Bioactive Molecules for Discriminating Robinia and Helianthus Honey: High-Performance Liquid Chromatography–Electron Spray Ionization–Mass Spectrometry Polyphenolic Profile and Physicochemical Determinations
Abstract
1. Introduction
2. Results and Discussions
2.1. Quality General Parameter Results for Investigated Honey Samples
2.2. Total Polyphenolic and Flavonoid Content
2.3. Antioxidant Activity
2.4. LC–MS Analysis of Phenolic Acids and Flavonoids from Robinia Pseudoacacia Honey
2.5. Abscisic Acid in Robinia Pseudoacacia Honey
2.6. LC–MS Analysis of Phenolic Acids and Flavonoids from Helianthus Annuus Honey
3. Materials and Methods
3.1. Chemicals
3.2. Honey Samples
3.3. Physicochemical Quality Determinations
3.4. Pollen Analysis
3.5. Total Polyphenol and Flavonoid Content
3.6. Antioxidant Activity
3.7. Polyphenols (Flavonoids and Phenolic Acid) Isolation
3.8. HPLC Analysis of Flavonoids and Phenolic Acids
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gheldorf, N.; Engeseth, N.J. Antioxidant capacity of honeys from various sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J. Agric. Food Chem. 2002, 50, 3050–3055. [Google Scholar] [CrossRef] [PubMed]
- Ciulu, M.; Solinas, S.; Floris, I.; Panzanelli, A.; Pilo, M.I.; Piu, P.C.; Spano, N.; Sanna, G. RP-HPLC determination of water-soluble vitamins in honey. Talanta 2011, 83, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Halgarada, M.; Groth, S.; Popek, S.; Rohn, S.; Pedan, V. Antioxidant Activity and Phenolic Profile of Selected Organic and Conventional Honeys from Poland. Antioxidants 2020, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J.; Sojka, M.; Palenikova, H.; Bucekova, M.; Majtan, V. Vitamin C Enhances the Antibacterial Activity of Honey against Planktonic and Biofilm-Embedded Bacteria. Molecules 2020, 25, 992. [Google Scholar] [CrossRef]
- Bodó, A.; Radványi, L.; Kőszegi, T.; Csepregi, R.; Nagy, D.U.; Farkas, A.; Kocsis, M. Quality Evaluation of Light- and Dark-Colored Hungarian Honeys, Focusing on Botanical Origin, Antioxidant Capacity and Mineral Content. Molecules 2021, 26, 2825. [Google Scholar] [CrossRef]
- Terio, V.; Bozzo, G.; Ceci, E.; Savarino, A.E.; Barrasso, R.; Di Pinto, A.; Mottola, A.; Marchetti, P.; Tantillo, G.; Bonerba, E. Methylglyoxal (MGO) in Italian Honey. Appl. Sci. 2021, 11, 831. [Google Scholar] [CrossRef]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivar of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Bunea, A.; Andjelkovic, M.; Socaciu, C.; Bobiş, O.; Neacşu, M.; Verhe, R.; Van Camp, J. Total and individual carotenoids and phenolic acids content in fresh, refrigerated and processed spinach (Spinacia oleracea L.). Food Chem. 2008, 108, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.A.; Martos, I.; Ferreres, F.; Radovic, B.S.; Anklam, E. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J. Sci.Food Agric. 2001, 81, 485–496. [Google Scholar] [CrossRef]
- Nayik, G.A.; Nanda, V. A chemometric approach to evaluate the phenolic compounds, antgioxidant activity and mineral content of different unifloral honey types from Kashmir, India. LWT Food Sci Technol. 2016, 74, 504–513. [Google Scholar] [CrossRef]
- Ciulu, M.; Spano, N.; Pilo, M.I.; Sanna, G. Recent advances in the analysis of phenolic compounds in unifloral honeys. Molecules 2016, 24, 451. [Google Scholar] [CrossRef]
- Yao, L.; Jiang, Y.; Singanusong, R.; Datta, N.; Raymont, K. Phenolic acids and abscisic acid in Australian Eucalyptus honeys and their potential for floral authentication. Food Chem. 2004, 86, 169–177. [Google Scholar] [CrossRef]
- Kenjerić, D.; Mandić, M.L.; Primorac, L.; Bubalo, D.; Perl, A. Flavonoid profile of Robinia honeys produced in Croatia. Food Chem. 2007, 102, 683–690. [Google Scholar] [CrossRef]
- Gašić, U.M.; Milojković-Opsenica, D.M.; Tešić, Z.L. Polyphenols as Possible Markers of Botanical Origin of Honey. J. AOAC Int. 2017, 100, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Šarić, G.; Vahčić, N.; Bursać Kovačević, D.; Putnik, P. The Changes of Flavonoids in Honey during Storage. Processes 2020, 8, 943. [Google Scholar] [CrossRef]
- Wali, A.F.; Jabnoum, S.; Razmpoor, M.; Akbar, I.; Al Dhaheri, Y.; Khan, A.; Alshahrani, S.; Alhazmi, H.A.; Imtiyaz, Z. Chrysin, an Important Active Ingredient of Honey: Beneficial Pharmacological Activities and Molecular Mechanism of Action. In Therapeutic Applications of Honey and Its Phytochemicals; Rehman, M.U., Majid, S., Eds.; Springer: Singapore, 2020; pp. 409–432. [Google Scholar] [CrossRef]
- Mărghitaş, L.A.; Dezmirean, D.S.; Bobiş, O.; Moise, A.; Bogdanov, S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Battion, M. Honey as a source of dietary antioxidants: Structures, bioavailability and evidence of protective effects against human chronic diseases. Curr. Med. Chem. 2013, 20, 621–638. [Google Scholar] [CrossRef]
