Ruthenium Complexes as Promising Candidates against Lung Cancer
Abstract
:1. Introduction
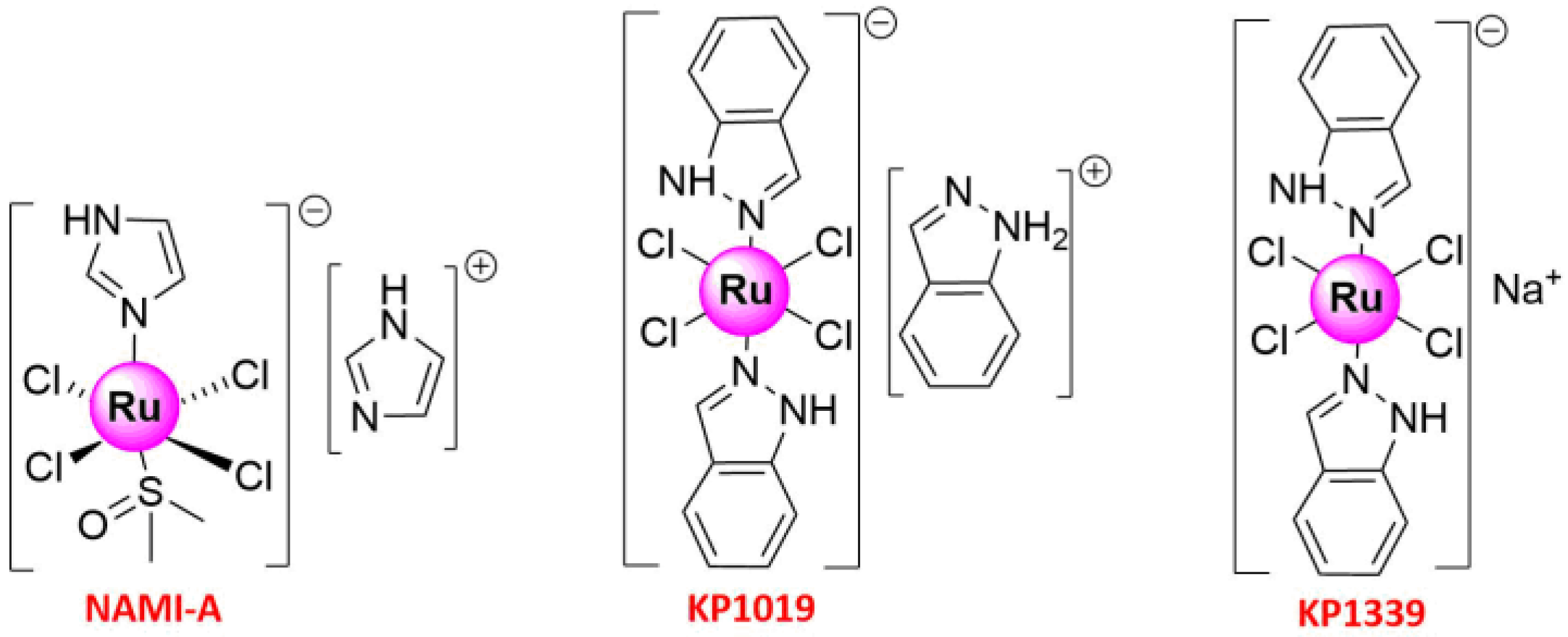
2. Ru(III) Complexes
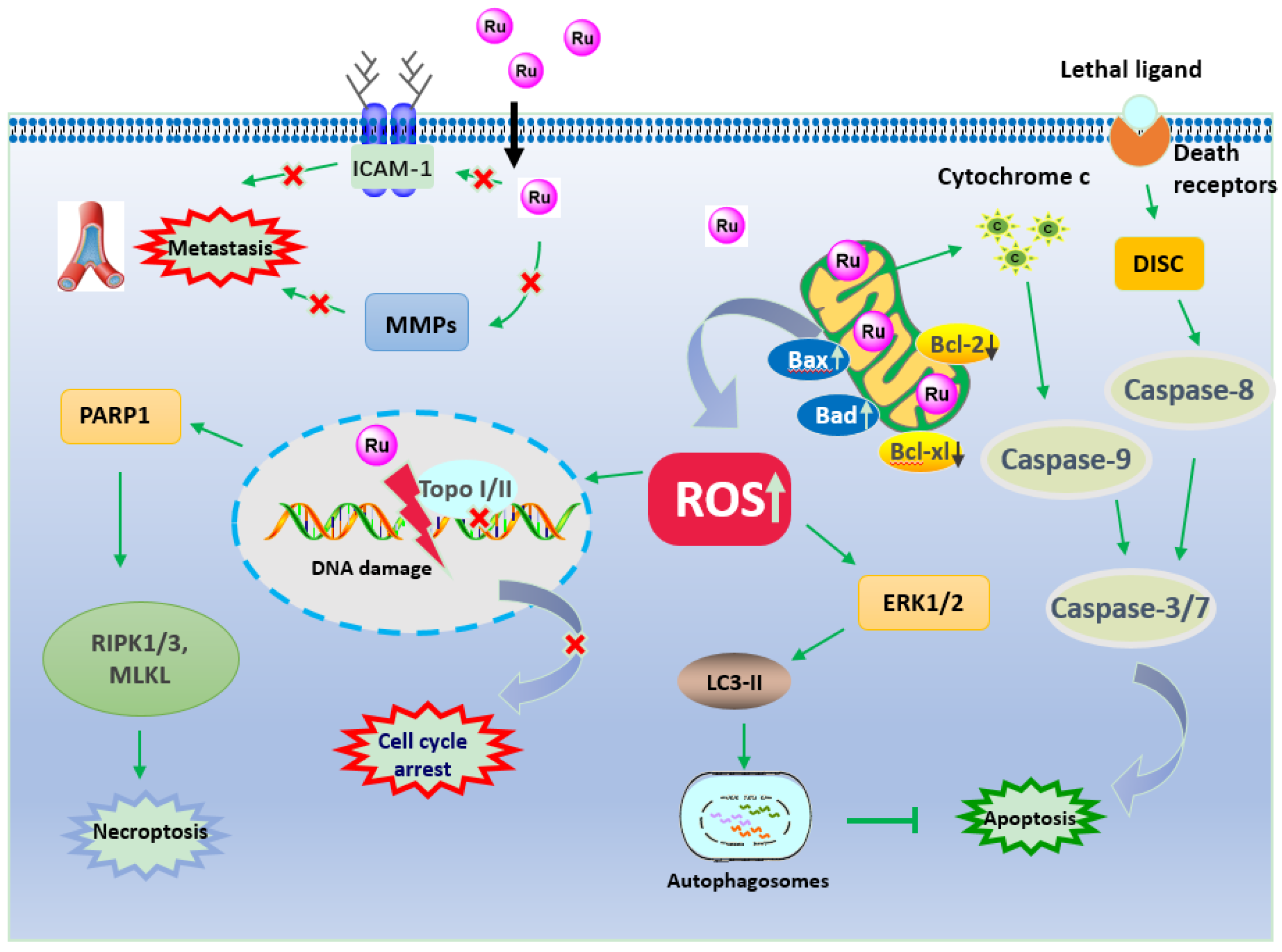
2.1. Apoptosis
2.2. Anti-Metastasis
3. Ru(II) Complexes
3.1. Apoptosis
3.2. Autophagy
3.3. Necroptosis
3.4. Anti-Metastasis
3.5. Cell Cycle Arrest
4. Conclusions/Discussions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung. Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, A.; Di Maio, M. Platinum-based chemotherapy in advanced non-small-cell lung cancer: Optimal number of treatment cycles. Expert Rev. Anticancer Ther. 2016, 16, 653–660. [Google Scholar] [CrossRef]
- Giaccone, G.; Splinter, T.A.; Debruyne, C.; Kho, G.S.; Lianes, P.; van Zandwijk, N.; Pennucci, M.C.; Scagliotti, G.; van Meerbeeck, J.; van Hoesel, Q.; et al. Randomized study of paclitaxel-cisplatin versus cisplatin-teniposide in patients with advanced non-small-cell lung cancer. The European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. J. Clin. Oncol. 1998, 16, 2133–2141. [Google Scholar] [CrossRef]
- Jamieson, E.R.; Lippard, S.J. Structure, Recognition, and Processing of Cisplatin-DNA Adducts. Chem. Rev. 1999, 99, 2467–2498. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Boulikas, T.; Vougiouka, M. Cisplatin and platinum drugs at the molecular level (Review). Oncol. Rep. 2003, 10, 1663–1682. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Ma, D.L.; He, H.Z.; Leung, K.H.; Chan, D.S.; Leung, C.H. Bioactive luminescent transition-metal complexes for biomedical applications. Angew. Chem. Int. Ed. Engl. 2013, 52, 7666–7682. [Google Scholar] [CrossRef]
- Ma, D.L.; Wu, C.; Wu, K.J.; Leung, C.H. Iridium(III) Complexes Targeting Apoptotic Cell Death in Cancer Cells. Molecules 2019, 24, 2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohata, J.; Ball, Z.T. Rhodium at the chemistry-biology interface. Dalton Trans. 2018, 47, 14855–14860. [Google Scholar] [CrossRef] [PubMed]
- Omondi, R.O.; Ojwach, S.O.; Jaganyi, D. Review of comparative studies of cytotoxic activities of Pt(II), Pd(II), Ru(II)/(III) and Au(III) complexes, their kinetics of ligand substitution reactions and DNA/BSA interactions. Inorg. Chim. Acta 2020, 512, 119883. [Google Scholar] [CrossRef]
- Koiri, R.K.; Mehrotra, A.; Trigun, S.K. Targetting cancer with Ru(III/II)-phosphodiesterase inhibitor adducts: A novel approach in the treatment of cancer. Med. Hypotheses 2013, 80, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zheng, W.; Luo, Q.; Li, X.; Zhao, Y.; Xiong, S.; Wang, F. Transferrin serves as a mediator to deliver organometallic ruthenium(II) anticancer complexes into cells. Inorg. Chem. 2013, 52, 5328–5338. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, C.Y.; Nam, T.G. Ruthenium Complexes as Anticancer Agents: A Brief History and Perspectives. Drug Des. Dev. Ther. 2020, 14, 5375–5392. [Google Scholar] [CrossRef]
- Jablonska-Wawrzycka, A.; Rogala, P.; Michalkiewicz, S.; Hodorowicz, M.; Barszcz, B. Ruthenium complexes in different oxidation states: Synthesis, crystal structure, spectra and redox properties. Dalton Trans. 2013, 42, 6092–6101. [Google Scholar] [CrossRef]
- Suss-Fink, G. Arene ruthenium complexes as anticancer agents. Dalton Trans. 2010, 39, 1673–1688. [Google Scholar] [CrossRef]
- Hairat, S.; Zaki, M. Half sandwiched Ruthenium(II) complexes: En Route towards the targeted delivery by Human Serum Albumin (HSA). J. Organomet. Chem. 2021, 937, 121732, Corrigendum in 2021, 943, 121734. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, D.; Gui, L.; Li, Y.; Wang, W.; Liu, J.; Wang, Y. A dual-targeting ruthenium nanodrug that inhibits primary tumor growth and lung metastasis via the PARP/ATM pathway. J. Nanobiotechnol. 2021, 19, 115. [Google Scholar] [CrossRef]
- Lin, K.; Zhao, Z.Z.; Bo, H.B.; Hao, X.J.; Wang, J.Q. Applications of Ruthenium Complex in Tumor Diagnosis and Therapy. Front. Pharmacol. 2018, 9, 1323. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Lai, H.; Xiong, Z.; Chen, B.; Chen, T. Functionalization and cancer-targeting design of ruthenium complexes for precise cancer therapy. Chem. Commun. 2019, 55, 9904–9914. [Google Scholar] [CrossRef]
- Jakubaszek, M.; Goud, B.; Ferrari, S.; Gasser, G. Mechanisms of action of Ru(ii) polypyridyl complexes in living cells upon light irradiation. Chem. Commun. 2018, 54, 13040–13059. [Google Scholar] [CrossRef] [Green Version]
