Effect of Microbial Inoculation on Carbon Preservation during Goat Manure Aerobic Composting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Composting Experimental Design
2.2. Sample Collection and Analysis of Physicochemical Indicators
2.3. Bacterial Composition Analysis
2.3.1. DNA Extractions
2.3.2. PCR Amplification and 16S rDNA Sequencing
2.3.3. Processing of Sequencing Data
2.4. Statistical Analysis
3. Results
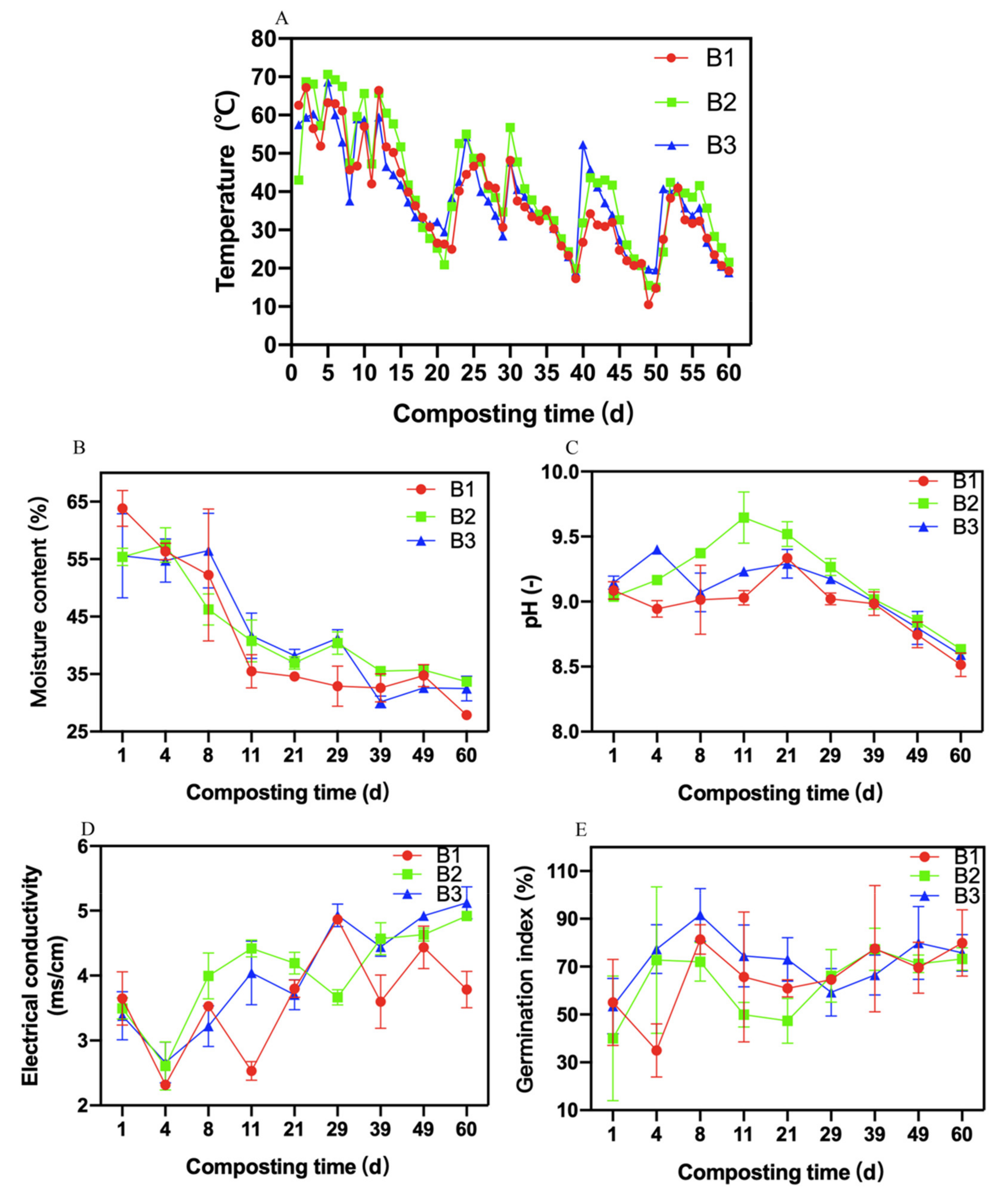
3.1. Changes in Physicochemical Indicators
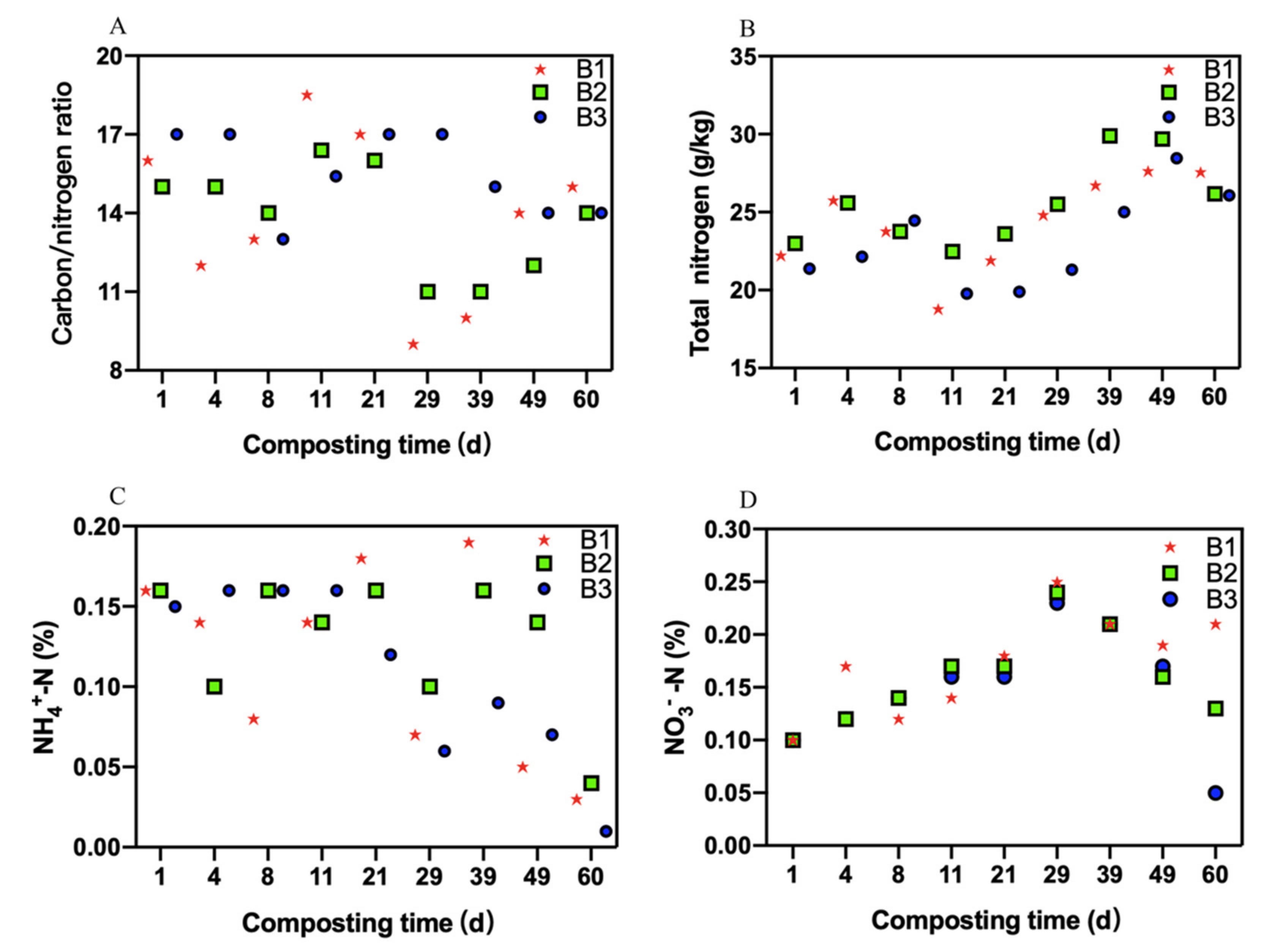
3.2. Changes in Nitrogen
3.3. Changes in Carbon
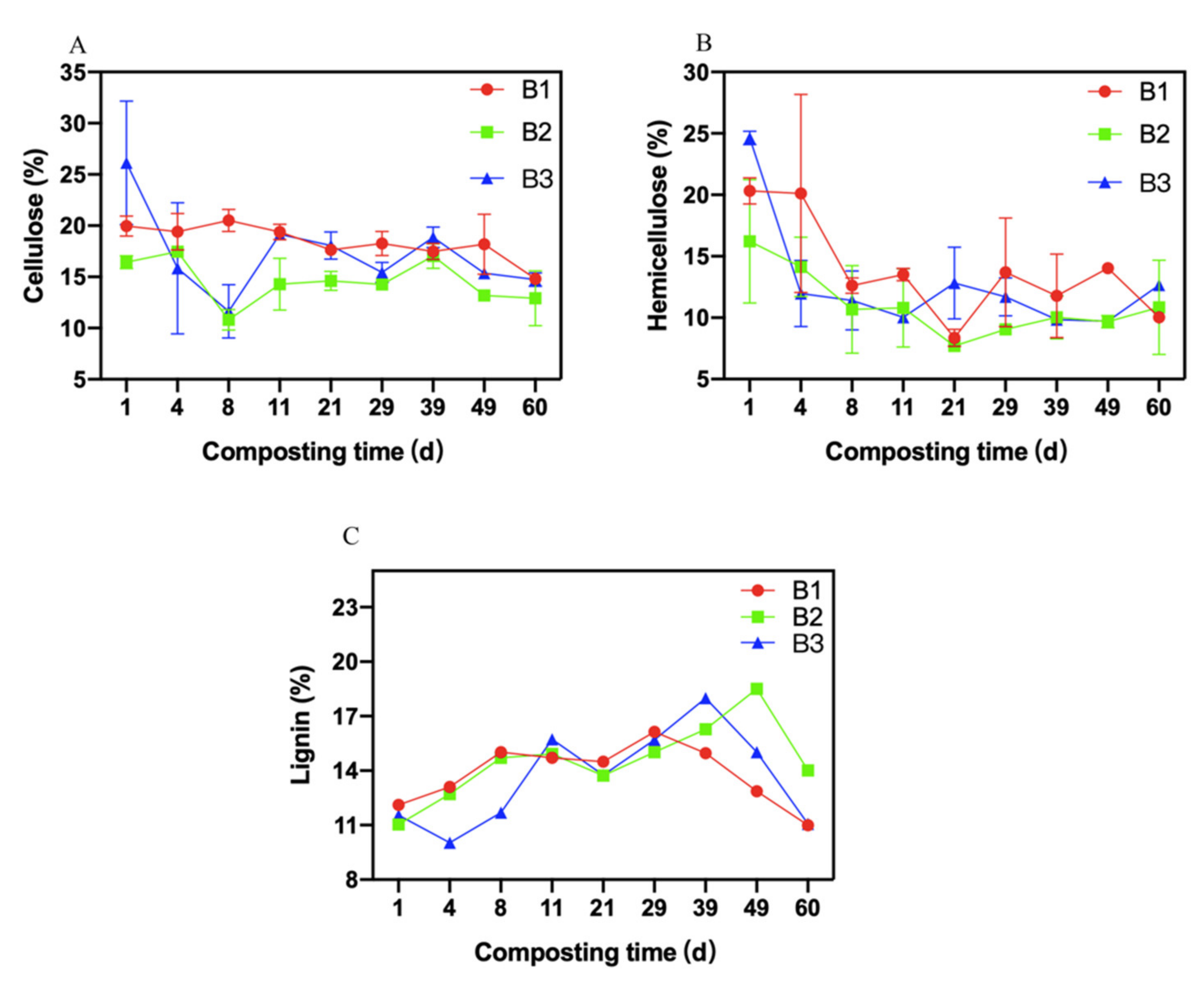
3.4. Changes in Fiber
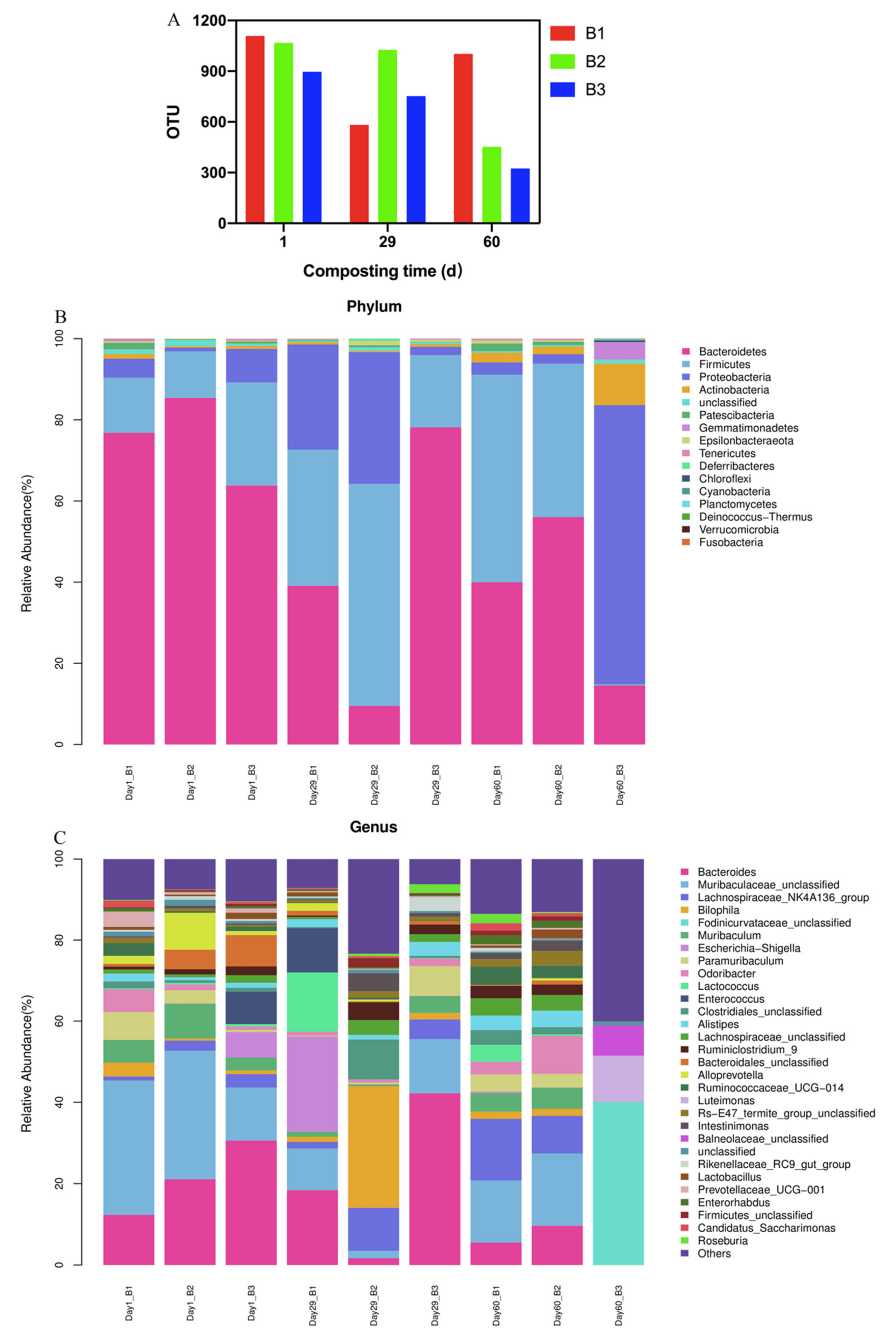
3.5. Changes in Bacterial Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ferichani, M. The potential of ettawa goat manure and urine management to support the productive and sustain-able farming. J. Crop Weed 2013, 9, 76–80. [Google Scholar]
- Awodun, M.A. Effect of goat manure and urea fertilizer on soil, growth and yield of okra (Abelmoschus esculentus (L.) Moench). Int. J. Agric. Res. 2007, 2, 632–636. [Google Scholar]
- Yu, H.; Xie, B.; Khan, R. The changes in carbon, nitrogen components and humic substances during organic-inorganic aerobic co-composting. Bioresour. Technol. 2019, 271, 228–235. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, C.; Gu, J.; Wang, X.; Zheng, W.; Wang, R. Roles of nxrA-like oxidizers and nirS-like reducers in nitrite conversion during swine manure composting. Bioresour. Technol. 2019, 297, 122426. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Hou, Q.; Huang, W.; Zheng, H.; Gao, X.; Yu, J.; Kwok, L.; Zhang, H.; Sun, Z. Lactobacillus plantarum improves the efficiency of sheep manure composting and the quality of the final product. Bioresour. Technol. 2020, 297, 122456. [Google Scholar] [CrossRef]