- Kavanagh, S.; Gunnoo, J.; Passos, T.M.; Stout, J.C.; White, B. Physicochemical properties and phenolic content of honey from different floral origins and from rural versus urban landscapes. Food Chem. 2019, 272, 66–75. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant Activity, Total Phenolic Content, Individual Phenolics and Physicochemical Parameters Suitability for Romanian Honey Authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Mazzoni, L.; Giampieri, F. The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Mărghitaş, L.A.; Dezmirean, D.; Pocol, C.; Ilea, M.; Bobiş, O.; Gergen, I. The Development of a Biochemical Profile of Acacia Honey by identifying Biochemical Determinants of its Quality. Not. Bot. Hort. Agrobot. 2010, 38, 9–15. [Google Scholar]
- Dobre, I.; Escuredo, O.; Rodriguez-Flores, S.; Seijo, C.M. Evaluation of several Romanian honeys based on their palynological and biochemical profiles. Int. J. Food Prop. 2014, 17, 1850–1860. [Google Scholar] [CrossRef][Green Version]
- Isopescu, R.D.; Josceanu, A.M.; Colţa, T.; Spulber, R. Romanian Honey: Characterization and Classification. In Honey Analysis; De Alencar, V., De Toledo, A., Eds.; IntechOpen Publisher: London, UK, 2017; pp. 1491–1528. [Google Scholar] [CrossRef]
- Bobiş, O.; Dezmirean, D.; Moise, A.R. Honey and Diabetes: The Importance of Natural Simple Sugars in Diet for Preventing and Treating Different Type of Diabetes. Oxid. Med. Cell. Longev. 2018, 4757893. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Ropciuc, S.; Padureţ, S.; Sanduleac, E.T. Authentication of Romanian honeys based on physicochemical properties, texture and chemometric. J. Food. Sci. Technol. 2014, 54, 4240–4250. [Google Scholar] [CrossRef] [PubMed]
- Ciucure, C.T.; Geană, E.I. Phenolic compounds profile and biochemical properties of honeys in relationship to the honey floral sources. Phytochem. Anal. 2019, 1–12. [Google Scholar] [CrossRef]
- Mădaş, N.M.; Mărghitaş, L.A.; Dezmirean, D.S.; Bonta, V.; Bobiş, O.; Fauconnier, M.L.; Francis, F.; Haubruge, E.; Nguyen, K.B. Volatile Profile and Physico-Chemical Analysis of Acacia Honey for Geographical Origin and Nutritional Value Determination. Foods 2019, 8, 445. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Ropciuc, S. Romanian honey authentication using voltammetric electronic tongue. Correlation of voltammetric data with physic-chemical parameters and phenolic compounds. Comp. Electron. Agric. 2019, 157, 371–379. [Google Scholar] [CrossRef]
- Geană, E.I.; Ciucure, C.T.; Costinel, D.; Ionete, R.E. Evaluation of honey in terms of Quality and authenticity based on the general physicochemical pattern, major sugar composition and δ13C signature. Food Control. 2020, 109, 106919. [Google Scholar] [CrossRef]
- Pauliuc, D.; Oroian, M. Organic acids and physic-chemical parameters of Romanian Sunflower honey. Food Environ. Saf. J. Fac. Food Eng. 2020, XIX, 148–155. [Google Scholar]
- Pauliuc, D.; Dranca, F.; Oroian, M. Raspberry, Rape, Thyme, Sunflower and Mint Honeys Authentication Using Voltammetric Tongue. Sensors 2020, 20, 2565. [Google Scholar] [CrossRef]
- Mădaş, N.; Mărghitaş, L.A.; Dezmirean, D. Black locust (Robinia pseudoacacia): The most valuable source for monofloral honey in Romania. Agricultura 2012, 83, 162–168. [Google Scholar] [CrossRef]
- Erler, S.; Moritz, R.F.A. Pharmacophagy and pharmacophory: Mechanisms of self-medication and disease prevention in the honeybee colony (Apis mellifera). Apigologie 2016, 47, 389–411. [Google Scholar] [CrossRef]
- Gherman, B.; Denner, A.; Bobiş, O.; Dezmireran, D.S.; Mărghitaş, L.A.; Schlüns, H.; Moritz, R.F.A.; Erler, S. Pathogen-associated self-medication behavior in the honeybee Apis mellifera. Behav. Ecol. Sociobiol. 2014, 68, 1777–1784. [Google Scholar] [CrossRef]
- Geana, I.E.; Ciucure, C.T. Establishing authenticity of honey via comprehensive Romanian honey analysis. Food Chem. 2020, 306, 125595. [Google Scholar] [CrossRef] [PubMed]
- Vranic, D.; Petronijevic, R.; Koricanac, V.; Djinovic Stojanovic, J.; Lilic, S.; Borovic, B.; Lukic, M. Evaluation of Serbian black locust honey quality parameters as a contribution to conformation of its botanical origin. IOP Conf. Ser. Earth Environ. Sci. 2019, 333, 012113. [Google Scholar] [CrossRef]
- Karapetsas, A.; Voulagaridou, G.-P.; Iliadi, D.; Tsochantaridis, O.; Michail, P.; Kynigopoulos, S.; Lambropoulou, M.; Stavropoulou, M.-I.; Stathopoulou, K.; Karabournioti, S.; et al. Honey Extract s Exhibit Cytoprotective Properties against UVB-Induced Photodamage in Human Experimental Skin Models. Antioxidants 2020, 9, 566. [Google Scholar] [CrossRef] [PubMed]
- Gośliński, M.; Nowak, D.; Szwengiel, A. Multidimensional Comparative Analysis of Bioactive Phenolic Compounds of Honey of Various Origin. Antioxidants 2021, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Nayik, G.A.; Suhag, Y.; Majid, I.; Nanda, V. Discrimination of high altitude Indian honey by chemometric approach according to their antioxidant properties and macro minerals. J.Saudi Soc. Agric.Sci. 2018, 17, 200–207. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F. Antioxidant Activity in Bee Products: A review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef]
- SR 784-1–3:2009 Bee Honey, Part 1: Quality Requirements on Acquisition from Beekeepers; Part 2: Quality Requirements on the Market; Part 3: Analysis Method; Asociația de Standardizare din România: Bucharest, Romania, 2009.