- Mede, T.; Jager, M.; Schubert, U.S. “Chemistry-on-the-complex”: Functional Ru(II) polypyridyl-type sensitizers as divergent building blocks. Chem. Soc. Rev. 2018, 47, 7577–7627. [Google Scholar] [CrossRef]
- Oliveira, K.M.; Honorato, J.; Goncalves, G.R.; Cominetti, M.R.; Batista, A.A.; Correa, R.S. Ru(II)/diclofenac-based complexes: DNA, BSA interaction and their anticancer evaluation against lung and breast tumor cells. Dalton Trans. 2020, 49, 12643–12652. [Google Scholar] [CrossRef]
- Rilak Simović, A.; Masnikosa, R.; Bratsos, I.; Alessio, E. Chemistry and reactivity of ruthenium(II) complexes: DNA/protein binding mode and anticancer activity are related to the complex structure. Coord. Chem. Rev. 2019, 398, 113011. [Google Scholar] [CrossRef]
- Mari, C.; Gasser, G. Lightening up Ruthenium Complexes to Fight Cancer? Chimia 2015, 69, 176–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Nat. Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carruth, J.A. Clinical applications of photodynamic therapy. Int. J. Clin. Pract. 1998, 52, 39–42. [Google Scholar] [CrossRef]
- Thota, S.; Rodrigues, D.A.; Crans, D.C.; Barreiro, E.J. Ru(II) Compounds: Next-Generation Anticancer Metallotherapeutics? J. Med. Chem. 2018, 61, 5805–5821. [Google Scholar] [CrossRef]
- Alessio, E.; Messori, L. NAMI-A and KP1019/1339, Two Iconic Ruthenium Anticancer Drug Candidates Face-to-Face: A Case Story in Medicinal Inorganic Chemistry. Molecules 2019, 24, 1995. [Google Scholar] [CrossRef] [Green Version]
- Alessio, E. Thirty Years of the Drug Candidate NAMI-A and the Myths in the Field of Ruthenium Anticancer Compounds: A Personal Perspective. Eur. J. Inorg. Chem. 2017, 1549–1560. [Google Scholar] [CrossRef]
- Coverdale, J.; Laroiya-McCarron, T.; Romero-Canelón, I. Designing Ruthenium Anticancer Drugs: What Have We Learnt from the Key Drug Candidates? Inorganics 2019, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Leijen, S.; Burgers, S.A.; Baas, P.; Pluim, D.; Tibben, M.; van Werkhoven, E.; Alessio, E.; Sava, G.; Beijnen, J.H.; Schellens, J.H. Phase I/II study with ruthenium compound NAMI-A and gemcitabine in patients with non-small cell lung cancer after first line therapy. Investig. New Drugs 2015, 33, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Pluim, D.; van Waardenburg, R.C.; Beijnen, J.H.; Schellens, J.H. Cytotoxicity of the organic ruthenium anticancer drug Nami-A is correlated with DNA binding in four different human tumor cell lines. Cancer Chemother. Pharmacol. 2004, 54, 71–78. [Google Scholar] [CrossRef]
- Hartinger, C.G.; Jakupec, M.A.; Zorbas-Seifried, S.; Groessl, M.; Egger, A.; Berger, W.; Zorbas, H.; Dyson, P.J.; Keppler, B.K. KP1019, a new redox-active anticancer agent--preclinical development and results of a clinical phase I study in tumor patients. Chem. Biodivers. 2008, 5, 2140–2155. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, C.G.; Zorbas-Seifried, S.; Jakupec, M.A.; Kynast, B.; Zorbas, H.; Keppler, B.K. From bench to bedside--preclinical and early clinical development of the anticancer agent indazolium trans-[tetrachlorobis(1H-indazole)ruthenate(III)] (KP1019 or FFC14A). J. Inorg. Biochem. 2006, 100, 891–904. [Google Scholar] [CrossRef]
- Jakupec, M.A.; Arion, V.B.; Kapitza, S.; Reisner, E.; Eichinger, A.; Pongratz, M.; Marian, B.; Graf von Keyserlingk, N.; Keppler, B.K. KP1019 (FFC14A) from bench to bedside: Preclinical and early clinical development—An overview. Int. J. Clin. Pharmacol. Ther. 2005, 43, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Lentz, F.; Drescher, A.; Lindauer, A.; Henke, M.; Hilger, R.A.; Hartinger, C.G.; Scheulen, M.E.; Dittrich, C.; Keppler, B.K.; Jaehde, U.; et al. Pharmacokinetics of a novel anticancer ruthenium complex (KP1019, FFC14A) in a phase I dose-escalation study. Anticancer Drugs 2009, 20, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kapitza, S.; Pongratz, M.; Jakupec, M.A.; Heffeter, P.; Berger, W.; Lackinger, L.; Keppler, B.K.; Marian, B. Heterocyclic complexes of ruthenium(III) induce apoptosis in colorectal carcinoma cells. J. Cancer Res. Clin. Oncol. 2005, 131, 101–110. [Google Scholar] [CrossRef]
