A multifunctional compound with potent therapeutic effects. J. Food Biochem. 2021, 45, 1–10. [Google Scholar] [CrossRef]
- Ilić, V.; Vukmirović, S.; Stilinović, N.; Čapo, I.; Arsenović, M.; Milijašević, B. Insight into anti-diabetic effect of low dose of stevioside. Biomed. Pharmacother. 2017, 90, 216–221. [Google Scholar] [CrossRef]
- Saravanan, R.; Ramachandran, V. Effect of Rebaudioside A, a diterpenoid on glucose homeostasis in STZ-induced diabetic rats. J. Physiol. Biochem. 2012, 68, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Siddique, H.R.; Saleem, M. Beneficial health effects of lupeol triterpene: A review of preclinical studies. Life Sci. 2011, 88, 285–293. [Google Scholar] [CrossRef]
- Hung, H.-Y.; Qian, K.; Morris-Natschke, S.L.; Hsu, C.-S.; Lee, K.-H. Recent discovery of plant-derived anti-diabetic natural products. Nat. Prod. Rep. 2012, 29, 580–606. [Google Scholar] [CrossRef]
- Lee, H.-A.; Kim, M.-J.; Han, J.-S. Alleviating effects of lupeol on postprandial hyperglycemia in diabetic mice. Toxicol. Res. 2021, 10, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.; Saravanan, R. Glucose uptake through translocation and activation of GLUT4 in PI3K/Akt signaling pathway by asiatic acid in diabetic rats. Hum. Exp. Toxicol. 2015, 34, 884–893. [Google Scholar] [CrossRef]
- Li, Y.; Mai, Y.; Qiu, X.; Chen, X.; Li, C.; Yuan, W.; Hou, N. Effect of long-term treatment of Carvacrol on glucose metabolism in Streptozotocin-induced diabetic mice. BMC Complement. Med. Ther. 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Sarabi, M.M.; Gholami, M.; Assadollahi, V.; Khorramabadi, R.M.; Moradi, F.H.; Ahmadvand, H. D-limonene in diabetic rats. J. Ren. Inj. Prev. 2021, 10, 1–8. [Google Scholar]