- Persanno Oddo, L.; Piro, R. Main European unifloral honeys: Descriptive sheets. Apidologie 2004, 35, S38–S81. [Google Scholar] [CrossRef]
- Codex Alimentarius Commision. Codex Alimentarius Commision Standards, Codex Stan 12-1981; Codex Alimentarius Commision: Rome, Italy, 2001. [Google Scholar]
- Council, E.U. Council Directive 2001/110/EC of 20 December 2001 relating to honey. Communities 2002, 10, 47–52. [Google Scholar]
- Kádár, M.; Juan-Borrás, M.; Hellebrandova, M.; Doménech, E.; Escheriche, I. Differentiation of Acacia, Sunflower and Tilia Honeys from Different Countries Based on Sugar Composition, Physicochemical and Color Parameters. Bull. USAMV Agric. 2010, 67, 252–258. [Google Scholar]
- Lazarević, K.B.; Andrić, F.; Trifković, J.; Tešić, Ž.; Milojković-Opsenica, D. Characterization of Serbian unifloral honeys according to their physicochemical parameters. Food Chem. 2012, 132, 2060–2064. [Google Scholar] [CrossRef]
- Gismondi, A.; De Rossi, S.; Canuti, L.; Novelli, S.; Di Marco, G.; Fattorini, L.; Canini, A. From Robinia pseudoacacia L. nectar to Acacia monofloral honey: Biochemical changes and variation of biological properties. J. Sci. Food Agric. 2018, 98, 4312–4322. [Google Scholar] [CrossRef] [PubMed]
- Predescu, C.; Papus, C.; Nicorescu, V. Antioxidant activity of sunflower and meadow honey. Sci. Works Ser. C Vet. Med. 2015, LXI, 45–50. [Google Scholar]
- Wesołowska, M.; Dżugan, M. The use of the PHOTOCHEM Device in Evaluation of Antioxidant Activity of Polish Honey. Food Anal. Meth. 2017, 10, 1568–1574. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plantg Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Duda, S.; Mărghitas, L.A.; Dezmirean, D.; Duda, M.; Mărgăoan, R.; Bobiş, O. Changes in major bioactive compounds with antioxidant activity of Agastache foeniculum, Lavandula angustifolia, Melissa officinalis and Nepeta cataria: Effect of harvest time ad plant species. Ind. Crop. Prod. 2015, 77, 499–507. [Google Scholar] [CrossRef]
- Ferreres, F.; Andrade, P.; Tomas-Barberan, F.A. Natural occurrence of Abscisic acid in heather honey and floral nectar. J. Agric. Food Chem. 1996, 44, 2053–2056. [Google Scholar] [CrossRef]
- Koulis, G.A.; Tsagkaris, A.S.; Aalizadeh, R.; Dasenaki, M.E.; Panagopoulou, E.I.; Drivelos, S.; Halagarda, M.; Georgiou, C.G.; Proestos, C.; Thomaidis, N.S. Honey phenolic compound profiling and authenticity assessment using HRMS targeted and untargeted metabolomics. Molecules 2021, 26, 2769. [Google Scholar] [CrossRef]
- Pulcini, P.; Allegrini, F.; Festuccia, N. Fast SPE extraction and LC-ESI-MS-MS analysis of flavonoids and phenolic acids in honey. Apiacta 2006, 41, 21–27. [Google Scholar]