- Biersack, B.; Schobert, R. Current State of Metal-Based Drugs for the Efficient Therapy of Lung Cancers and Lung Metastases. Adv. Exp. Med. Biol. 2016, 893, 211–224. [Google Scholar]
- Alessio, E.; Mestroni, G.; Bergamo, A.; Sava, G. Ruthenium antimetastatic agents. Curr. Top. Med. Chem. 2004, 4, 1525–1535. [Google Scholar] [CrossRef]
- Zheng, K.; Wu, Q.; Wang, C.; Tan, W.; Mei, W. Ruthenium(II) Complexes as Potential Apoptosis Inducers in Chemotherapy. Anticancer Agents Med. Chem. 2017, 17, 29–39. [Google Scholar]
- Sun, R.W.; Ng, M.F.; Wong, E.L.; Zhang, J.; Chui, S.S.; Shek, L.; Lau, T.C.; Che, C.M. Dual anti-angiogenic and cytotoxic properties of ruthenium(III) complexes containing pyrazolato and/or pyrazole ligands. Dalton Trans. 2009, 48, 10712–10716. [Google Scholar] [CrossRef]
- Riccardi, C.; Musumeci, D.; Irace, C.; Paduano, L.; Montesarchio, D. RuIIIComplexes for Anticancer Therapy: The Importance of Being Nucleolipidic. Eur. J. Org. Chem. 2017, 2017, 1100–1119. [Google Scholar] [CrossRef]
- Riccardi, C.; Musumeci, D.; Trifuoggi, M.; Irace, C.; Paduano, L.; Montesarchio, D. Anticancer Ruthenium(III) Complexes and Ru(III)-Containing Nanoformulations: An Update on the Mechanism of Action and Biological Activity. Pharmaceuticals 2019, 12, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.S. The development of anticancer ruthenium(ii) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Raza, M.K.; Dar, O.A.; Amadudin, A.; Abid, M.; Wani, M.Y.; Al-Bogami, A.S.; Hashmi, A.A. Probing the antibacterial and anticancer potential of tryptamine based mixed ligand Schiff base Ruthenium(III) complexes. Bioorg. Chem. 2019, 87, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Richert, M.; Walczyk, M.; Cieslak, M.J.; Kazmierczak-Baranska, J.; Krolewska-Golinska, K.; Wrzeszcz, G.; Muziol, T.; Biniak, S. Synthesis, X-ray structure, physicochemical properties and anticancer activity of mer and fac Ru(iii) triphenylphosphine complexes with a benzothiazole derivative as a co-ligand. Dalton Trans. 2019, 48, 10689–10702. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, E.; Kalaiselvi, S.; Padma, V.V.; Bhuvanesh, N.S.; Dharmaraj, N. Solvent assisted formation of ruthenium(III) and ruthenium(II) hydrazone complexes in one-pot with potential in vitro cytotoxicity and enhanced LDH, NO and ROS release. Dalton Trans. 2016, 45, 1693–1707. [Google Scholar] [CrossRef]
- Gu, L.; Li, X.; Ran, Q.; Kang, C.; Lee, C.; Shen, J. Antimetastatic activity of novel ruthenium (III) pyridine complexes. Cancer Med. 2016, 5, 2850–2860. [Google Scholar] [CrossRef] [PubMed]
- Lomzik, M.; Mazuryk, O.; Rutkowska-Zbik, D.; Stochel, G.; Gros, P.C.; Brindell, M. New ruthenium compounds bearing semicarbazone 2-formylopyridine moiety: Playing with auxiliary ligands for tuning the mechanism of biological activity. J. Inorg. Biochem. 2017, 175, 80–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurgul, I.; Mazuryk, O.; Lomzik, M.; Gros, P.C.; Rutkowska-Zbik, D.; Brindell, M. Unexplored features of Ru(ii) polypyridyl complexes towards combined cytotoxic and antimetastatic activity. Metallomics 2020, 12, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.S.; Goncalves, Y.G.; Borges, B.C.; Silva, M.J.B.; Amstalden, M.K.; Costa, T.R.; Antunes, L.M.G.; Rodrigues, R.S.; Rodrigues, V.M.; de Faria Franca, E.; et al. Ruthenium (II) complex cis-[Ru(II)(ŋ(2)-O2CC7H7O2)(dppm)2]PF6-hmxbato induces ROS-mediated apoptosis in lung tumor cells producing selective cytotoxicity. Sci. Rep. 2020, 10, 15410. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Deng, Y.; Wang, T.; Miao, T.; Li, C.; Cai, X.; Liu, Y.; Henri, J.; Chen, L. Ru(II) Complexes Bearing O, O-Chelated Ligands Induced Apoptosis in A549 Cells through the Mitochondrial Apoptotic Pathway. Bioinorg. Chem. Appl. 2020, 2020, 8890950. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.H.; Jiang, G.B.; Yao, J.H.; Li, W.; Han, B.J.; Zhang, C.; Zeng, C.C.; Liu, Y.J. Cytotoxic activity, DNA damage, cellular uptake, apoptosis and western blot analysis of ruthenium(II) polypyridyl complex against human lung decarcinoma A549 cell. J. Inorg. Biochem. 2015, 152, 1–9. [Google Scholar] [CrossRef]