- Bhasker, S.; Madhav, H.; Chinnamma, M. Molecular evidence of insulinomimetic property exhibited by steviol and stevioside in diabetes induced L6 and 3T3L1 cells. Phytomedicine 2015, 22, 1037–1044. [Google Scholar] [CrossRef]
- Saleh, O.M.; Awad, N.S.; Soliman, M.M.; Mansour, A.A.; Nassan, M.A. Insulin-mimetic activity of stevioside on diabetic rats: Biochemical, molecular and histopathological study. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Shreenithi, S.; Vishnupriya, V.; Ponnulakshmi, R.; Gayathri, R.; Madhan, K.; Shyamaladevi, B.; Selvaraj, J. In silico and in vivo approach to identify the antidiabetic activity of lupeol. Drug Invent. Today 2019, 11, 1113–1116. [Google Scholar]
- Dhivyadharshini, A.; Vishnpriya, V.; Ponnulakshmi, R.; Gayathri, R.; Madhan, K.; Shyamaladevi, B.; Selvaraj, J. Effect of lupeol on insulin receptor substrate-1 and AKt expression in adipose tissue of type-2 diabetic rats. Drug Invent. Today 2019, 12, 801–805. [Google Scholar]
- Birgani, G.A.; Ahangarpour, A.; Khorsandi, L.; Moghaddam, H.F. Anti-diabetic effect of betulinic acid on streptozotocin-nicotinamide induced diabetic male mouse model. Braz. J. Pharm. Sci. 2018, 54, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ahangarpour, A.; Shabani, R.; Farbood, Y. The effect of betulinic acid on leptin, adiponectin, hepatic enzyme levels and lipid profiles in streptozotocin–nicotinamide-induced diabetic mice. Res. Pharm. Sci. 2018, 13, 142–148. [Google Scholar] [CrossRef]