- International Honey Commission. Harmonised Methods of the International Honey Commission. World Network of Honey Science, 2009. Available online: https://www.ihc-platform.net/ihcmethods2009.pdf (accessed on 21 July 2021).
- Bonta, V.; Mărghitaş, L.A.; Stanciu, O.; Laslo, L.; Dezmirean, D.; Bobiş, O. High-performance liquid chromatographic analysis of sugars in Transilvanian honeydew honey. Bull. USAMV-CN 2008, 65, 229–232. [Google Scholar]
- Louvreaux, J.; Maurizio, A.; Vorwohl, G. Methods in Melissopalinology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization d’une extrait de propolis et identification des principaux constituents. J. Pharm. Belgique 1994, 49, 462–468. [Google Scholar] [PubMed]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Blasa, M.; Candiracci, M.; Accorsi, A.; Piacentini, P.M.; Albertini, M.C.; Piatti, E. Raw Millefiori honey is packed full of antioxidants. Food Chem. 2005, 97, 217–222. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm.Wiss.Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing antioxidant power assay: Direct measure of total antioxidant activity of biological fluidsand modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Meth. Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Chen, L.; Mehta, A.; Berenbaum, M.; Zangerl, A.R.; Engeseth, N.J. Honeys from different floral sources as inhibitors of enzymatic browning in fruit and vegetable homogenates. J. Agric. Food Chem. 2000, 48, 4997–5000. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Rad. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidant and the degenerative diseases of aging. Proc. Nat. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Aljadi, A.M.; Kamaruddin, M.Y. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004, 85, 513–518. [Google Scholar] [CrossRef]
- Ferreres, F.; Tomas-Barberan, F.A.; Soler, C.; Garcia-Viguera, C.; Ortiz, A.; Tomas-Lorente, F. A simple extractive technique for honey flavonoid HPLC analysis. Apidologie 1994, 25, 21–30. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Van Camp, J.; Pedra, M.; Renders, K.; Socaciu, C.; Verhé, R. Correlations of the Phenolic Compounds and the Phenolic Content in Some Spanish and French Olived Oils. J. Agric. Food Chem. 2008, 56, 5181–5187. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
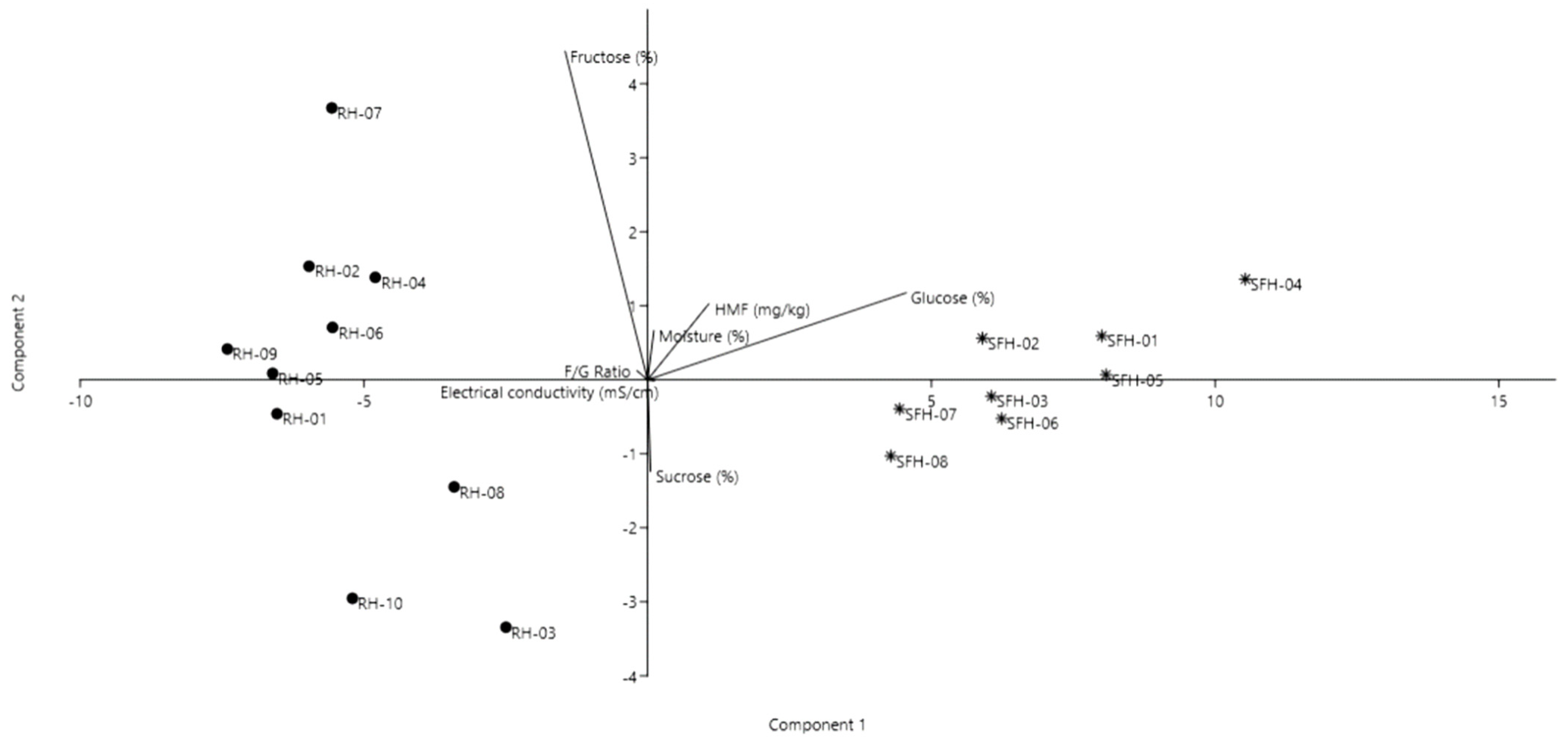
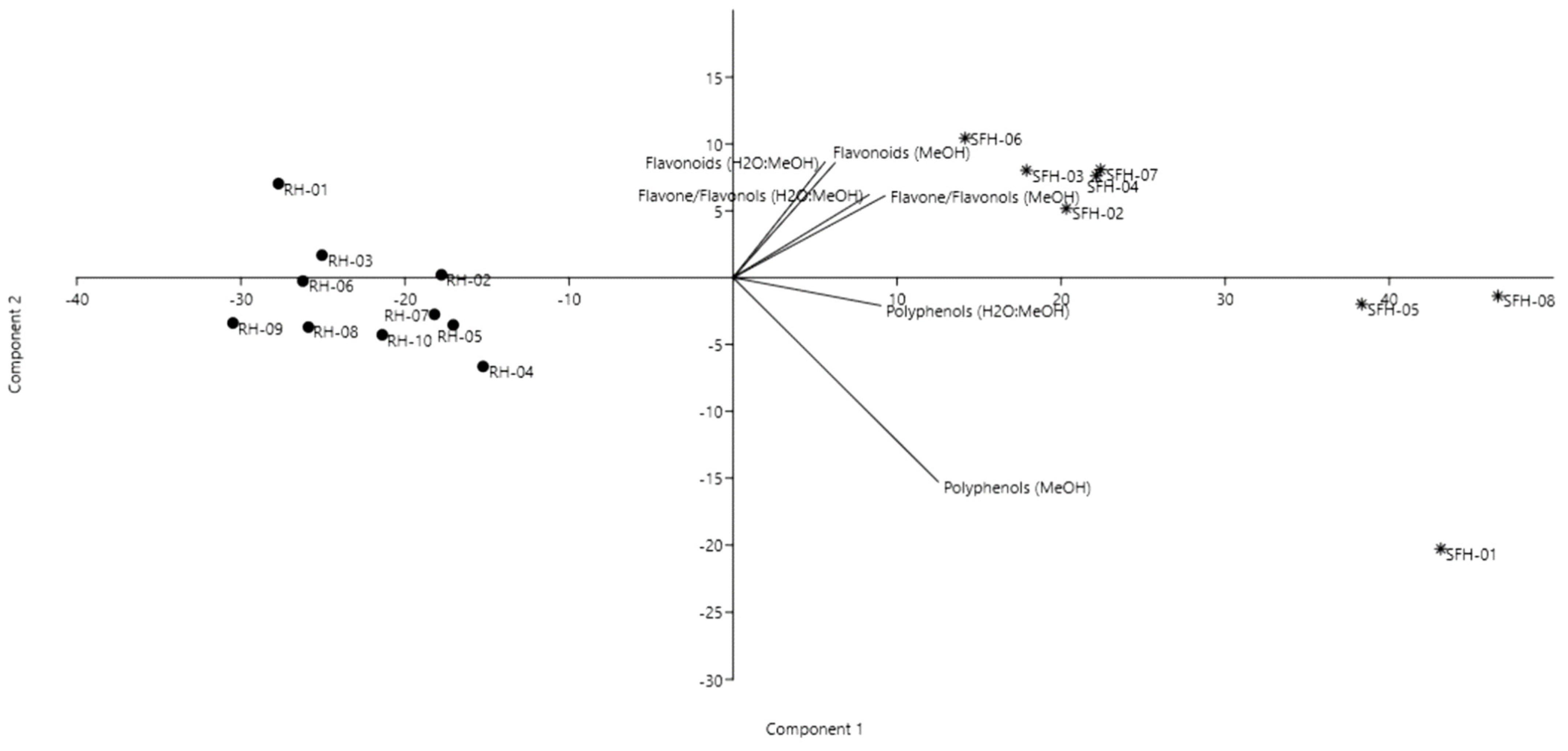
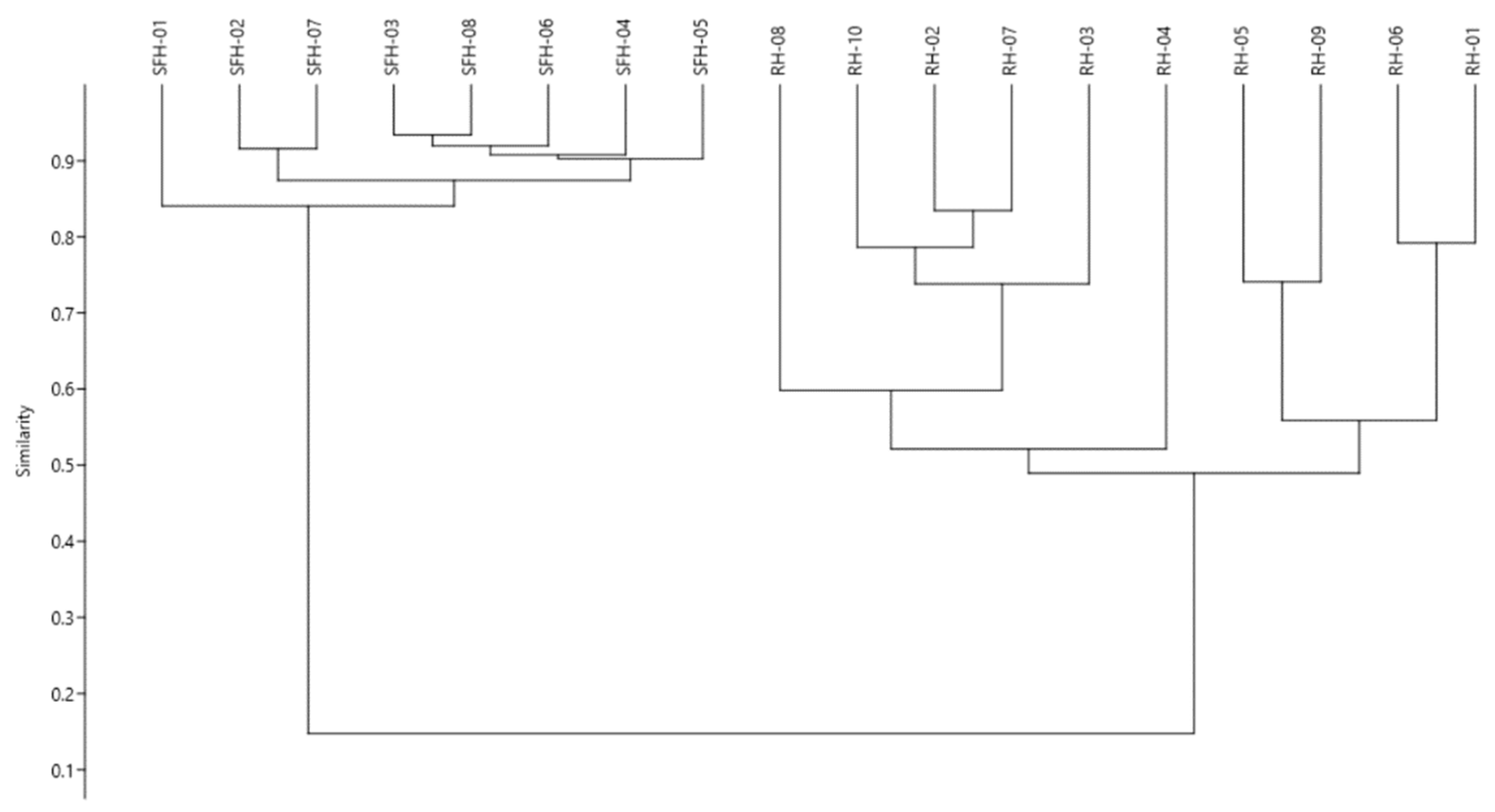
| Sample | Moisture (%) | Electrical Conductivity (mS/cm) | HMF (mg/kg) | Glucose (%) | Fructose (%) | F/G Ratio | Sucrose (%) |