- Fayad, C.; Audi, H.; Khnayzer, R.S.; Daher, C.F. The anti-cancer effect of series of strained photoactivatable Ru(II) polypyridyl complexes on non-small-cell lung cancer and triple negative breast cancer cells. J. Biol. Inorg. Chem. 2021, 26, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Peng, F.; Li, G.D.; Jie, X.M.; Cai, K.R.; Cai, C.; Zhong, Y.; Zeng, H.; Li, W.; Zhang, Z.; et al. The studies on the cytotoxicity in vitro, cellular uptake, cell cycle arrest and apoptosis-inducing properties of ruthenium methylimidazole complex [Ru(MeIm)4(p-cpip)](2.). J. Inorg. Biochem. 2016, 156, 64–74. [Google Scholar] [CrossRef]
- Chen, L.; Li, G.; Peng, F.; Jie, X.; Dongye, G.; Cai, K.; Feng, R.; Li, B.; Zeng, Q.; Lun, K.; et al. The induction of autophagy against mitochondria-mediated apoptosis in lung cancer cells by a ruthenium (II) imidazole complex. Oncotarget 2016, 7, 80716–80734. [Google Scholar] [CrossRef] [Green Version]
- Xiong, K.; Qian, C.; Yuan, Y.; Wei, L.; Liao, X.; He, L.; Rees, T.W.; Chen, Y.; Wan, J.; Ji, L.; et al. Necroptosis Induced by Ruthenium(II) Complexes as Dual Catalytic Inhibitors of Topoisomerase I/II. Angew. Chem. Int. Ed. Engl. 2020, 59, 16631–16637. [Google Scholar] [CrossRef]
- Mazuryk, O.; Suzenet, F.; Kieda, C.; Brindell, M. The biological effect of the nitroimidazole derivative of a polypyridyl ruthenium complex on cancer and endothelial cells. Metallomics 2015, 7, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.C.; Kljun, J.; Turel, I.; Di Virgilio, A.L.; Leon, I.E. Comparative antitumor studies of organoruthenium complexes with 8-hydroxyquinolines on 2D and 3D cell models of bone, lung and breast cancer. Metallomics 2019, 11, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Silva, G.A.; Ortega, M.M.; Banionis, M.A.; Garavelli, G.Y.; Martins, F.T.; Dias, J.S.M.; Viegas, C., Jr.; Oliveira, J.C.; Nascimento, F.B.D.; Doriguetto, A.C.; et al. [Ru(pipe)(dppb)(bipy)]PF6: A novel ruthenium complex that effectively inhibits ERK activation and cyclin D1 expression in A549 cells. Toxicol. In Vitro 2017, 44, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Sathiya Kamatchi, T.; Mohamed Subarkhan, M.K.; Ramesh, R.; Wang, H.; Malecki, J.G. Investigation into antiproliferative activity and apoptosis mechanism of new arene Ru(ii) carbazole-based hydrazone complexes. Dalton Trans. 2020, 49, 11385–11395. [Google Scholar] [CrossRef]
- Rademaker-Lakhai, J.M.; van den Bongard, D.; Pluim, D.; Beijnen, J.H.; Schellens, J.H. A Phase I and pharmacological study with imidazolium-trans-DMSO-imidazole-tetrachlororuthenate, a novel ruthenium anticancer agent. Clin. Cancer Res. 2004, 10, 3717–3727. [Google Scholar] [CrossRef] [Green Version]
- Fleisher, T.A. Apoptosis. Ann. Allergy Asthma Immunol. 1997, 78, 245–249; quiz 249–250. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [Green Version]
- Tait, S.W.; Green, D.R. Caspase-independent cell death: Leaving the set without the final cut. Oncogene 2008, 27, 6452–6461. [Google Scholar] [CrossRef] [Green Version]
- Domotor, O.; de Almeida, R.F.; Corte-Real, L.; Matos, C.P.; Marques, F.; Matos, A.; Real, C.; Kiss, T.; Enyedy, E.A.; Helena Garcia, M.; et al. Studies on the mechanism of action of antitumor bis(aminophenolate) ruthenium(III) complexes. J. Inorg. Biochem. 2017, 168, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.P.; Massague, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wu, Y.; Guo, J.; Fei, X.; Yu, L.; Ma, S. Adipocyte-derived exosomes promote lung cancer metastasis by increasing MMP9 activity via transferring MMP3 to lung cancer cells. Oncotarget 2017, 8, 81880–81891. [Google Scholar] [CrossRef]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Sambi, M.; Qorri, B.; Harless, W.; Szewczuk, M.R. Therapeutic Options for Metastatic Breast Cancer. Adv. Exp. Med. Biol. 2019, 1152, 131–172. [Google Scholar]
- Sava, G.; Bergamo, A. Drug control of solid tumour metastases: A critical view. Anticancer Res. 1999, 19, 1117–1124. [Google Scholar] [PubMed]
- Sava, G.; Clerici, K.; Capozzi, I.; Cocchietto, M.; Gagliardi, R.; Alessio, E.; Mestroni, G.; Perbellini, A. Reduction of lung metastasis by ImH[trans-RuCl4(DMSO)Im]: Mechanism of the selective action investigated on mouse tumors. Anticancer Drugs 1999, 10, 129–138. [Google Scholar] [CrossRef]