- Ding, H.; Wu, X.; Pan, J.; Hu, X.; Gong, D.; Zhang, G. New insights into the inhibition mechanism of betulinic acid on α-glucosidase. J. Agric. Food Chem. 2018, 66, 7065–7075. [Google Scholar] [CrossRef] [PubMed]
- Brusotti, G.; Montanari, R.; Capelli, D.; Cattaneo, G.; Laghezza, A.; Tortorella, P.; Loiodice, F.; Peiretti, F.; Bonardo, B.; Paiardini, A.; et al. Betulinic acid is a PPARγ antagonist that improves glucose uptake, promotes osteogenesis and inhibits adipogenesis. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellano, J.; Guinda, A.; Macías, L.; Santos-Lozano, J.; Lapetra, J.; Rada, M. Free radical scavenging and α-glucosidase inhibition, two potential mechanisms involved in the anti-diabetic activity of oleanolic acid. Grasas y Aceites 2016, 67, 142–153. [Google Scholar] [CrossRef] [Green Version]
- Castro, A.J.G.; Frederico, M.J.S.; Cazarolli, L.H.; Mendes, C.P.; Bretanha, L.C.; Schmidt, É.C.; Bouzon, Z.L.; de Medeiros Pinto, V.A.; da Fonte Ramos, C.; Pizzolatti, M.G.; et al. The mechanism of action of ursolic acid as insulin secretagogue and insulinomimetic is mediated by cross-talk between calcium and kinases to regulate glucose balance. Biochim. Biophys. Acta BBA Gen. Subj. 2015, 1850, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Tripathi, R.; Mohammed, A.; Rizvi, S.I.; Mishra, N. Effects of thymol supplementation against type 2 diabetes in streptozocin- induced rat model. Plant. Arch. 2020, 20, 863–869. [Google Scholar]
- Qi, L.-W.; Liu, E.-H.; Chu, C.; Peng, Y.-B.; Cai, H.-X.; Li, P. Anti-diabetic agents from natural products—an update from 2004 to 2009. Curr. Top. Med. Chem. 2010, 10, 434–457. [Google Scholar] [CrossRef]