|---|---|---|---|---|---|---|---|
| RH-01 | 17.33 ± 0.12 ef | 0.14 ± 0.01 ef | 0.997 ± 0.03 g | 29.86 ± 0.35 ef | 42.29 ± 0.51 c | 1.42 ± 0.02 c | 0.70 ± 0.12 d |
| RH-02 | 17.83 ± 0.05 de | 0.20 ± 0.00 d | 0.18 ± 0.03 h | 31.24 ± 0.56 ef | 44.11 ± 0.22 a | 1.41 ± 0.02 c | 0.21 ± 0.10 fg |
| RH-03 | 17.37 ± 0.15 ef | 0.15 ± 0.03 e | 0.88 ± 0.06 g | 33.0 ± 0.11 e | 38.67 ± 0.50 e | 1.70 ± 0.01 a | 2.12 ± 0.23 a |
| RH-04 | 18.90 ± 0.10 a | 0.10 ± 0.00 g | 1.50 ± 0.04 f | 31.93 ± 0.39 e | 43.29 ± 0.38 b | 1.35 ± 0.00 d | 0.20 ± 0.02 g |
| RH-05 | 18.30 ± 0.10 c | 0.18 ± 0.00 d | 1.74 ± 0.04 de | 29.66 ± 0.50 ef | 42.53 ± 0.13 c | 1.43 ± 0.02 c | 0.35 ± 0.05 f |
| RH-06 | 17.43 ± 0.05 e | 0.15 ± 0.00 ef | 1.12 ± 0.10 g | 31.16 ± 0.77 f | 43.18 ± 0.25 b | 1.38 ± 0.02 c | 0.65 ± 0.03 de |
| RH-07 | 17.86 ± 0.11 de | 0.14 ± 0.00 ef | 1.38 ± 0.07 fg | 31.98 ± 0.44 e | 45.99 ± 1.54 a | 1.43 ± 0.04 c | 0.19 ± 0.06 g |
| RH-08 | 18.30 ± 0.10 c | 0.19 ± 0.00 d | 2.18 ± 0.32 cd | 32.20 ± 0.24 e | 40.06 ± 0.27 d | 1.24 ± 0.01 e | 0.35 ± 0.05 f |
| RH-09 | 18.63 ± 0.15 b | 0.13 ± 0.00 f | 2.04 ± 0.13 cd | 28.86 ±0.31 g | 42.96 ± 0.24 bc | 1.48 ± 0.02 b | 0.27 ± 0.04 f |
| RH-10 | 17.30 ± 0.10 f | 0.14 ± 0.00 ef | 1.85 ± 0.09 cd | 30.19 ± 0.14 ef | 39.51 ± 0.48 d | 1.30 ± 0.01 e | 1.63 ± 0.19 b |
| SFH-01 | 18.13 ± 0.20 de | 0.56 ± 0.05 a | 2.79 ± 0.59 c | 44.08 ± 0.67 a | 39.27 ± 0.23 d | 0.89 ± 0.01 g | 1.06 ± 0.15 c |
| SFH-02 | 18.36 ± 0.15 c | 0.52 ± 0.02 a | 4.29 ± 0.48 b | 41.46 ± 0.52 c | 39.26 ± 0.24 d | 0.94 ± 0.00 g | 0.03 ± 0.05 h |
| SFH-03 | 18.36 ± 0.41 c | 0.28 ± 0.03 c | 1.31 ± 0.33 fg | 42.22 ± 0.35 c | 38.88 ± 0.10 de | 0.92 ± 0.00 g | 0.06 ± 0.05 h |
| SFH-04 | 19.03 ± 0.40 a | 0.26 ± 0.05 c | 9.03 ± 0.51 a | 44.96 ± 0.77 a | 38.17 ± 0.30 e | 0.85 ± 0.01 h | 0.58 ± 0.03 e |
| SFH-05 | 17.83 ± 0.25 de | 0.58 ± 0.02 a | 5.15 ± 0.21 b | 43.30 ± 0.61 b | 38.31 ± 0.43 e | 0.88 ± 0.00 g | 0.77 ± 0.05 d |
| SFH-06 | 17.53 ± 0.41 e | 0.46 ± 0.03 b | 3.28 ± 0.19 c | 41.87 ± 0.29 c | 38.61 ± 0.27 e | 0.92 ± 0.01 g | 1.14 ± 0.17 c |
| SFH-07 | 18.06 ± 0.15 d | 0.55 ± 0.02 a | 1.53 ± 0.31 f | 40.61 ± 0.37 d | 39.49 ± 0.14 d | 0.97 ± 0.00 f | 1.41 ± 0.18 b |
| SFH-08 | 18.20 ± 0.30 cd | 0.54 ± 0.04 a | 1.82 ± 0.23 cde | 40.11 ± 0.41 d | 38.65 ± 0.39 de | 0.96 ± 0.00 f | 0.74 ± 0.21 d |
| Sample Code | Total Polyphenols (mg GAE/100 g Honey) | Flavone/Flavonol Content (mg QE/100 g Honey) | Total Flavonoids Content (mg QE/100 g Honey) | |||
|---|---|---|---|---|---|---|
| MeOH | H2O:MeOH | MeOH | H2O:MeOH | MeOH | H2O:MeOH | |