- Bergamo, A.; Gava, B.; Alessio, E.; Mestroni, G.; Serli, B.; Cocchietto, M.; Zorzet, S.; Sava, G. Ruthenium-based NAMI-A type complexes with in vivo selective metastasis reduction and in vitro invasion inhibition unrelated to cell cytotoxicity. Int. J. Oncol. 2002, 21, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Tanski, J.M.; Tibbetts, M.F.; Anderson, C.M. Synthesis, characterization, and in vitro evaluation of a potentially selective anticancer, mixed-metal [ruthenium(III)-platinum(II)] trinuclear complex. Inorg. Chem. 2008, 47, 274–280. [Google Scholar] [CrossRef]
- Anderson, C.M.; Taylor, I.R.; Tibbetts, M.F.; Philpott, J.; Hu, Y.; Tanski, J.M. Hetero-multinuclear ruthenium(III)/platinum(II) complexes that potentially exhibit both antimetastatic and antineoplastic properties. Inorg. Chem. 2012, 51, 12917–12924. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lim, Z.J.; Gwee, Y.Y.; Levina, A.; Lay, P.A. Characterization of a ruthenium(III)/NAMI-A adduct with bovine serum albumin that exhibits a high anti-metastatic activity. Angew. Chem. Int. Ed. Engl. 2010, 49, 1661–1664. [Google Scholar] [CrossRef]
- Biancalana, L.; Pampaloni, G.; Marchetti, F. Arene Ruthenium(II) Complexes with Phosphorous Ligands as Possible Anticancer Agents. Chimia 2017, 71, 573–579. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kundu, S.; Bhattacharyya, A.; Hartinger, C.G.; Dyson, P.J. The ruthenium(II)-arene compound RAPTA-C induces apoptosis in EAC cells through mitochondrial and p53-JNK pathways. J. Biol. Inorg. Chem. 2008, 13, 1149–1155. [Google Scholar] [CrossRef]
- Bhatti, M.Z.; Ali, A.; Duong, H.Q.; Chen, J.; Rahman, F.U. Anticancer activity and mechanism of bis-pyrimidine based dimetallic Ru(II)(eta(6)-p-cymene) complex in human non-small cell lung cancer via p53-dependent pathway. J. Inorg. Biochem. 2019, 194, 52–64. [Google Scholar] [CrossRef]
- Sachdeva, M.; Zhu, S.; Wu, F.; Wu, H.; Walia, V.; Kumar, S.; Elble, R.; Watabe, K.; Mo, Y.Y. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc. Natl. Acad. Sci. USA 2009, 106, 3207–3212. [Google Scholar] [CrossRef] [Green Version]
- Balaji, S.; Mohamed Subarkhan, M.K.; Ramesh, R.; Wang, H.; Semeril, D. Synthesis and Structure of Arene Ru(II) N∧O-Chelating Complexes: In Vitro Cytotoxicity and Cancer Cell Death Mechanism. Organometallics 2020, 39, 1366–1375. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.Y.; Li, S.; Wang, F.X.; Li, J.; Qian, Y.; Su, Z.; Mao, Z.W.; Sadler, P.J.; Liu, H.K. Rigid dinuclear ruthenium-arene complexes showing strong DNA interactions. J. Inorg. Biochem. 2018, 189, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Subarkhan, M.K.; Ren, L.; Xie, B.; Chen, C.; Wang, Y.; Wang, H. Novel tetranuclear ruthenium(II) arene complexes showing potent cytotoxic and antimetastatic activity as well as low toxicity in vivo. Eur. J. Med. Chem. 2019, 179, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.G.; Belisario, D.C.; Fontrodona, X.; Romero, I.; Tomaz, A.I.; Garcia, M.H.; Riganti, C.; Valente, A. Unprecedented collateral sensitivity for cisplatin-resistant lung cancer cells presented by new ruthenium organometallic compounds. Inorg. Chem. Front. 2021, 8, 1983–1996. [Google Scholar] [CrossRef]
- Yang, X.; Chen, L.; Liu, Y.; Yang, Y.; Chen, T.; Zheng, W.; Liu, J.; He, Q.Y. Ruthenium methylimidazole complexes induced apoptosis in lung cancer A549 cells through intrinsic mitochondrial pathway. Biochimie 2012, 94, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, X.; Tang, M.; Li, L.; Lei, Y.; Cheng, P.; Guo, W.; Zheng, Y.; Wang, W.; Luo, N.; et al. The role of ROS and subsequent DNA-damage response in PUMA-induced apoptosis of ovarian cancer cells. Oncotarget 2017, 8, 23492–23506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Costa, M.S.; Goncalves, Y.G.; Teixeira, S.C.; Nunes, D.C.O.; Lopes, D.S.; da Silva, C.V.; da Silva, M.S.; Borges, B.C.; Silva, M.J.B.; Rodrigues, R.S.; et al. Increased ROS generation causes apoptosis-like death: Mechanistic insights into the anti-Leishmania activity of a potent ruthenium(II) complex. J. Inorg. Biochem. 2019, 195, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.S.; Goncalves, Y.G.; Nunes, D.C.O.; Napolitano, D.R.; Maia, P.I.S.; Rodrigues, R.S.; Rodrigues, V.M.; Von Poelhsitz, G.; Yoneyama, K.A.G. Anti-Leishmania activity of new ruthenium(II) complexes: Effect on parasite-host interaction. J. Inorg. Biochem. 2017, 175, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef]