- Park, J.; Jang, H.-J. Anti-diabetic effects of natural products an overview of therapeutic strategies. Mol. Cell. Toxicol. 2017, 13, 1–20. [Google Scholar] [CrossRef]
- Belete, T.M. A recent achievement in the discovery and development of novel targets for the treatment of type-2 diabetes mellitus. J. Exp. Pharmacol. 2020, 12, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Osadebe, P.O.; Odoh, E.U.; Uzor, P.F. Natural products as potential sources of antidiabetic drugs. J. Pharm. Res. Int. 2014, 4, 2075–2095. [Google Scholar] [CrossRef]
- Jugran, A.K.; Rawat, S.; Devkota, H.P.; Bhatt, I.D.; Rawal, R.S. Diabetes and plant-derived natural products: From ethnopharmacological approaches to their potential for modern drug discovery and development. Phytother. Res. 2021, 35, 223–245. [Google Scholar] [CrossRef]
- Li, W.; Yuan, G.; Pan, Y.; Wang, C.; Chen, H. Network pharmacology studies on the bioactive compounds and action mechanisms of natural products for the treatment of diabetes mellitus: A review. Front. Pharmacol. 2017, 8, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2020, 338, 128119. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef]
- Sayem, A.S.M.; Arya, A.; Karimian, H.; Krishnasamy, N.; Ashok Hasamnis, A.; Hossain, C.F. Action of phytochemicals on insulin signaling pathways accelerating glucose transporter (GLUT4) protein translocation. Molecules 2018, 23, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proença, C.; Freitas, M.; Ribeiro, D.; Sousa, J.L.; Carvalho, F.; Silva, A.M.; Fernandes, P.A.; Fernandes, E. Inhibition of protein tyrosine phosphatase 1B by flavonoids: A structure-activity relationship study. Food Chem. Toxicol. 2018, 111, 474–481. [Google Scholar] [CrossRef]