- Zhao, G.H.; Yu, Y.L.; Zhou, X.T.; Lu, B.Y.; Li, Z.M. Effects of drying pretreatment and particle size adjustment on the composting process of discarded flue-cured tobacco leaves. Waste Manag. Res. 2017, 35, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, Y.; Yang, T.; Wei, Z.; Li, Y.; Wei, Y. Effects of exogenous protein-like precursors on humification process during lignocellulose-like biomass composting: Amino acids as the key linker to promote humification process. Bioresour. Technol. 2019, 291, 121882. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Gan, J.; Chen, Q. Effect of carbon resources conditioner on composting process and carbon and nitrogen loss during composting of cucumber stalk. Trans. Chin. Soc. Agric. Eng. 2016, 32, 254–259. [Google Scholar]
- Chang, R.; Li, Y.; Chen, Q.; Guo, Q.; Jia, J. Comparing the effects of three in situ methods on nitrogen loss control, temperature dynamics and maturity during composting of agricultural wastes with a stage of temperatures over 70 °C. J. Environ. Manag. 2019, 230, 119–127. [Google Scholar] [CrossRef]
- Chang, R.; Wang, Q.; Gan, J.; Bernal, M.P.; Wang, Q.; Li, Y. Influence of easily-degraded organic matter content on maturity and nitrogen loss during composting of cucumber vine. Trans. Chin. Soc. Agric. Eng. 2017, 33, 231–237. [Google Scholar]
- Karhu, K.; Mattila, T.; Bergström, I.; Regina, K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity–results from a short-term pilot field study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Du, X.Y.; Liu, J.L.; Huang, G.H. Formation of struvite crystals in a simulated food waste aerobic composting process. Chem. Res. Chin. Univ. 2010, 26, 210–216. [Google Scholar]