- Han, J.Y.; Hong, E.K.; Choi, B.G.; Park, J.N.; Kim, K.W.; Kang, J.H.; Jin, J.Y.; Park, S.Y.; Hong, Y.S.; Lee, K.S. Death receptor 5 and Bcl-2 protein expression as predictors of tumor response to gemcitabine and cisplatin in patients with advanced non-small-cell lung cancer. Med. Oncol. 2003, 20, 355–362. [Google Scholar] [CrossRef]
- Riccardi, C.; Musumeci, D.; Capuozzo, A.; Irace, C.; King, S.; Russo Krauss, I.; Paduano, L.; Montesarchio, D. “Dressing up” an Old Drug: An Aminoacyl Lipid for the Functionalization of Ru(III)-Based Anticancer Agents. ACS Biomater. Sci. Eng. 2018, 4, 163–174. [Google Scholar] [CrossRef]
- Webb, M.I.; Chard, R.A.; Al-Jobory, Y.M.; Jones, M.R.; Wong, E.W.; Walsby, C.J. Pyridine analogues of the antimetastatic Ru(III) complex NAMI-A targeting non-covalent interactions with albumin. Inorg. Chem. 2012, 51, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Onorati, A.V.; Dyczynski, M.; Ojha, R.; Amaravadi, R.K. Targeting autophagy in cancer. Cancer 2018, 124, 3307–3318. [Google Scholar] [CrossRef] [Green Version]
- Kocaturk, N.M.; Akkoc, Y.; Kig, C.; Bayraktar, O.; Gozuacik, D.; Kutlu, O. Autophagy as a molecular target for cancer treatment. Eur. J. Pharm. Sci. 2019, 134, 116–137. [Google Scholar] [CrossRef]
- Li, Y.J.; Lei, Y.H.; Yao, N.; Wang, C.R.; Hu, N.; Ye, W.C.; Zhang, D.M.; Chen, Z.S. Autophagy and multidrug resistance in cancer. Chin. J. Cancer 2017, 36, 52. [Google Scholar] [CrossRef]
- Antonaci, G.; Cossa, L.G.; Muscella, A.; Vetrugno, C.; De Pascali, S.A.; Fanizzi, F.P.; Marsigliante, S. [Pt(O,O’-acac)(gamma-acac)(DMS)] Induces Autophagy in Caki-1 Renal Cancer Cells. Biomolecules 2019, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimaldi, M.; Bo, V.D.; Ferrari, B.; Roda, E.; De Luca, F.; Veneroni, P.; Barni, S.; Verri, M.; De Pascali, S.A.; Fanizzi, F.P.; et al. Long-term effects after treatment with platinum compounds, cisplatin and [Pt(O,O’-acac)(gamma-acac)(DMS)]: Autophagy activation in rat B50 neuroblastoma cells. Toxicol. Appl. Pharmacol. 2019, 364, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, M.; Misso, G.; Ferraro, M.G.; Riccardi, C.; Capuozzo, A.; Zarone, M.R.; Maione, F.; Trifuoggi, M.; Stiuso, P.; D’Errico, G.; et al. Exploring cellular uptake, accumulation and mechanism of action of a cationic Ru-based nanosystem in human preclinical models of breast cancer. Sci. Rep. 2019, 9, 7006. [Google Scholar] [CrossRef] [Green Version]
- Irace, C.; Misso, G.; Capuozzo, A.; Piccolo, M.; Riccardi, C.; Luchini, A.; Caraglia, M.; Paduano, L.; Montesarchio, D.; Santamaria, R. Antiproliferative effects of ruthenium-based nucleolipidic nanoaggregates in human models of breast cancer in vitro: Insights into their mode of action. Sci. Rep. 2017, 7, 45236. [Google Scholar] [CrossRef] [Green Version]
- Montel, A.M.; Dos Santos, R.G.; da Costa, P.R.; Silveira-Lacerda, E.P.; Batista, A.A.; Dos Santos, W.G. Neutron activation increases activity of ruthenium-based complexes and induces cell death in glioma cells independent of p53 tumor suppressor gene. Biometals 2017, 30, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manda, S.; Lee, N.K.; Oh, D.C.; Lee, J. Design, Synthesis, and Biological Evaluation of Proteolysis Targeting Chimeras (PROTACs) for the Dual Degradation of IGF-1R and Src. Molecules 2020, 25, 1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Kepp, O.; Chan, F.K.; Kroemer, G. Necroptosis: Mechanisms and Relevance to Disease. Annu. Rev. Pathol. 2017, 12, 103–130. [Google Scholar] [CrossRef] [PubMed]
- Sharapova, T.N.; Romanova, E.A.; Sashchenko, L.P.; Yashin, D.V. FasL on the surface of Tag7 (PGRP-S)-activated lymphocytes induces necroptosis in HLA-negative tumor cells with the involvement of lysosomes and mitochondria. Biochimie 2018, 152, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef] [Green Version]
- Suntharalingam, K.; Awuah, S.G.; Bruno, P.M.; Johnstone, T.C.; Wang, F.; Lin, W.; Zheng, Y.R.; Page, J.E.; Hemann, M.T.; Lippard, S.J. Necroptosis-inducing rhenium(V) oxo complexes. J. Am. Chem. Soc. 2015, 137, 2967–2974. [Google Scholar] [CrossRef] [Green Version]
- Flamme, M.; Cressey, P.B.; Lu, C.; Bruno, P.M.; Eskandari, A.; Hemann, M.T.; Hogarth, G.; Suntharalingam, K. Induction of Necroptosis in Cancer Stem Cells using a Nickel(II)-Dithiocarbamate Phenanthroline Complex. Chemistry 2017, 23, 9674–9682. [Google Scholar] [CrossRef] [Green Version]