| RH-01 | 75.87 ± 0.50 k | 60.19 ± 0.85 i | 10.81 ± 0.53 fg | 10.01 ± 0.10 ef | 38.81 ± 0.30 e | 42.49 ± 0.50 d |
| RH-02 | 82.35 ± 0.51 gh | 75.18 ± 0.28 g | 12.27 ±.26 f | 10.51 ± 0.60 ef | 36.14 ± 0.32 ef | 41.88 ± 0.39 d |
| RH-03 | 76.44 ± 0.48 ij | 72.32 ± 0.58 h | 10.11 ± 0.27 g | 10.12 ± 0.25 ef | 33.48 ± 1.02 g | 38.84 ± 0.50 e |
| RH-04 | 85.44 ± 0.76 g | 83.87 ± 1.19 e | 11.18 ± 0.25 fg | 10.20 ± 0.29 ef | 32.66 ± 0.47 g | 36.85 ± 0.54 f |
| RH-05 | 83.69 ± 0.30 gh | 79.77 ± 0.78 f | 10.74 ± 0.50 fg | 10.04 ± 0.11 ef | 36.22 ± 0.39 ef | 37.36 ± 0.44 ef |
| RH-06 | 76.65 ± 0.48 ij | 72.32 ± 0.34 h | 10.05 ± 0.18 g | 9.37 ± 0.57 f | 34.30 ± 0.89 fg | 34.16 ± 0.42 gh |
| RH-07 | 82.30 ± 0.27 h | 79.11 ± 0.55 f | 11.27 ± 0.25 fg | 9.85 ± 0.29 f | 37.17 ± 0.28 e | 35.53 ± 0.22 g |
| RH-08 | 77.91 ± 0.26 j | 75.48 ± 0.51 g | 9.81 ± 0.60 gh | 8.94 ± 0.23 f | 30.62 ± 0.54 h | 32.71 ± 0.48 hi |
| RH-09 | 75.66 ± 0.45 k | 71.33 ± 0.44 h | 9.01 ± 0.50 h | 7.81 ± 0.51 g | 29.19 ± 0.27 h | 31.31 ± 0.12 i |
| RH-10 | 80.52 ± 0.43 i | 79.14 ± 0.35 f | 10.16 ± 0.12 fg | 10.15 ± 0.15 e | 32.49 ± 0.47 g | 33.82 ± 0.51 gh |
| SFH-01 | 134.21 ± 0.80 a | 96.81 ± 0.44 c | 34.13 ± 0.21 c | 30.64 ± 0.63 c | 46.55 ± 0.49 d | 47.58 ± 0.54 c |
| SFH-02 | 98.75 ± 0.39 d | 94.26 ± 0.97 c | 31.06 ± 0.37 d | 28.47 ± 0.19 d | 48.33 ± 0.11 c | 49.54 ± 0.03 b |
| SFH-03 | 95.79 ± 0.56 e | 92.38 ± 1.59 cd | 30.06 ± 0.18 e | 28.10 ± 0.18 d | 49.69 ± 0.49 b | 50.55 ± 0.83 ab |
| SFH-04 | 99.32 ± 1.11 d | 92.23 ± 1.87 cd | 32.09 ± 0.13 d | 30.02± ± 0.23 c | 51.03 ± 0.39 ab | 51.57 ± 0.60 a |
| SFH-05 | 115.78 ± 0.48 c | 99.97 ± 1.61 b | 36.13 ± 0.30 b | 32.29 ± 0.36 b | 52.33 ± 0.39 a | 52.93 ± 0.68 a |
| SFH-06 | 92.40 ± 0.04 f | 88.65 ± 0.53 e | 30.20 ± 0.21 e | 28.15 ± 0.13 d | 48.96 ± 0.43 c | 50.25 ± 0.40 b |
| SFH-07 | 98.79 ± 0.49 d | 92.12 ± 0.34 d | 34.13 ± 0.27 c | 30.42 ± 0.21 c | 50.15 ± 0.41 b | 50.91 ± 0.43 ab |
| SFH-08 | 118.08 ± 0.55 b | 107.55 ± 2.77 a | 40.64 ± 0.40 a | 35.97 ± 0.40 a | 53.26 ± 0.42 a | 53.56 ± 0.38 a |
| Sample Code | Radical Scavenging Activity | Total Antioxidant Power | ||
|---|---|---|---|---|
| % Inhibition | mmol Trolox/100 g Honey | IC50 | FRAP Value (mM Fe2+) | |
| RH-01 | 19.6 ± 0.70 ef | 4.34 ± 0.15 ef | 25.53 ± 0.93 ab | 0.700 ± 0.02 ef |
| RH-02 | 20.7 ± 0.62 de | 4.58 ± 0.14 de | 24.17 ± 0.72 ab | 0.730 ± 0.00 e |
| RH-03 | 19.9 ± 0.31 e | 4.40 ± 0.07 de | 25.17 ± 0.39 ab | 0.709 ± 0.03 ef |
| RH-04 | 21.7 ± 0.70 d | 4.80 ± 0.16 d | 23.02 ± 0.75 b | 0.766 ± 0.06 de |
| RH-05 | 17.9 ± 0.56 g | 3.97 ± 0.12 g | 27.95 ± 0.86 a | 0.652 ± 0.00 h |