- Wang, K.; Li, W.; Guo, J.; Zou, J.; Li, Y.; Zhang, L. Spatial distribution of dynamics characteristic in the intermittent aeration static composting of sewage sludge. Bioresour. Technol. 2011, 102, 5528–5532. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Zhang, Y.; Zhou, B.; Qin, Z.; Wu, J.; Wang, Q.; Yin, Y. Effects of Bacillus subtilis on carbon components and microbial functional metabolism during cow manure-straw composting. Bioresour. Technol. 2020, 303, 122868. [Google Scholar] [CrossRef] [PubMed]
- Siu-Rodas, Y.; de los Angeles Calixto-Romo, M.; Guillén-Navarro, K.; Sánchez, J.E.; Zamora-Briseno, J.A. Bacillus subtilis with endocellulase and exocellulase activities isolated in the thermophilic phase from composting with coffee residues. Rev. Argent. Microbiol. 2018, 50, 234–243. [Google Scholar] [CrossRef]
- Choi, K.Y.; Wernick, D.G.; Tat, C.A.; Liao, J.C. Consolidated conversion of protein waste into biofuels and ammonia using Bacillus Subtilis. Metab. Eng. 2014, 23, 53–61. [Google Scholar] [CrossRef]
- Jurado, M.; López, M.J.; Suárez-Estrella, F.; Vargas-García, M.C.; López-González, J.A.; Moreno, J. Exploiting composting biodiversity: Study of the persistent and biotechnologically relevant microorganisms from lignocellulose-based composting. Bioresour. Technol. 2014, 162, 283–293. [Google Scholar] [CrossRef]
- Gautam, S.P.; Bundela, P.S.; Pandey, A.K.; Jamaluddin; Awasthi, M.K.; Sarsaiya, S. Diversity of cellulolytic microbes and the biodegradation of municipal solid waste by a potential strain. Int. J. Microbiol. 2012, 2012, 325907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellejero, G.; Ardizzi, C.; Aschkar, G.; Chorolque, A.; Ballesta, R. Quality assessment by Aspergillus niger of an onion, cattle manure and alfalfa waste compost blend. IJPS Sci. 2015, 8, 1–8. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, X.; Liao, X.; Wu, Y.; Mi, J.; Wang, Y. Effect of different proportions of three microbial agents on ammonia mitigation during the composting of layer manure. Molecules 2019, 24, 2513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Z.; Zeng, Y.; Wang, S.; Sun, Z.; Tang, Y.; Kida, K. Insight into the microbiology of nitrogen cycle in the dairy manure composting process revealed by combining high-throughput sequencing and quantitative PCR. Bioresour. Technol. 