- Champoux, J.J. DNA topoisomerases: Structure, function, and mechanism. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, K.; Liang, J.; Wang, Y.; Kou, J.; Qian, C.; Ji, L.; Chao, H. Dual inhibition of topoisomerases I and IIalpha by ruthenium(II) complexes containing asymmetric tridentate ligands. Dalton Trans. 2014, 43, 17303–17316. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, M.; Gunther, S.D.; Schwarzer, R.; Albert, M.C.; Schorn, F.; Werthenbach, J.P.; Schiffmann, L.M.; Stair, N.; Stocks, H.; Seeger, J.M.; et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 2019, 575, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Mazuryk, O.; Maciuszek, M.; Stochel, G.; Suzenet, F.; Brindell, M. 2-Nitroimidazole-ruthenium polypyridyl complex as a new conjugate for cancer treatment and visualization. J. Inorg. Biochem. 2014, 134, 83–91. [Google Scholar] [CrossRef]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Peerlings, J.; Van De Voorde, L.; Mitea, C.; Larue, R.; Yaromina, A.; Sandeleanu, S.; Spiegelberg, L.; Dubois, L.; Lambin, P.; Mottaghy, F.M. Hypoxia and hypoxia response-associated molecular markers in esophageal cancer: A systematic review. Methods 2017, 130, 51–62. [Google Scholar] [CrossRef]
- Gao, S.W.; Liu, F. Novel insights into cell cycle regulation of cell fate determination. J. Zhejiang Univ. Sci. B 2019, 20, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, M.; La Monica, S.; Fumarola, C.; Alfieri, R. Multiple effects of CDK4/6 inhibition in cancer: From cell cycle arrest to immunomodulation. Biochem. Pharmacol. 2019, 170, 113676. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.C.; Kolb, E.A.; Sampson, V.B. Development of Chemotherapy with Cell-Cycle Inhibitors for Adult and Pediatric Cancer Therapy. Cancer Res. 2018, 78, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.L.; Meng, T.; Chen, Z.F.; Xie, X.L.; Qin, Q.P.; He, X.J.; Huang, K.B.; Liang, H. Facile total synthesis of lysicamine and the anticancer activities of the Ru(II), Rh(III), Mn(II) and Zn(II) complexes of lysicamine. Oncotarget 2017, 8, 59359–59375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
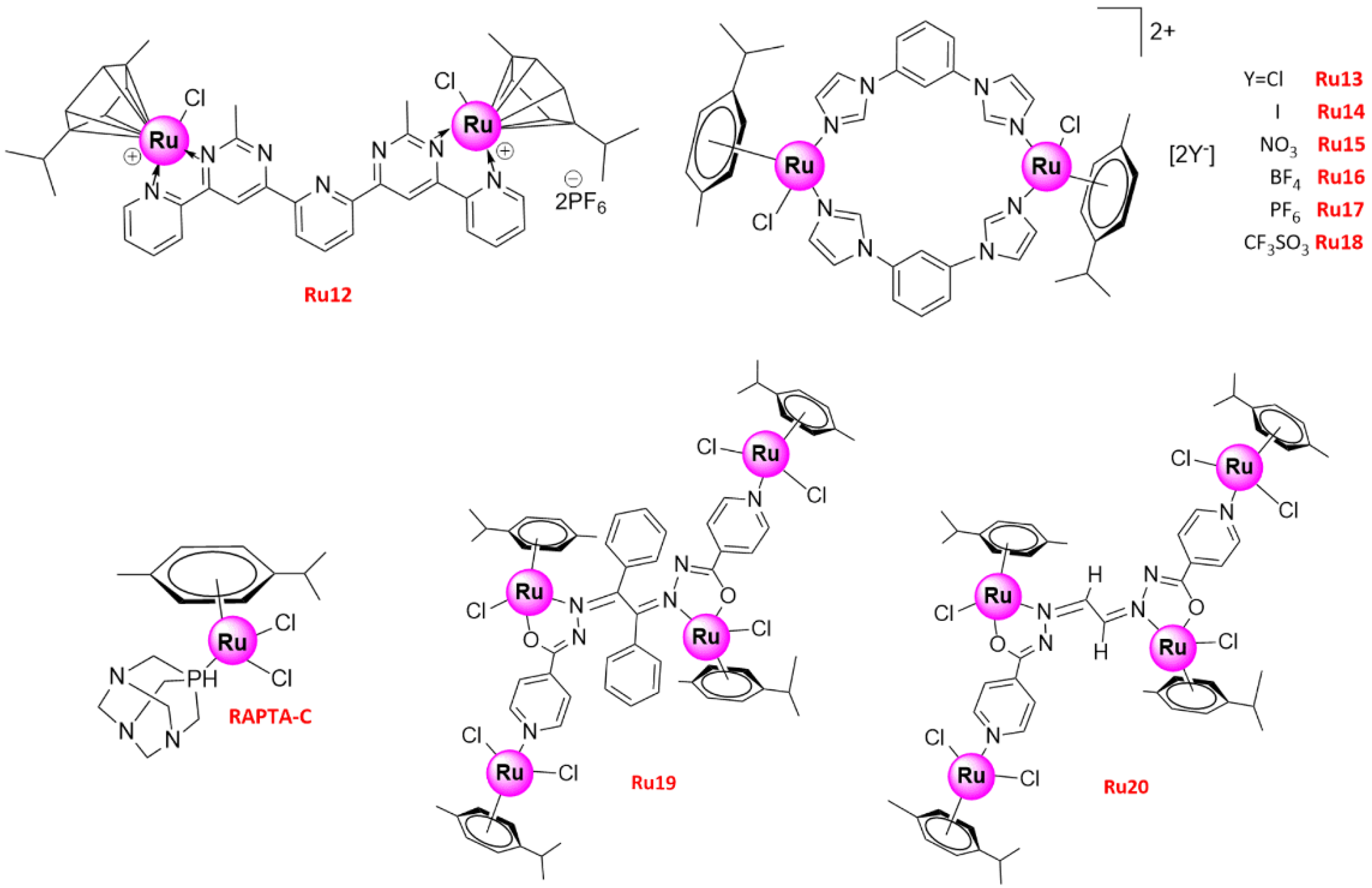
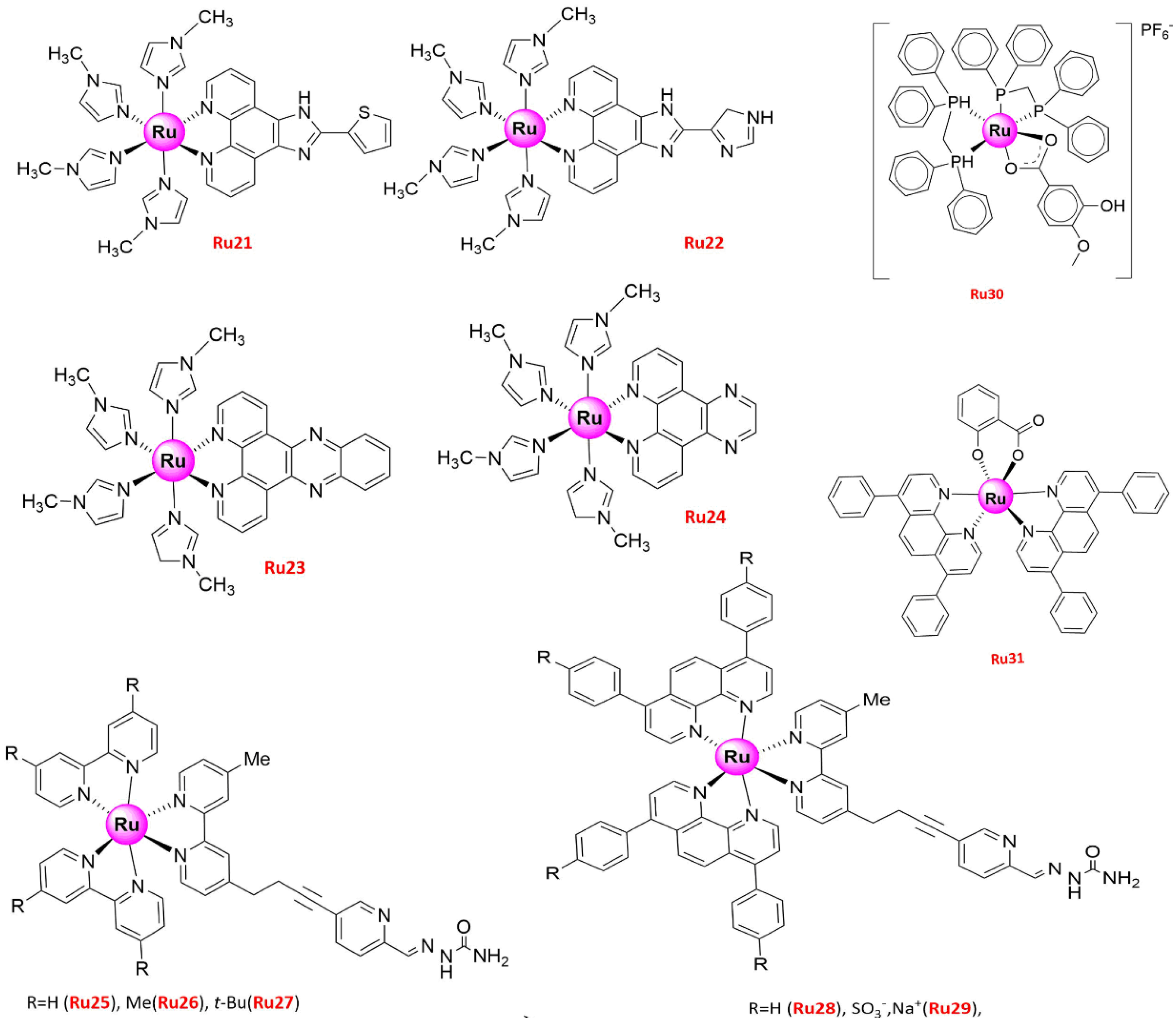
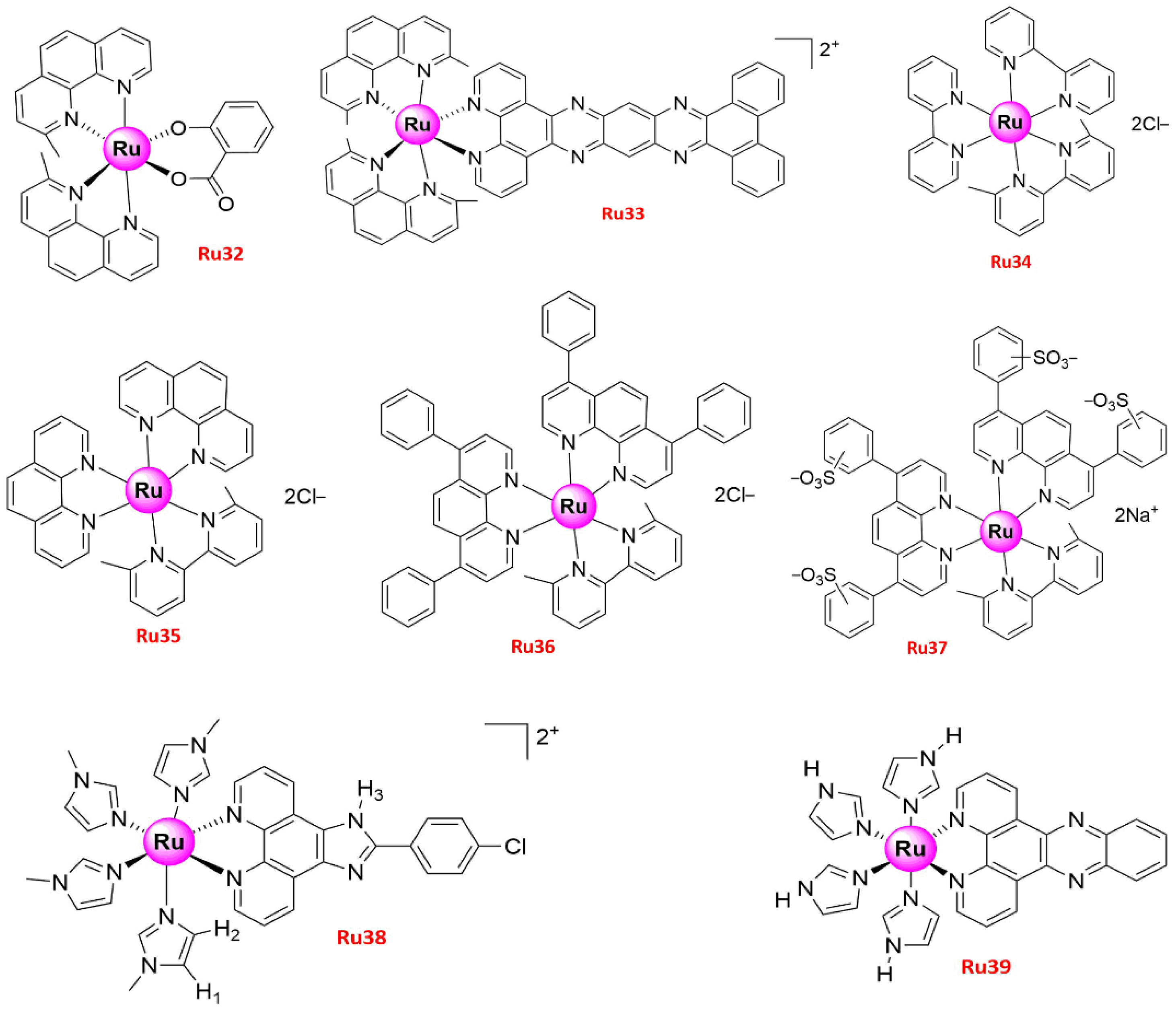
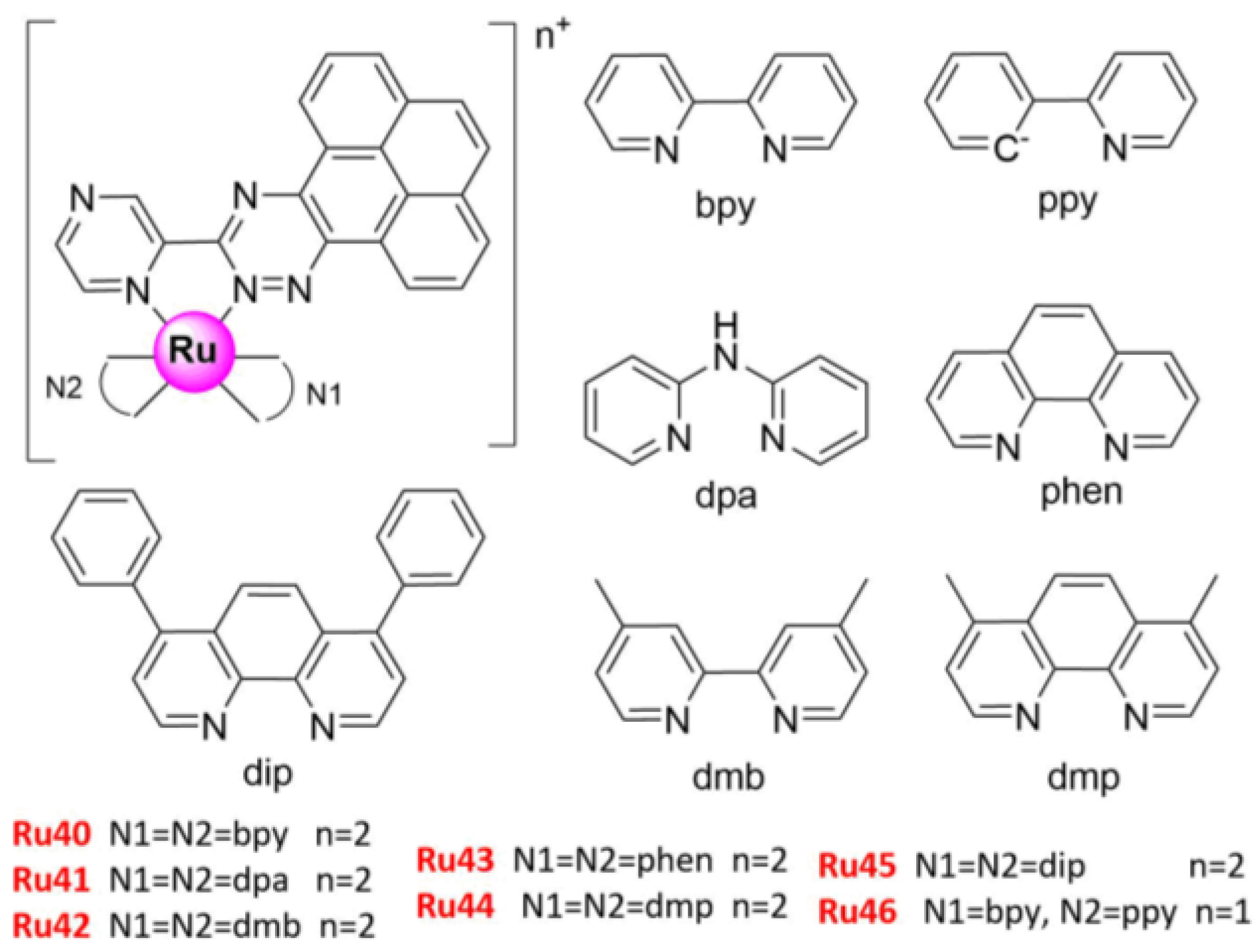
| No. | IC50 (μ/M) | Cell Lines | Biology and Mechanism | Ref. |
|---|---|---|---|---|
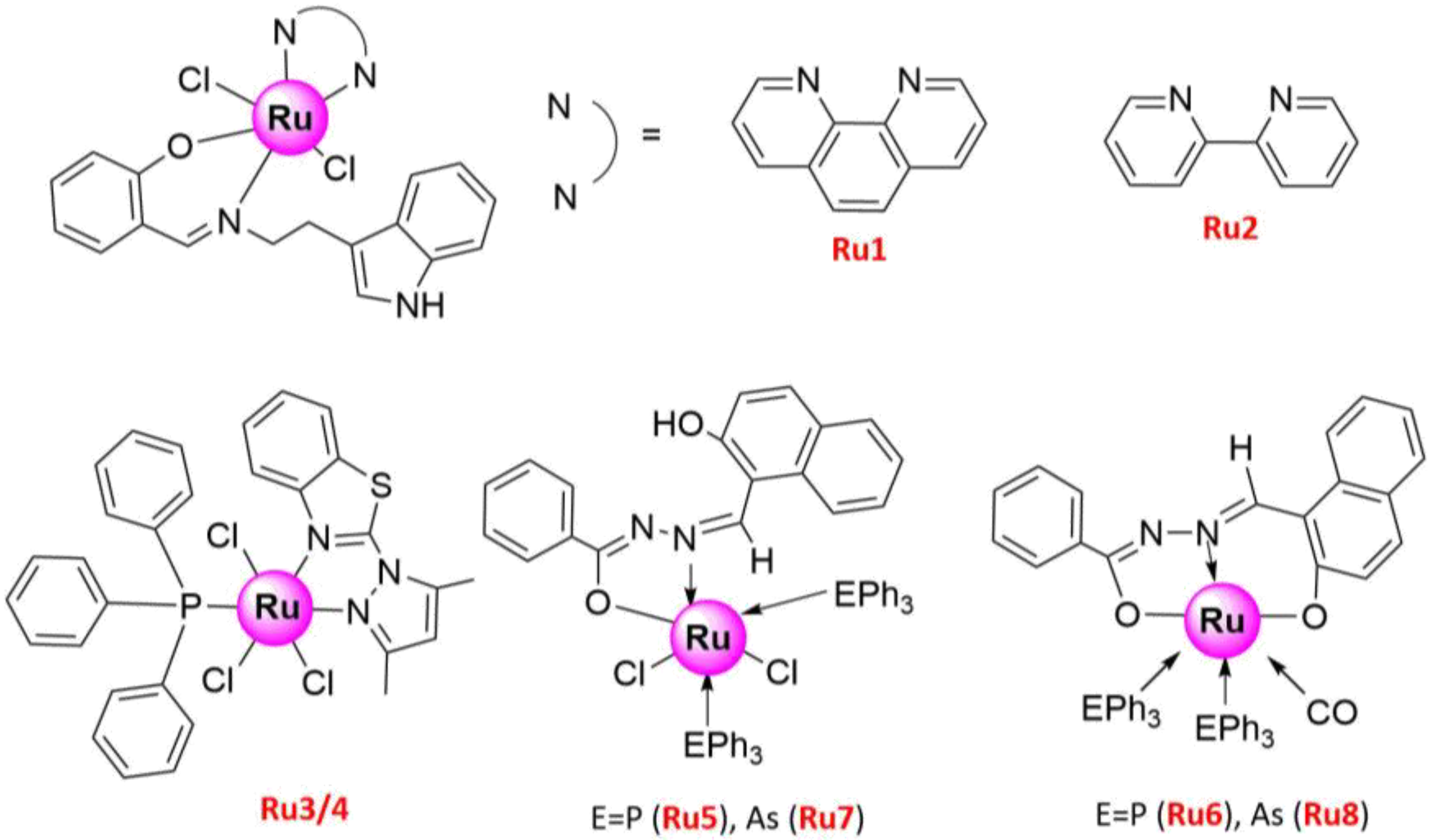
| Ru1 | 10–12.5 ± 0.5 | H1299 | Cytotoxicity | [48] |
| Ru2 | 15–20 ± 0.5 | H1299 | ||
| Ru3 | 30 ± 5 | A549 | (1) Anti-proliferation (2) Pro-apoptosis (3) Caspase 3/7-dependent apoptosis | [49] |
| Ru4 | 5 ± 2.6 | A549 | ||
| Ru5 | 18 ± 0.67 | A549 | (1) Anti-proliferation (2) Pro-apoptosis (3) Enhanced the LDH, NO, and ROS release | [50] |
| Ru6 | 24 ± 1.0 | A549 | ||
| Ru7 | 22 ± 1.17 | A549 | ||
| Ru8 | 24 ± 0.93 | A549 | ||
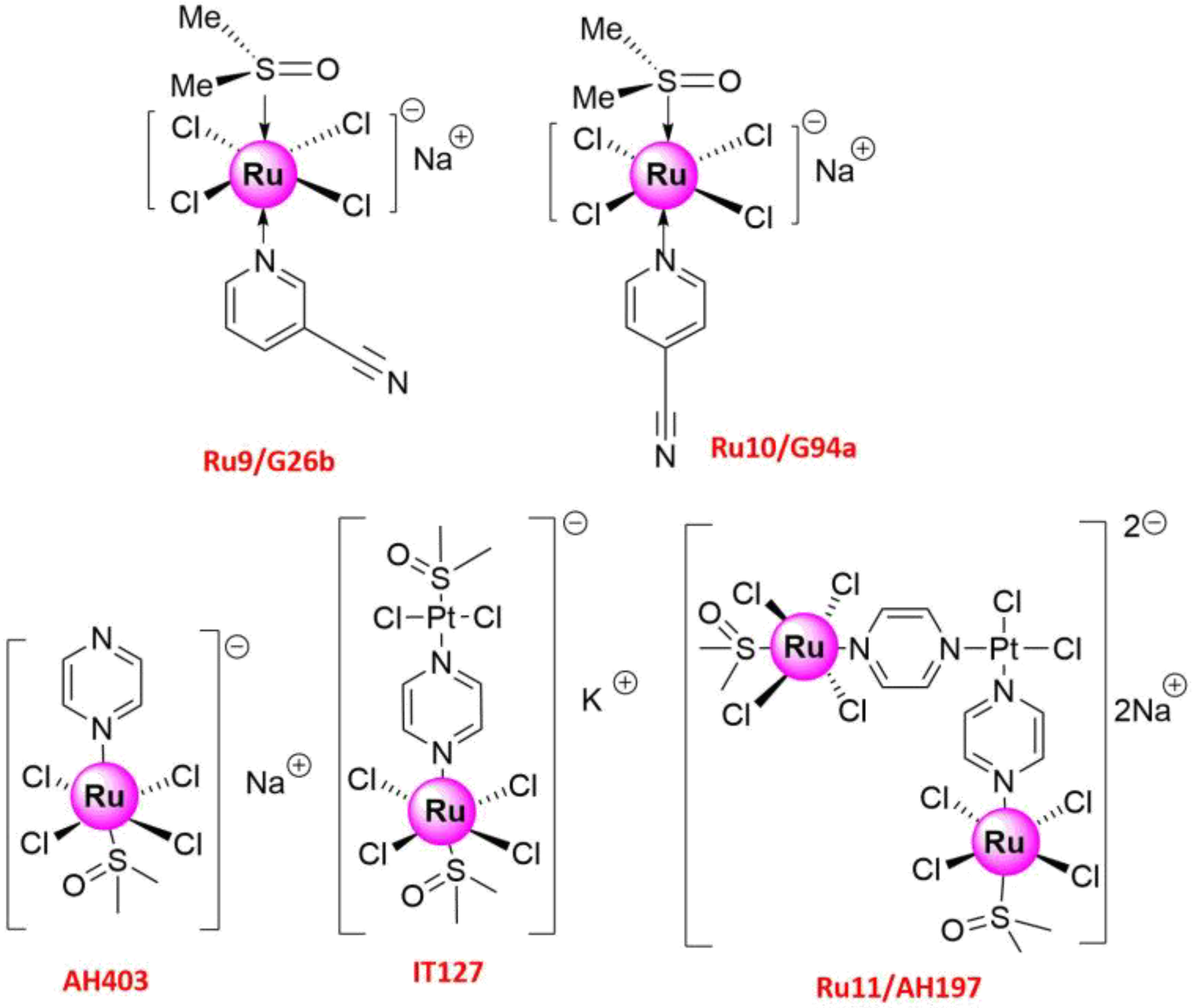
| Ru9/G26b Ru10/G94a | >200 | A549 | (1) Anti-proliferation (2) Anti-metastasis (3) Suppressed MMP-2, MMP-9 and VEGF | [51] |
| Ru11/AH197 | 5.0 | HOP-62 | Anti-metastasis and inhibited cell motility | [51] |
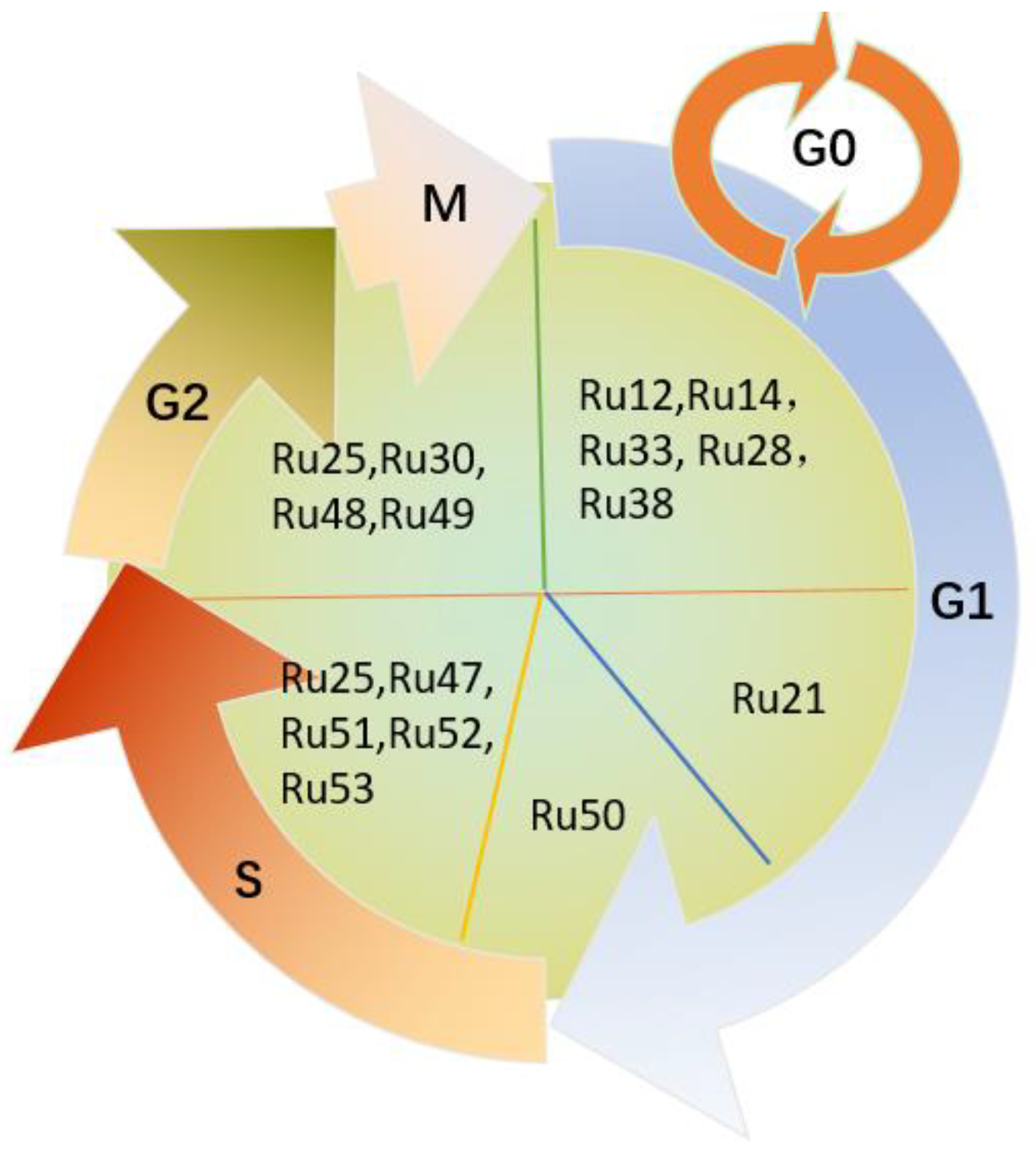
| Ru12 | 0–20 0–20 | A549 A427 | (1) Anti-proliferation (2) Pro-apoptosis (3) G0/G1 phase arrest (4) DNA damage, caspase-dependent apoptosis involving PARP activation and induction of p53-dependent pathway | [51] |
| Ru13 | - | A549 | (1) Anti-proliferation | [51] |
| Ru14 | 30.9 | A549 | (1) Anti-proliferation (2) G0/G1 phase arrest (3) induced late apoptosis | [51] |
| Ru15–18 | - | A549 | (1) Anti-proliferation | [51] |
| Ru19 | 1.39 ± 0.15 3.39 ± 0.47 | A549 A549cisR | (1) Anti-proliferation (2) Induced apoptosis (3) Anti-migration and anti-invasion | [51] |
| Ru20 | 1.41 ± 0.23 5.70 ± 0.33 | A549 A549cisR | (1) Anti-proliferation (2) Induced apoptosis | [51] |
| Ru21 | 17.34 ± 0.42 51.59 ± 2.57 | A549 HBE | (1) Anti-proliferation (2) Pro-apoptosis (3) Generated a peak of apoptosis in sub-G1 phase, via both mitochondrial and death receptor apoptotic pathways | [51] |
| Ru22 | 21.97 ± 2.31 | A549 | Anti-proliferation | [51] |
| Ru23 | 444.38 ± 3.19 | A549 | ||
| Ru24 | 37.62 ± 2.83 | A549 | ||
| Ru25 | >240 | A549 | (1) Anti-proliferation (2) Apoptosis induction (3) S-phase arrest, G2/M phase arrest (4) Anti-metastasis (5) Inhibited MMP2 and MMP9 enzyme activities, intrinsic mitochondrial pathway-triggered apoptosis | [52] |