- Zhao, B.T.; Nguyen, D.H.; Le, D.D.; Choi, J.S.; Min, B.S.; Woo, M.H. Protein tyrosine phosphatase 1B inhibitors from natural sources. Arch. Pharm. Res. 2018, 41, 130–161. [Google Scholar] [CrossRef]
- Jung, H.A.; Paudel, P.; Seong, S.H.; Min, B.-S.; Choi, J.S. Structure-related protein tyrosine phosphatase 1B inhibition by naringenin derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 2274–2280. [Google Scholar] [CrossRef]
- Oh, Y.S. Plant-derived compounds targeting pancreatic beta cells for the treatment of diabetes. Evid. Based Complement. Altern. Med. 2015, 2015, 629863. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Park, S.-Y. Can antioxidants be effective therapeutics for type 2 diabetes? Yeungnam Univ. J. Med. 2021, 38, 83. [Google Scholar] [CrossRef]
- Matos, A.L.; Bruno, D.F.; Ambrósio, A.F.; Santos, P.F. The benefits of flavonoids in diabetic retinopathy. Nutrients 2020, 12, 3169. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Chaturvedi, N.; Singh, A.; Mishra, A. Investigation of hypoglycemic effects, oxidative stress potential and xanthine-oxidase activity of polyphenols (gallic acid, catechin) derived from faba bean on 3T3-L1 cell line: Insights into molecular docking and simulation study. Toxicol. Res. 2020, 9, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Nwabueze, P.O.; Ekeuku, S.O.; Chan, H.K.; Eluri, K.; Fromming, G.R.A. Palmatine inhibits up-regulation of GRP78 and CALR protein in an STZ-induced diabetic rat model. Curr. Pharm. Biotechnol. 2021, 22, 288–298. [Google Scholar]
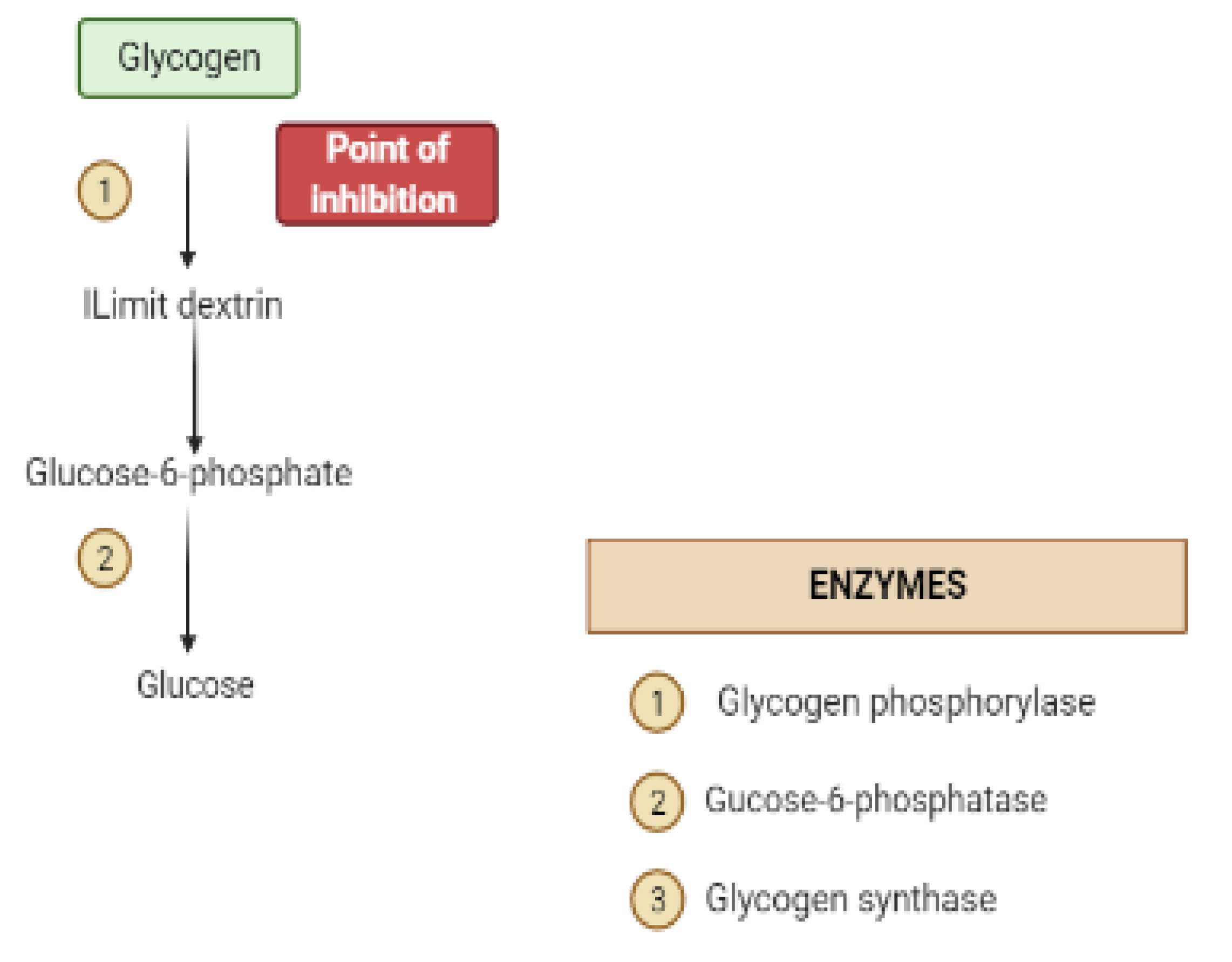