2020, 301, 122760. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bi, L.; Liao, Y.; Lu, D.; Zhang, H.; Liao, X.; Liang, J.B.; Wu, Y. Influence and characteristics of Bacillus stearothermophilus in ammonia reduction during layer manure composting. Ecotoxicol. Environ. Saf. 2019, 180, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Rynk, R. Monitoring moisture in composting systems. Biocycle 2000, 41, 53. [Google Scholar]
- Zucconi, F.; Pera, A.; Forte, M.; de Bertoldi, M.D. Evaluating toxicity of immature compost. Biocycle 1981, 22, 54–57. [Google Scholar]
- Xu, Z.; Li, G.; Huda, N.; Zhang, B.; Wang, M.; Luo, W. Effects of moisture and carbon/nitrogen ratio on gaseous emissions and maturity during direct composting of cornstalks used for filtration of anaerobically digested manure centrate. Bioresour. Technol. 2020, 298, 122503. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, Y.; Zhao, W.; Yang, T.; Zhang, X.; Xie, X.; Cui, H.; Wei, Z. Effect of precursors combined with bacteria communities on the formation of humic substances during different materials composting. Bioresour. Technol. 2017, 226, 191–199. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Symposium: Carbohydrate methodology, metabolism, and nutritional implications in dairy cattle. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Garcia, C.; Hernandez, T.; Costa, F. Study on water extract of sewage sludge composts. Soil Sci. Plant Nutr. 1991, 37, 399–408. [Google Scholar] [CrossRef]
- Heidarzadeh, M.H.; Amani, H.; Javadian, B. Improving municipal solid waste compost process by cycle time reduction through inoculation of Aspergillus niger. J. Environ. Health Sci. Eng. 2019, 17, 295–303. [Google Scholar] [CrossRef]