| RH-06 | 18.6 ± 0.21 fg | 4.12 ± 0.05 fg | 26.89 ± 0.32 a | 0.670 ± 0.00 g h |
| RH-07 | 20.2 ± 0.25 de | 4.46 ± 0.05 de | 24.80 ± 0.31 ab | 0.793 ± 0.04 d |
| RH-08 | 18.0 ± 0.35 fg | 4.18 ± 0.08 fg | 26.51 ± 0.49 ab | 0.679 ± 0.02 gh |
| RH-09 | 19.2 ± 0.47 f | 4.26 ± 0.10 ef | 26.01 ± 0.65 ab | 0.710 ± 0.01 ef |
| RH-10 | 19.9 ± 0.20 e | 4.40 ± 0.04 de | 25.13 ± 0.25 ab | 0.770 ± 0.05 d |
| SFH-01 | 25.2 ± 0.55 ab | 5.55 ± 0.12 bc | 19.87 ± 0.44 cd | 1.141 ± 0.17 bc |
| SFH-02 | 23.1 ± 0.75 c | 5.11 ± 0.16 d | 21.63 ± 0.70 c | 0.991 ± 0.01 c |
| SFH-03 | 26.2 ± 0.25 ab | 5.77 ± 0.05 b | 19.11 ± 0.18 ef | 1.249 ± 0.12 b |
| SFH-04 | 24.9 ± 0.45 b | 5.50 ± 0.10 c | 20.06 ± 0.36 cd | 1.093 ± 0.06 c |
| SFH-05 | 27.1 ± 0.31 a | 5.98 ± 0.07 a | 18.43 ± 0.21 f | 1.398 ± 0.04 a |
| SFH-06 | 25.9 ± 0.38 ab | 5.70 ± 0.08 b | 19.33 ± 0.28 de | 1.173 ± 0.11 bc |
| SFH-07 | 24.6 ± 0.42 b | 5.50 ± 0.09 c | 20.06 ± 0.33 cd | 0.972 ± 0.03 c |
| SFH-08 | 26.4 ± 0.42 ab | 5.81 ± 0.09 ab | 18.97 ± 0.30 ef | 1.132 ± 0.11 bc |
| Sample Code | Phenolic Acids (mg/kg Honey) | Abscisic Acid | Flavonoids (mg/kg) | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-hBA | VA | p-CouA | FerA | t-CinA | PinoB | Api | Kae | PinoC | Chr | Aca | |||
| RH-01 | 0.38 | nd | 2.43 | 2.28 | nd | 2.95 | 2.19 | 1.42 | 0.59 | 0.38 | 0.95 | 1.15 | 9.46 |
| RH-02 | 0.61 | nd | nd | 0.72 | nd | 15.91 | 2.28 | nd | 1.12 | 1.48 | 1.23 | 0.49 | 22.31 |
| RH-03 | nd | nd | nd | 1.25 | 1.38 | 13.93 | 1.64 | 2.44 | nd | 0.56 | 0.69 | 1.14 | 21.03 |
| RH-04 | 0.58 | 2.91 | 6.36 | 8.66 | 1.05 | 23.05 | 1.06 | 1.55 | 1.45 | 0.74 | 0.71 | 1.2 | 59.82 |
| RH-05 | 0.75 | nd | 4.25 | 6.51 | 1.51 | 6.61 | 0.89 | 2.06 | 0.85 | 0.85 | 1.05 | 0.90 | 25.13 |
| RH-06 | 0.25 | nd | 3.02 | 1.89 | 0.85 | 3.95 | 2.23 | 0.89 | nd | 1.20 | 1.24 | 1.75 | 12.13 |
| RH-07 | nd | nd | nd | 4.25 | 1.40 | 15.45 | 1.90 | nd | 0.65 | 0.96 | 0.86 | 1.12 | 26.39 |
| RH-08 | nd | 1.85 | 2.51 | 1.15 | nd | 7.45 | 2.12 | nd | nd | 1.26 | 0.98 | 0.95 | 17.77 |
| RH-09 | nd | nd | nd | 6.02 | nd | 4.88 | 0.85 | 1.68 | 0.87 | 1.35 | 1.05 | 2.24 | 16.27 |
| RH-10 | nd | 2.45 | nd | 2.45 | nd | 18.25 | 0.94 | nd | nd | 0.89 | 0.87 | 1.05 | 27.08 |
| Average | 0.26 | 0.72 | 1.86 | 3.52 | 0.62 | 11.24 | 1.61 | 1.00 | 0.55 | 0.97 | 0.96 | 1.20 | 24.55 |
| SD | 0.30 | 1.19 | 2.24 | 2.71 | 0.68 | 6.94 | 0.61 | 0.95 | 0.53 | 0.35 | 0.19 | 0.48 | 9.78 |
| Sample Code | Content of Phenolic Acids (mg/kg Honey) | Content of Flavonoids (mg/kg) | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PrCatA | ChlA | p-hBA | CafA | p-CouA | Qe | Lut | PinoB | Api | Kae | PinoC | Chr | Gal | ||