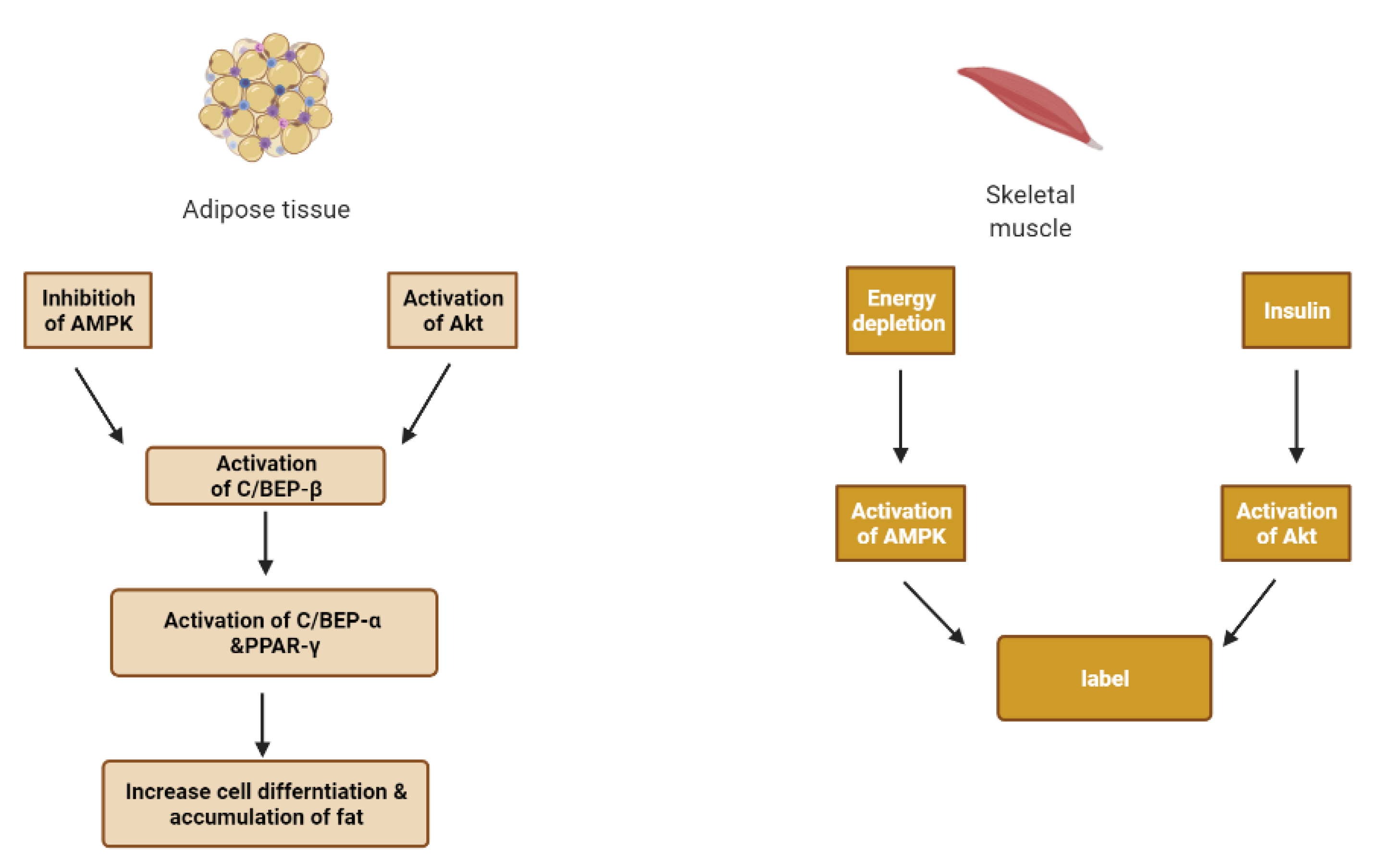
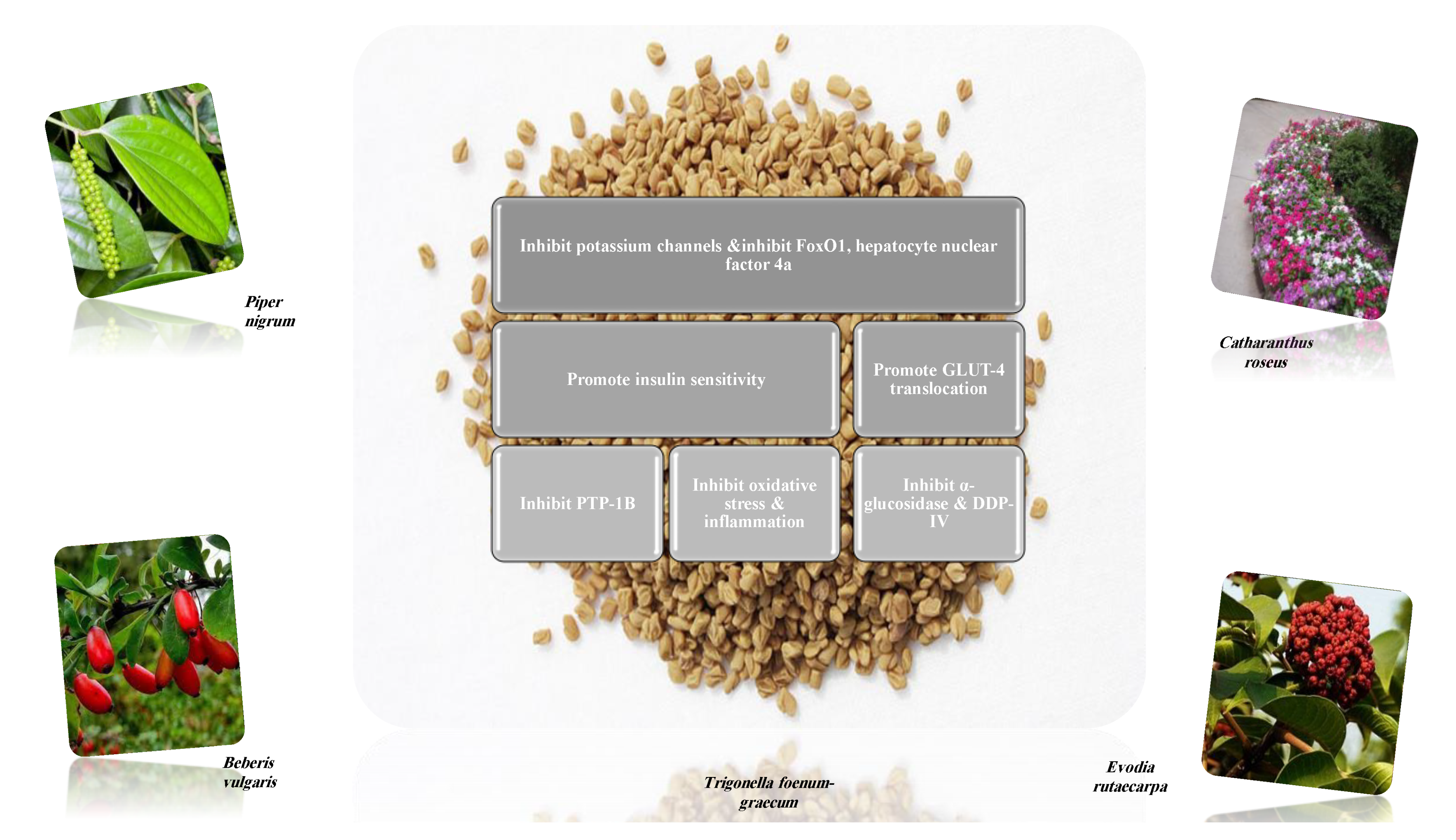
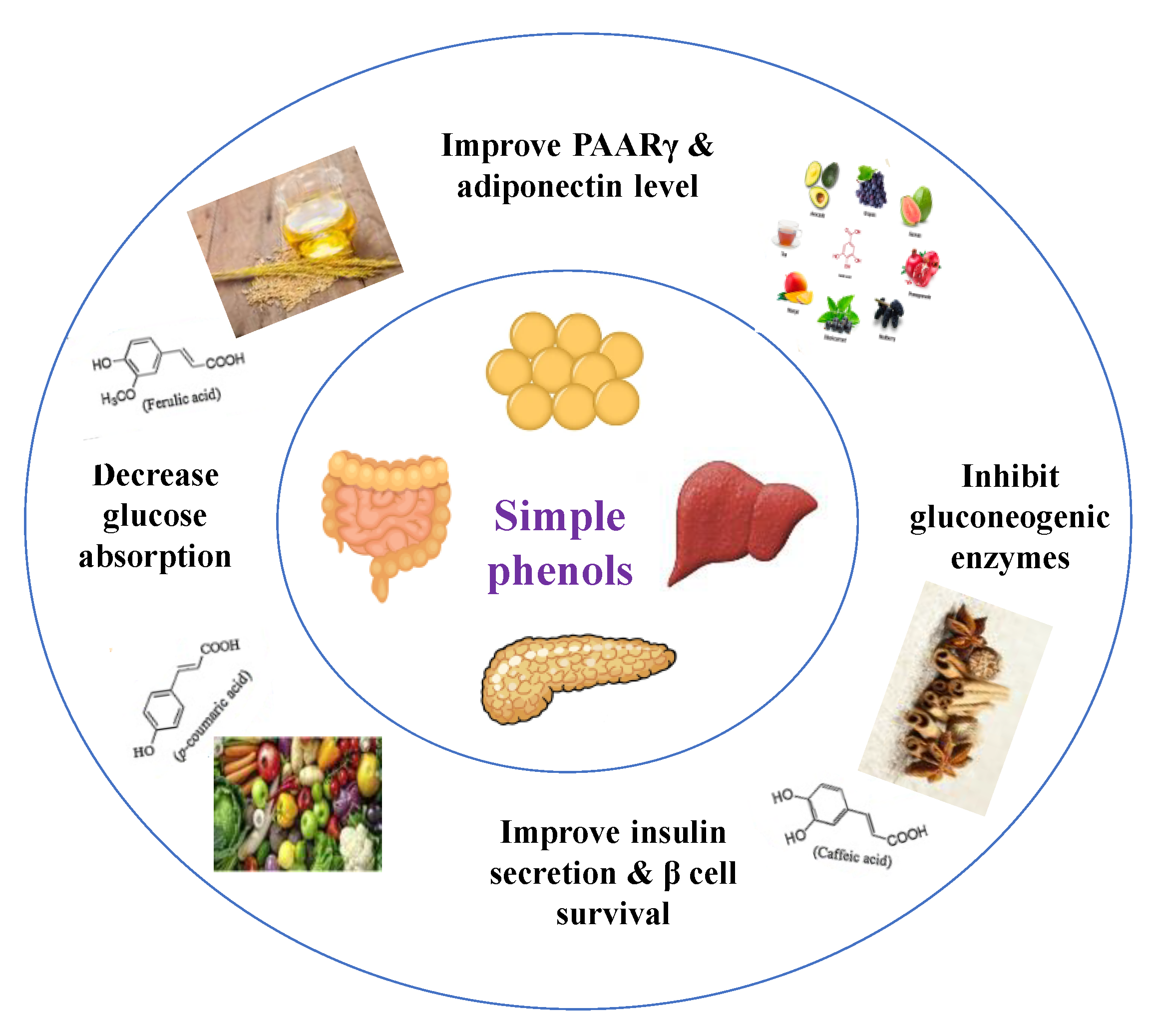
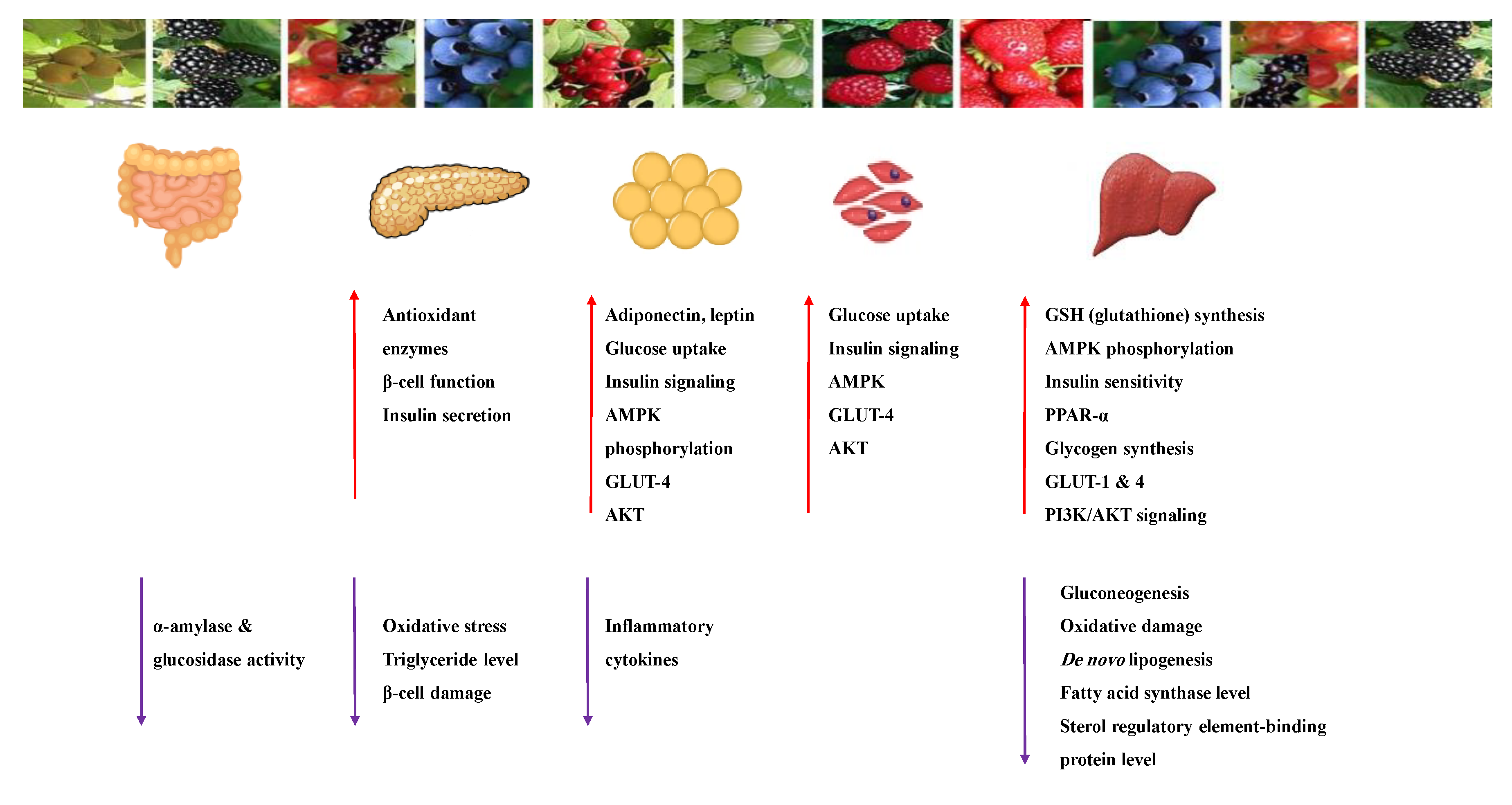

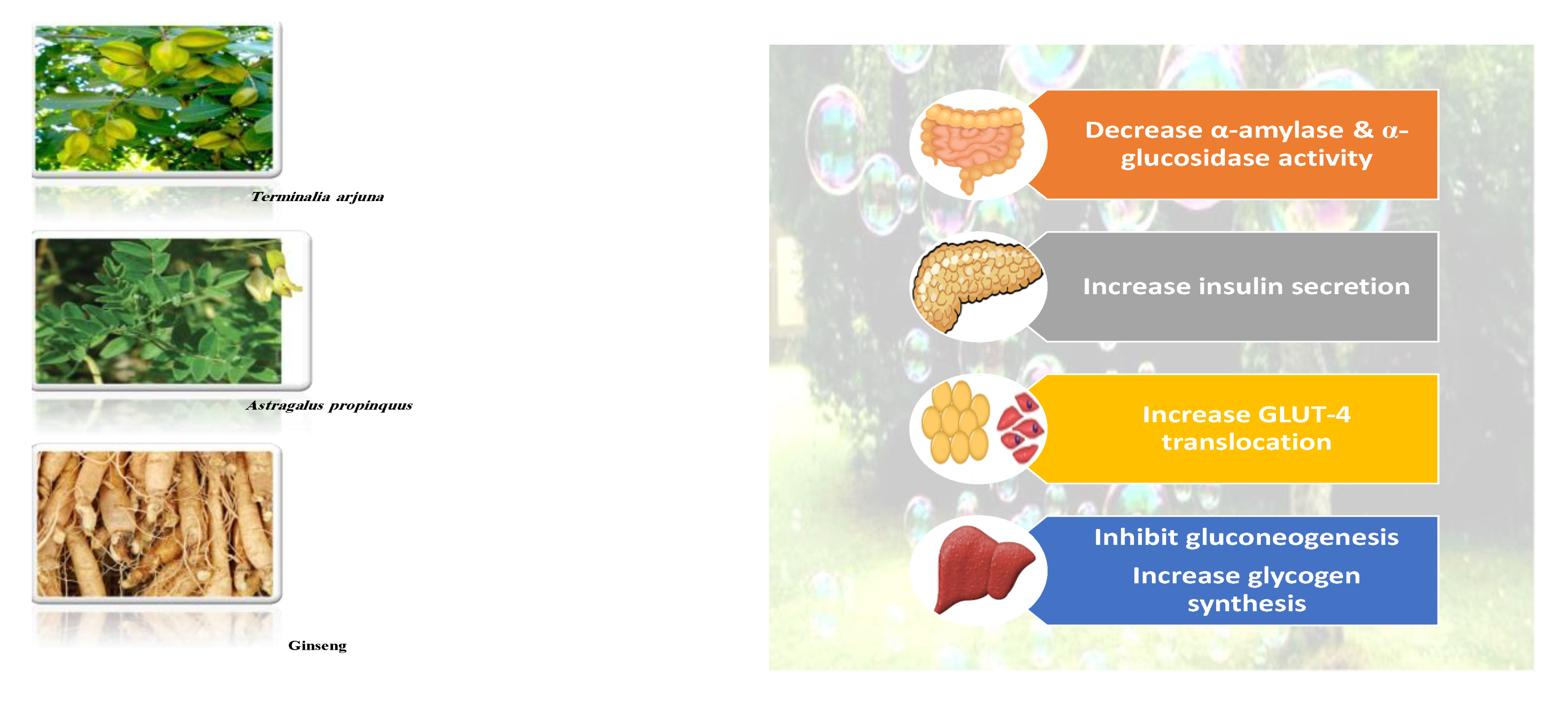
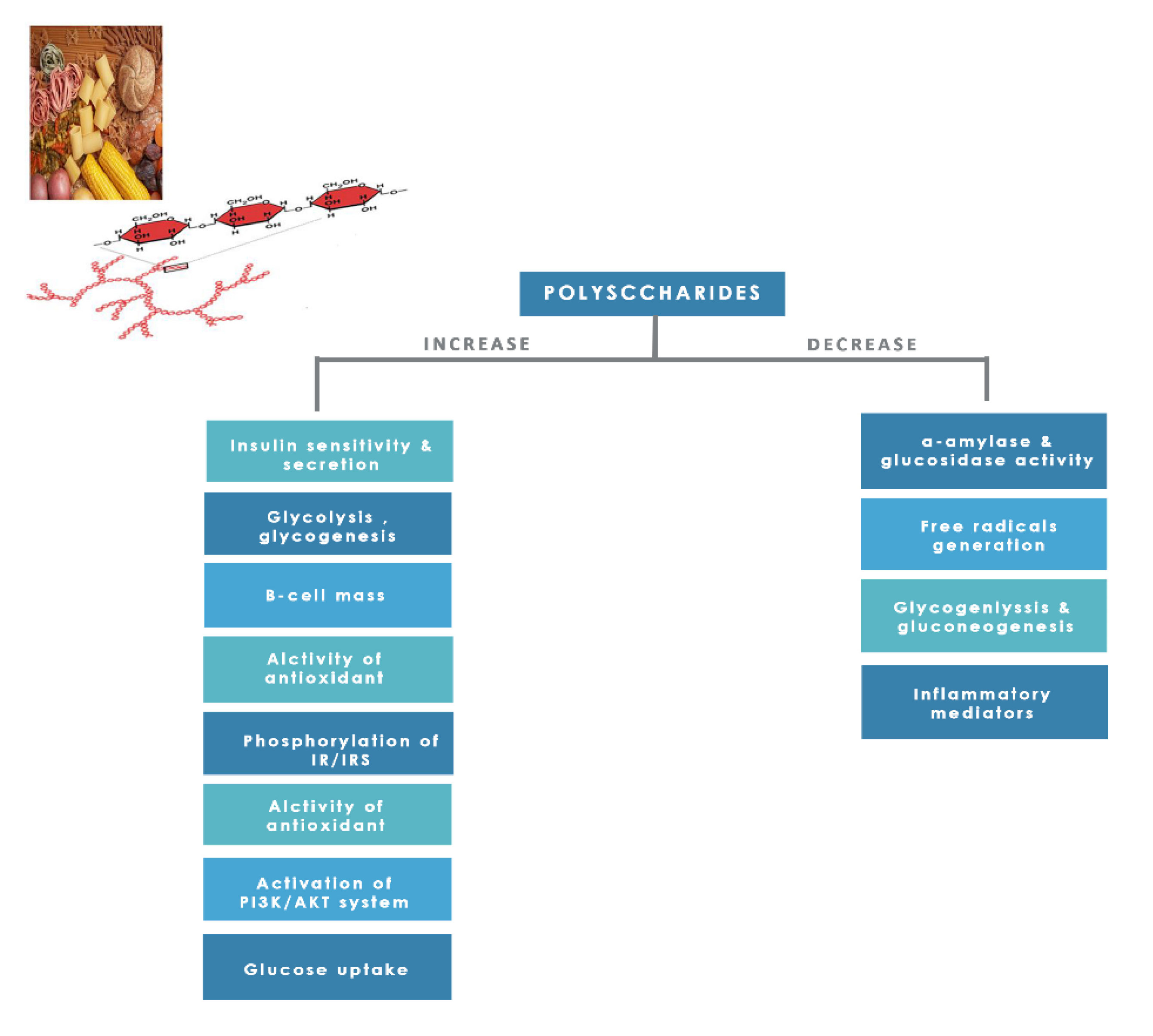
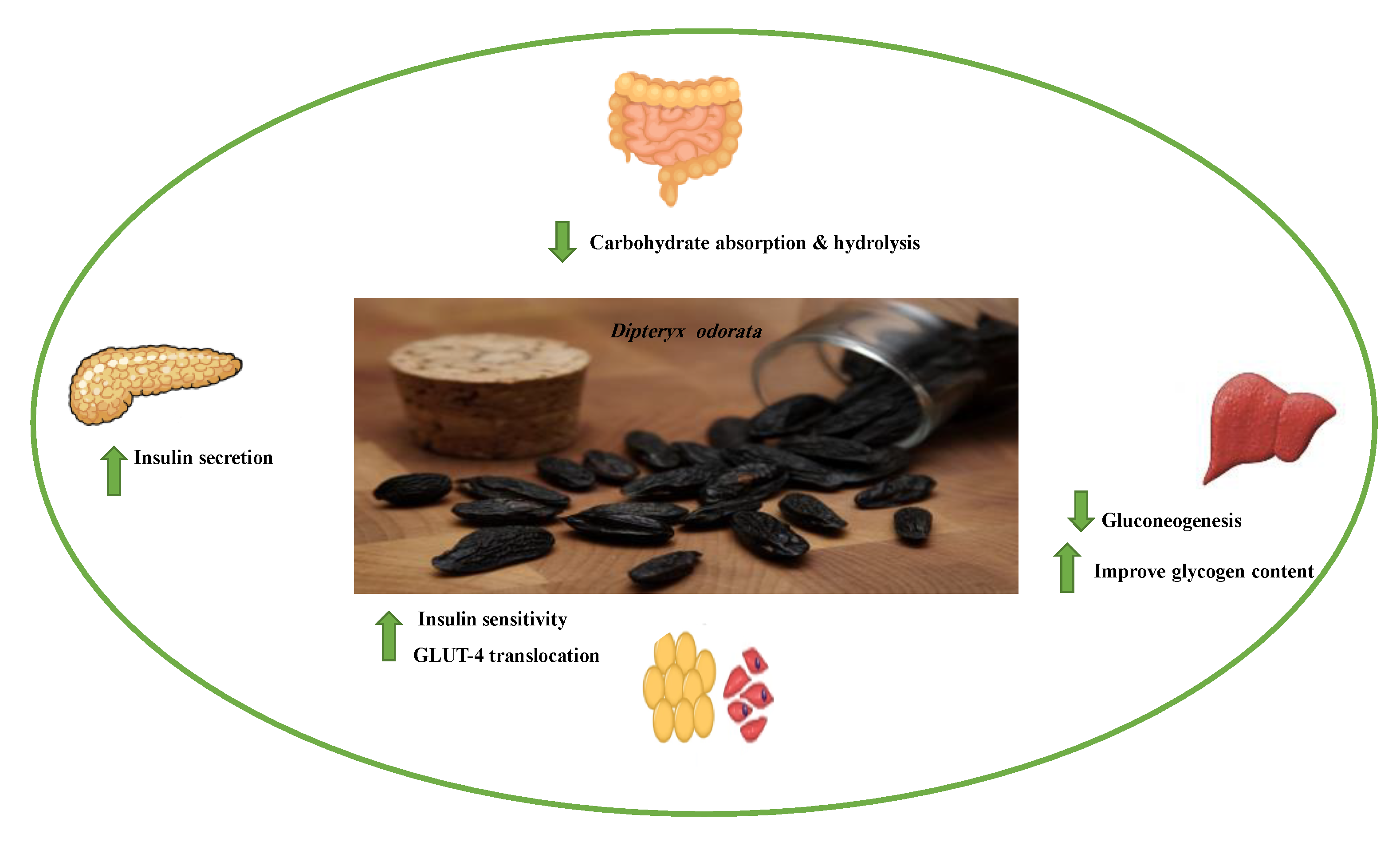
| Target | Glucose Transporter | Hepatic Enzymes | Β-Cells Apoptosis | PPAR | AMPK | Tyrosine Kinase Inhibitor | NF-κB | |
|---|---|---|---|---|---|---|---|---|
| Normal effects | Enhance glucose uptake by tissues | Enhance glucose metabolism | Control program cell death | Control of lipid metabolism | Energy homeostasis | Control growth factor signaling | Control of β-cells survival | |
| Changes by diabetes |  | GLUT translocation Glucose uptake | Insulin signaling Liver glycogen | Apoptotic regulatory genes Caspases | Lipid metabolism | AMPK activation Glucose homeostasis | Insulin sensitivity Insulin secretion | |
 | Insulin resistance | Insulin resistance Glucose production | Oxidative stress Insulin resistance Mitochondria dysfunction | Hyperglycemia Hyperlipidemia hyperinsulinemia | Insulin resistance | Islet cell function | Proinflammatory cytokines Oxidative stress NF-κB expression | |
| Name of Compound | Chemical Structure | Mechanism | References |
|---|---|---|---|
| Resveratrol | 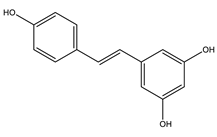 |
| [10,102,149] |
| Pterostilbene | 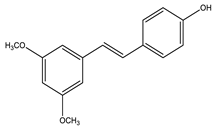 |
| [10,150] |
| Compound | Mechanism | Dose of the Tested Compound (Duration) | Model | References |
|---|---|---|---|---|
| Resveratrol | Suppressed oxidative stress and increased potential to internalize glucose by extrahepatic tissues. | 20 mg/kg (8 weeks) | Streptozocin-induced diabetic rats | [151] |