- Wan, L.; Wang, X.; Cong, C.; Li, J.; Xu, Y.; Li, X.; Hou, F.; Wu, Y.; Wang, L. Effect of inoculating microorganisms in chicken manure composting with maize straw. Bioresour. Technol. 2020, 301, 122730. [Google Scholar] [CrossRef]
- Zayed, G.; Abdel-Motaal, H. Bio-production of compost with low pH and high soluble phosphorus from sugar cane bagasse enriched with rock phosphate. World J. Microbiol. Biotechnol. 2005, 21, 747–752. [Google Scholar] [CrossRef]
- Requena, N.; Baca, T.M.; Azcón, R. Evolution of humic substances from unripe compost during incubation with lignolytic or cellulolytic microorganisms and effects on the lettuce growth promotion mediated by Azotobacter chroococcum. Biol. Fertil. Soils 1997, 24, 59–65. [Google Scholar] [CrossRef]
- Nakasaki, K.; Hirai, H. Temperature control strategy to enhance the activity of yeast inoculated into compost raw material for accelerated composting. Waste Manag. 2017, 65, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhao, Y.; Zhao, X.; Yang, T.; Dang, Q.; Wu, J. Effect of manganese dioxide on the formation of humin during different agricultural organic wastes compostable environments: It is meaningful carbon sequestration. Bioresour. Technol. 2019, 299, 122596. [Google Scholar] [CrossRef] [PubMed]
- Guizelini, B.P.; Vandenberghe, L.P.S.; Sella, S.R.B.R.; Soccol, C.R. Study of the influence of sporulation conditions on heat resistance of Geobacillus stearothermophilus used in the development of biological indicators for steam sterilization. Arch. Microbiol. 2012, 194, 991–999. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.C.; Zhang, H.; Shi, H.; Hu, T.; Ngo, H.H. Characteristics of nitrogen transformation and microbial community in an aerobic composting reactor under two typical temperatures. Bioresour. Technol. 2013, 137, 270–277. [Google Scholar] [CrossRef]