| Ru26 | 158 ± 15 | A549 | (1) Anti-proliferation (2) Anti-metastasis (3) Inhibited MMP2 and MMP9 enzyme activities | [53] |
| Ru27 | 14.1 ± 0.3 | A549 | ||
| Ru28 | 10.7 ± 0.7 | A549 | (1) Anti-proliferation (2) Anti-metastasis (3) G0/G1 phase arrest (4) Pro-apoptosis (5) Inhibited MMP2 and MMP9 enzyme activities, caspase-independent apoptosis | [52] |
| Ru29 | >240 | A549 | (1) Anti-proliferation (2) Anti-metastasis (3) Inhibited MMP2 and MMP9 enzyme activities | [53] |
| Ru30 | 3.8 (2.3–6.2) 40.3 (22.6–71.7) | A549 BEAS-2B | (1) Anti-proliferation (2) G2/M phase arrest (3) The changes in morphology and organization patterns of the actin cytoskeleton, apoptosis, mitochondrial membrane potential changes and DNA damage result from increased ROS | [54] |
| Ru31 | 11.3 ± 1.1 54.3 ± 3.4 | A549 BEAS-2B | (1) Anti-proliferation (2) Induced apoptosis (3) Through Mitochondrial Apoptotic Pathway, and ROS accumulation, DNA damage | [55] |
| Ru32 | 30.1 ± 1.2 48.1 ± 3.7 | A549 BEAS-2B | ||
| Ru33 | 1.5 ± 0.3 | A549 | (1) Anti-proliferation (2) Induced apoptosis (3) G0/G1 phase arrest (4) Through an intrinsic ROS-mediated mitochondrial dysfunction pathway | [56] |
| Ru34 | 12.94 ± 0.43 (Dark) 13.84 ± 3.57 (Light) | A549 | (1) Anti-proliferation | [57] |
| Ru35 | 17.42 ± 2.39 (Dark) 2.90 ± 0.83 (Light) | A549 | ||
| Ru36 | 6.03 ± 0.89 (Dark) 1.25 ± 0.17 (Light) | A549 | (1) Anti-proliferation (2) Phototoxicity (3) Induced apoptosis (4) ROS production and increased Bax/Bcl2 ratio and PERK levels | [57] |
| Ru37 | 89.30 ± 3.95 (Dark) 21.89 ± 4.53 (Light) | A549 | (1) Anti-proliferation | [57] |
| Ru38 | 18.3 ± 2.7 | A549 | (1) Anti-proliferation (2) Induced apoptosis (3) G0/G1 phase arrest (4) Via the mitochondrial pathway, ROS accumulation, the mitochondrial dysfunction and Bcl-2 and caspase correlative family member activation | [58] |
| Ru39 | 21.24 ± 1.24 23.10 ± 3.2 176.47 ± 13.4 | A549 NCI-H460 HBE | (1) Anti-proliferation (2) Apoptosis and autophagy induction (3) Mitochondrial dysfunction, ROS generation, caspase 3-dependent apoptosis, ERK mediated-autophagy | [59] |
| Ru46 | 3.0 ± 0.1 | A549 | (1) topo I and II inhibitors (2) induced necroptosis, via ROS burst, plasma membrane permeabilization, and cytosolic ATP reduction (3) induced DNA damage, activated PARP1, RIPK1, RIPK3, and MLKL | [60] |
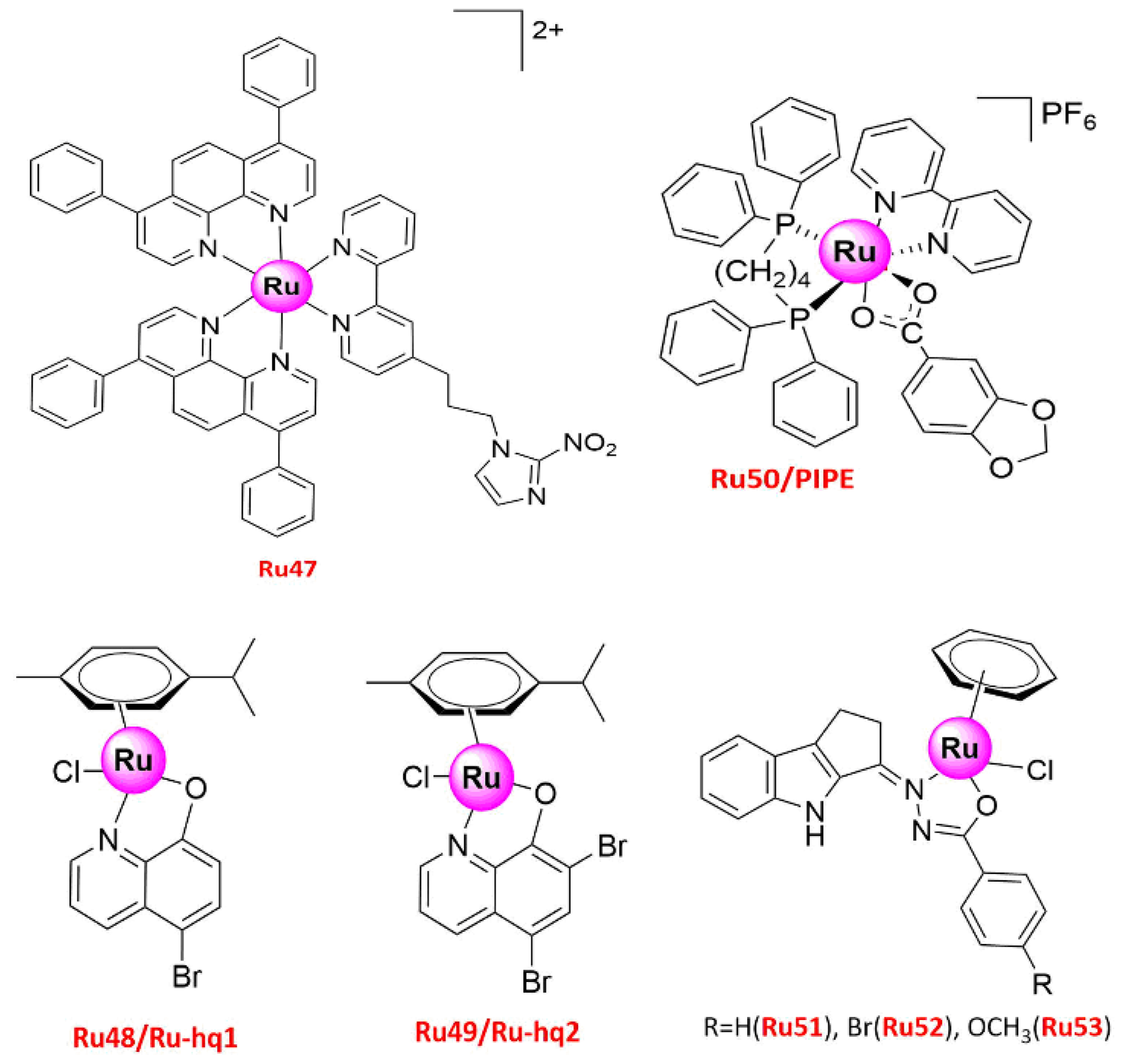
| Ru47 | Normoxia 17.5 ± 5.7 (24 h) 3.4 ± 0.5 (48 h) Hypoxia 10.9 ± 2.8 (24 h) 4.9 ± 1.6 (48 h) | A549 | (1) Anti-proliferation (2) Anti-metastasis, Anti-invasion (3) Pro-apoptosis (4) S-phase arrest (5) Decreased the number of adherent cells to different surfaces (fibronectin, collagen, plastic) and the expression of several MMPs and protein-lysine 6-oxidase, increased the expression of the extracellular matrix inhibitor | [61] |
| Ru48/Ru-hq1 | 50.9 ± 5.3 (2D) 103.9 ± 10.8 (3D) | A549 | (1) Anti-proliferation (2) Anti-migration, anti-invasion (3) Pro-apoptosis (4) G2/M phase arrest | [62] |
| Ru49/Ru-hq2 | 24.9 ± 6.5 (2D) 213.6.9 ± 8.6 (2D) | A549 | ||
| Ru50/PIPE | 17.99 ± 0.39 (24 h) 4.11 ± 0.27 (48 h) | A549 | (1) Anti-proliferation (2) Induced apoptosis (3) G1/S phase arrest (4) Reduced cyclin D1 expression and ERK phosphorylation levels, and induced apoptosis by intrinsic pathway | [63] |
| Ru51 | 10.0 ± 1.0 | A549 | (1) Anti-proliferation (2) S-phase arrest | [64] |
| Ru52 | 7.0 ± 0.5 | A549 | ||
| Ru53 | 3.0 ± 0.5 | A549 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Li, Y.; Shi, H.; Wang, Y.; Zhang, J.; Zhang, Q. Ruthenium Complexes as Promising Candidates against Lung Cancer. Molecules 2021, 26, 4389. https://doi.org/10.3390/molecules26154389
Sun Q, Li Y, Shi H, Wang Y, Zhang J, Zhang Q. Ruthenium Complexes as Promising Candidates against Lung Cancer. Molecules. 2021; 26(15):4389. https://doi.org/10.3390/molecules26154389
Chicago/Turabian StyleSun, Qi, Yingsi Li, Hongdong Shi, Yi Wang, Jitian Zhang, and Qianling Zhang. 2021. "Ruthenium Complexes as Promising Candidates against Lung Cancer" Molecules 26, no. 15: 4389. https://doi.org/10.3390/molecules26154389
APA StyleSun, Q., Li, Y., Shi, H., Wang, Y., Zhang, J., & Zhang, Q. (2021). Ruthenium Complexes as Promising Candidates against Lung Cancer. Molecules, 26(15), 4389. https://doi.org/10.3390/molecules26154389