| Pterostilbene | Ameliorated morphological impairment of the pancreas, increased the protein expression of PPARγ, PI3K, p-Akt, GLUT4 and IRS-1 in adipose tissues. | 20, 40, 80 mg/kg (8 weeks) | Streptozocin-induced diabetic rats | [150] |
| Activated Nrf2, thereby reducing oxidative damage, reverted hexokinase, glucose-6-phosphatase, glucose-6-phosphate dehydrogenase, and fructose-1,6-bisphosphatase, to near-normal levels, improved insulin secretion. | 5, 10 mg/kg (5 weeks) | In vitro: MIN-6 cell line, streptozocin-induced diabetic rats | [152] |
| Compound | Mechanism | Dose of the Tested Compound (duration) | Model | References |
|---|---|---|---|---|
| Ginsenoside K | Inhibited the expression of PEPCK and G6Pase enzymes, increased the activation of AMPK. | 1, 2, 4, 8 μM, (24 h) 30 mg/kg, (4 weeks) | In vitro: HepG2 cell line, streptozocin-induced diabetic mice | [162] |
| Inhibited inflammation and improved insulin signaling in adipose tissue by suppressing ER stress-associated NLRP3 inflammation activation. | 10 µM (24 h) | In vitro: T3-L1 cell line, mice model | [163] | |
| Ginsenoside Rb1 | Increased GLUT-4 translocation through up-regulated adipoR1 and adipoR2 gene. | 0.001–100 mM (1–12 h) | In vitro: C2C12 myotubes cell line | [164] |
| Exhibited insulin-sensitizing effect. | 20 mg/kg (14 days) | Diabetic mice | [165] | |
| Reduced hepatic glucose production, increased glucose uptake in skeletal muscle. | 10 mg/kg every other day (One week) | High-fat-induced diabetic mice | [166] | |