- Wang, S.P.; Zhong, X.Z.; Wang, T.T.; Sun, Z.Y.; Tang, Y.Q. Aerobic composting of distilled grain waste eluted from a Chinese spirit-making process: The effects of initial pH adjustment. Bioresour. Technol. 2017, 245, 778–785. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.T.; Zhang, J.; Xu, T. Biochar influences the microbial community structure during tomato stalk composting with chicken manure. Bioresour. Technol. 2014, 154, 148–154. [Google Scholar]
- Caceres, R.; Coromina, N.; Malinska, K.; Martinez-Farre, F.X.; Lopez, M.; Sava, M.; Marfa, O. Nitrification during extended co-composting of extreme mixtures of green waste and solid fraction of cattle slurry to obtain growing media. Waste Manag. 2016, 58, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Sun, X.; Li, S.; Cai, L.; Zhang, P.; Kang, Y.; Yu, Z.; Tong, J.; Wang, L. Application of seasonal freeze-thaw to pretreat raw material for accelerating green waste composting. J. Environ. Manag. 2019, 239, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Amlinger, F.; Götz, B.; Dreher, P. Nitrogen in biowaste and yard waste compost: Dynamics of mobilisation and availability. A review. Eur. J. Soil Biol. 2003, 39, 107–116. [Google Scholar] [CrossRef]
- Yu, K.; Sun, X.; Li, S.; Cai, L.; Zhang, P.; Kang, Y.; Yu, Z.; Tong, J.; Wang, L. Application of quadratic regression orthogonal design to develop a composite inoculum for promoting lignocellulose degradation during green waste composting. Waste Manag. 2018, 79, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Danyang, L.; Shah, G.M.; Chen, W.; Wang, W.; Xu, Y. Hyperthermophilic pretreatment composting significantly accelerates humic substances formation by regulating precursors production and microbial communities. Waste Manag. 2019, 92, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, J.; Han, L.; Huang, G. Characterization of lignocellulosic compositions’ degradation during chicken manure composting with added biochar by phospholipid fatty acid (PLFA) and correlation analysis. Sci. Total Environ. 2017, 586, 1003–1011. [Google Scholar] [CrossRef]