| SFH-01 | 3.00 | 1.63 | 2.07 | 2.08 | 3.09 | 1.21 | 27.09 | 2.13 | 1.97 | 0.54 | 1.44 | 0.69 | 1.53 | 48.47 |
| SFH-02 | 3.74 | 2.37 | nd | 4.16 | 3.85 | 2.33 | 17.50 | 2.05 | 1.13 | 1.29 | 2.18 | 1.59 | 2.39 | 44.58 |
| SFH-03 | 2.21 | 1.98 | 2.05 | 3.02 | 2.65 | 1.58 | 21.05 | 1.56 | nd | 0.83 | 1.05 | 0.56 | 1.52 | 40.06 |
| SFH-04 | 1.98 | 2.15 | 1.79 | 2.56 | 2.96 | 1.35 | 18.75 | 1.68 | 1.24 | 1.14 | 1.67 | 0.95 | 1.96 | 40.18 |
| SFH-05 | 2.5 | 1.86 | 1.96 | 3.05 | 1.98 | 1.98 | 22.78 | 2.10 | 2.02 | 1.04 | 2.01 | 1.28 | 1.49 | 46.05 |
| SFH-06 | 2.95 | 2.21 | nd | 2.74 | 2.52 | 2.04 | 20.52 | 1.97 | nd | 0.75 | 1.89 | 0.84 | 2.07 | 40.50 |
| SFH-07 | 2.64 | 1.58 | nd | 3.22 | 2.68 | 2.42 | 18.89 | 2.11 | 2.02 | 1.14 | 2.02 | 1.22 | 2.21 | 42.15 |
| SFH-08 | 1.99 | 2.26 | 1.84 | 2.95 | 3.69 | 1.89 | 21.68 | 1.89 | nd | 0.98 | 2.01 | 1.49 | 1.98 | 44.65 |
| Average | 2.63 | 2.01 | 1.94 | 2.97 | 2.93 | 1.85 | 21.03 | 1.94 | 1.68 | 0.96 | 1.78 | 1.08 | 1.89 | 2.63 |
| SD | 0.60 | 0.29 | 0.12 | 0.60 | 0.62 | 0.44 | 2.99 | 0.21 | 0.45 | 0.24 | 0.38 | 0.38 | 0.34 | 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobiş, O.; Bonta, V.; Cornea-Cipcigan, M.; Nayik, G.A.; Dezmirean, D.S. Bioactive Molecules for Discriminating Robinia and Helianthus Honey: High-Performance Liquid Chromatography–Electron Spray Ionization–Mass Spectrometry Polyphenolic Profile and Physicochemical Determinations. Molecules 2021, 26, 4433. https://doi.org/10.3390/molecules26154433
Bobiş O, Bonta V, Cornea-Cipcigan M, Nayik GA, Dezmirean DS. Bioactive Molecules for Discriminating Robinia and Helianthus Honey: High-Performance Liquid Chromatography–Electron Spray Ionization–Mass Spectrometry Polyphenolic Profile and Physicochemical Determinations. Molecules. 2021; 26(15):4433. https://doi.org/10.3390/molecules26154433
Chicago/Turabian StyleBobiş, Otilia, Victoriţa Bonta, Mihaiela Cornea-Cipcigan, Gulzar Ahmad Nayik, and Daniel Severus Dezmirean. 2021. "Bioactive Molecules for Discriminating Robinia and Helianthus Honey: High-Performance Liquid Chromatography–Electron Spray Ionization–Mass Spectrometry Polyphenolic Profile and Physicochemical Determinations" Molecules 26, no. 15: 4433. https://doi.org/10.3390/molecules26154433
APA StyleBobiş, O., Bonta, V., Cornea-Cipcigan, M., Nayik, G. A., & Dezmirean, D. S. (2021). Bioactive Molecules for Discriminating Robinia and Helianthus Honey: High-Performance Liquid Chromatography–Electron Spray Ionization–Mass Spectrometry Polyphenolic Profile and Physicochemical Determinations. Molecules, 26(15), 4433. https://doi.org/10.3390/molecules26154433