| Inhibited inflammation and improved insulin signaling in adipose tissue by suppressing ER stress-associated NLRP3 inflammation activation. | 10 µM (24 h) | In vitro: T3-L1 cell line, mice model | [163] | |
| Ginsenoside Rg1 | Reduced inflammation by inhibiting JNK activity, reduced caspase-3 and BAX (proapoptotic) proteins, increased BCL-2 (antiapoptotic) protein. | 25, 50 mg/kg (4 weeks) | Streptozocin-induced diabetic rats | [167] |
| Ginsenoside RK3 | Inhibited hepatic gluconeogenesis (inhibited PEPCK and G6pase protein expressions). | 10, 30, 60 mg/kg (4 weeks) | In vitro: HepG2 cell line, high-fat diet and streptozocin-induced mice | [168] |
| Name of Compound | Mechanism | References |
|---|---|---|
| Tannic acid |
| [170,171] |
| Condensed tannins |
| [172] |
| Hydrolyzable tannins |
| [173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehadeh, M.B.; Suaifan, G.A.R.Y.; Abu-Odeh, A.M. Plants Secondary Metabolites as Blood Glucose-Lowering Molecules. Molecules 2021, 26, 4333. https://doi.org/10.3390/molecules26144333
Shehadeh MB, Suaifan GARY, Abu-Odeh AM. Plants Secondary Metabolites as Blood Glucose-Lowering Molecules. Molecules. 2021; 26(14):4333. https://doi.org/10.3390/molecules26144333
Chicago/Turabian StyleShehadeh, Mayadah Bashir, Ghadeer A. R. Y. Suaifan, and Ala’ Mustafa Abu-Odeh. 2021. "Plants Secondary Metabolites as Blood Glucose-Lowering Molecules" Molecules 26, no. 14: 4333. https://doi.org/10.3390/molecules26144333
APA StyleShehadeh, M. B., Suaifan, G. A. R. Y., & Abu-Odeh, A. M. (2021). Plants Secondary Metabolites as Blood Glucose-Lowering Molecules. Molecules, 26(14), 4333. https://doi.org/10.3390/molecules26144333