- Hatakka, A. Lignin-modifying enzymes from selected white-rot fungi: Production and role from in lignin degradation. FEMS Microbiol. Rev. 2010, 13, 125–135. [Google Scholar] [CrossRef]
- Jurado, M.M.; Suárez-Estrella, F.; López, M.J.; Vargas-García, M.C.; López-González, J.A.; Moreno, J. Enhanced turnover of organic matter fractions by microbial stimulation during lignocellulosic waste composting. Bioresour. Technol. 2015, 186, 15–24. [Google Scholar] [CrossRef]
- Wang, H.B.; Han, L.R.; Feng, J.T.; Zhang, X. Evaluation of microbially enhanced composting of sophora flavescens residues. J. Environ. Sci. Health B 2016, 51, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.; Wang, X.; Wang, S.; Ma, L.; Ma, W. Transformation of nitrogen and carbon during composting of manure litter with different methods. Bioresour. Technol. 2019, 293, 122046. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of com-post. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef]
- Singh, J.; Kalamdhad, A.S. Assessment of bioavailability and leachability of heavy metals during rotary drum composting of green waste (Water hyacinth). Ecol. Eng. 2013, 52, 59–69. [Google Scholar] [CrossRef]
- Singh, J.; Kalamdhad, A.S. Effects of lime on bioavailability and leachability of heavy metals during agitated pile composting of water hyacinth. Bioresour. Technol. 2013, 138, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, Y.; Ge, J.; Zhu, L.; Wei, Z.; Wu, J. Effect of tricarboxylic acid cycle regulators on the formation of humic substance during composting: The performance in labile and refractory materials. Bioresour. Technol. 2019, 292, 121949. [Google Scholar] [CrossRef] [PubMed]
- Bhagavan, N.V.; Chung-Eun, H. Chapter 15: Protein and Amino Acid Metabolism. In Essentials of Medical Biochemistry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 227–268. [Google Scholar]
- Hartman, W.H.; Ye, R.; Horwath, W.R. A genomic perspective on stoichiometric regulation of soil carbon cycling. ISME J. 2017, 11, 2652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levesque, V.; Rochette Ziadi, N.; Dorais, M.; Antoun, H. Mitigation of CO2, CH4 and N2O from a fertigated horticultural growing medium amended with bio-chars and a compost. Appl. Soil Ecol. 2018, 126, 129–139. [Google Scholar] [CrossRef]

| Material | Moisture Content (%) | pH | TN (g/kg) | TOC (g/kg) | C/N | NO3−-N (mg/kg) | NH4+-N (mg/kg) |
|---|---|---|---|---|---|---|---|
| Goat manure | 62.42 ± 4.35 | 8.26 ± 0.21 | 18.60 ± 2.31 | 276.00 ± 6.72 | 5.00 ± 0.49 | 199.00 ± 10.48 | 591.00 ± 13.44 |
| Rape straw | 9.59 ± 0.43 | 6.65 ± 0.17 | 4.86 ± 0.28 | 328.00 ± 3.52 | 67.00 ± 2.17 | 247.00 ± 12.31 | 301.00 ± 11.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Wang, J.; Gao, Q.; Li, D.; Chen, Z.; Wei, Z.; Zhang, Y.; Wang, F. Effect of Microbial Inoculation on Carbon Preservation during Goat Manure Aerobic Composting. Molecules 2021, 26, 4441. https://doi.org/10.3390/molecules26154441
Lu J, Wang J, Gao Q, Li D, Chen Z, Wei Z, Zhang Y, Wang F. Effect of Microbial Inoculation on Carbon Preservation during Goat Manure Aerobic Composting. Molecules. 2021; 26(15):4441. https://doi.org/10.3390/molecules26154441
Chicago/Turabian StyleLu, Jiawei, Jingang Wang, Qin Gao, Dongxu Li, Zili Chen, Zongyou Wei, Yanli Zhang, and Feng Wang. 2021. "Effect of Microbial Inoculation on Carbon Preservation during Goat Manure Aerobic Composting" Molecules 26, no. 15: 4441. https://doi.org/10.3390/molecules26154441







