Biopharmaceutical Study on Nobiletin-Loaded Amorphous Solid Dispersion with Improved Hypouricemic Effect
Abstract
:1. Introduction
2. Results and Discussion
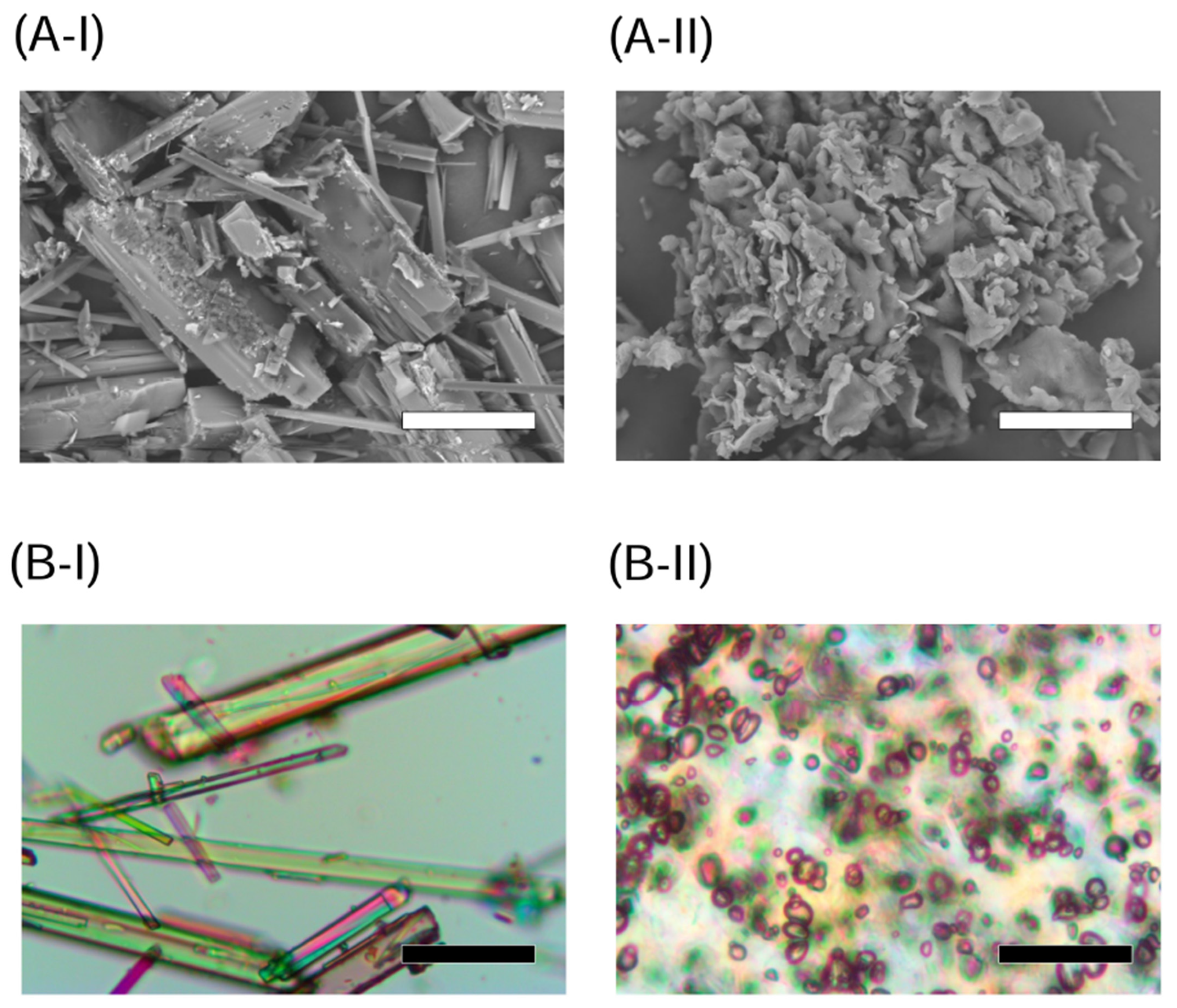
2.1. Appearance and Crystallinity of NOB Samples
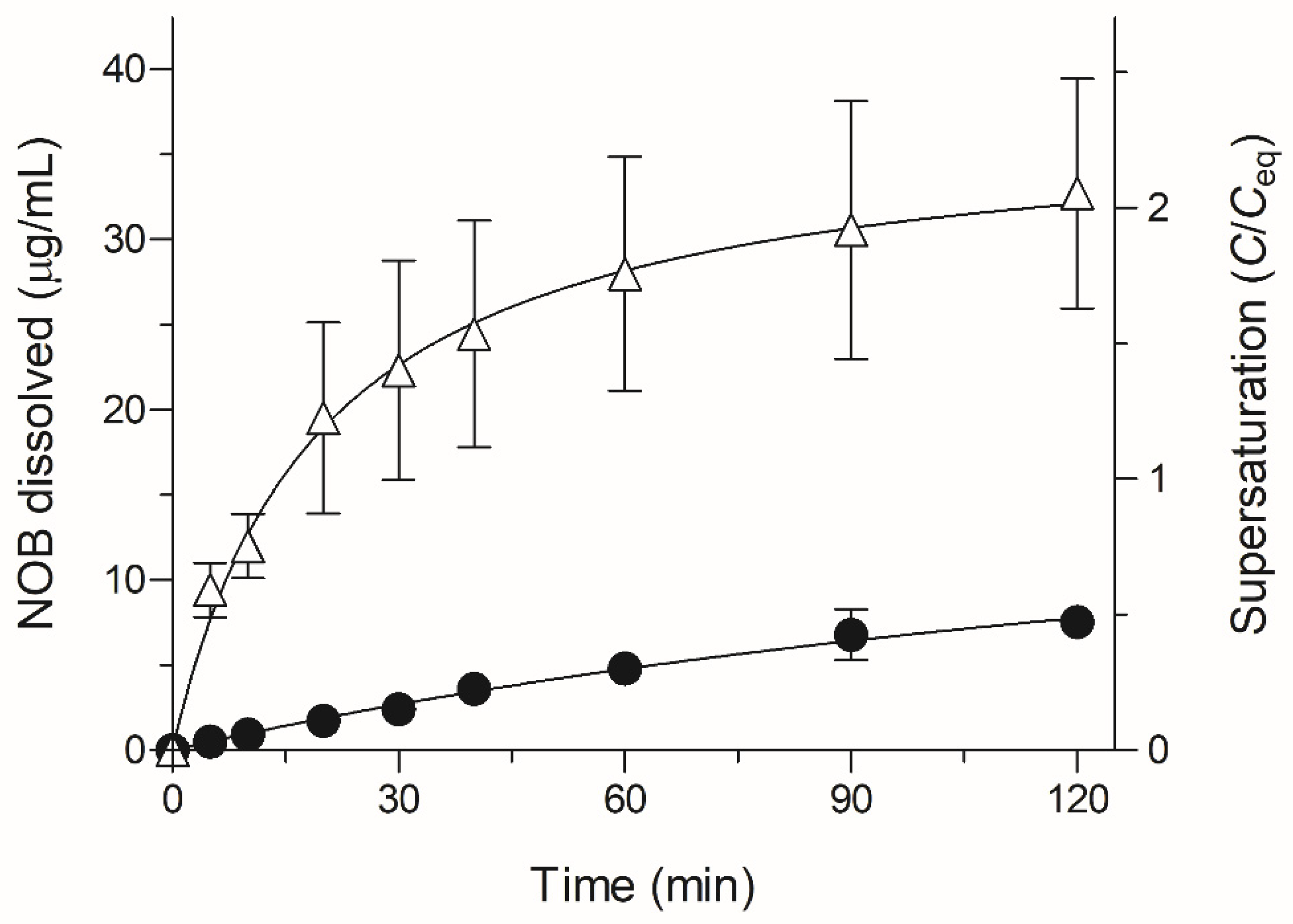
2.2. Dissolution Testing of NOB Samples
2.3. Pharmacokinetic Study of NOB Samples
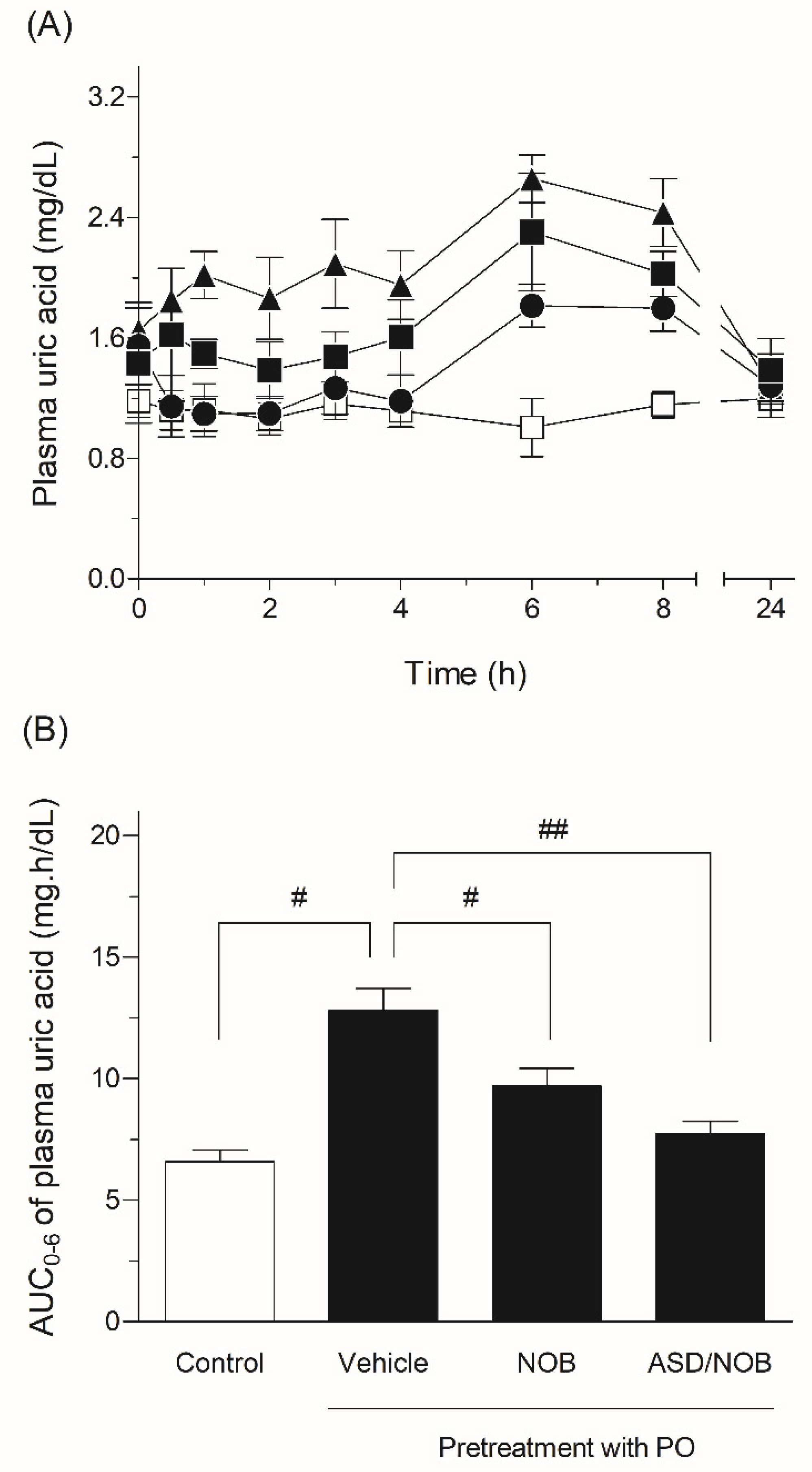
2.4. Hypouricemic Effects of NOB Samples in Rats
3. Material and Methods
3.1. Chemicals
3.2. Preparation for ASD Formulation of NOB
3.3. NOB Determination
3.4. Microscopic Experiments
3.5. Crystallinity
3.6. Dissolution Test
3.7. Pharmacokinetic Studies
3.7.1. Animals and Drug Administration
3.7.2. Plasma Concentration of NOB
3.8. Pharmacodynamic Studies
3.8.1. Rat Model of Hyperuricemia
3.8.2. Measurement of Uric Acid
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Gliozzi, M.; Malara, N.; Muscoli, S.; Mollace, V. The treatment of hyperuricemia. Int. J. Cardiol. 2016, 213, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Dalbeth, N.; Merriman, T.R.; Stamp, L.K. Gout. Lancet 2016, 388, 2039–2052. [Google Scholar] [CrossRef]
- Pacher, P.; Nivorozhkin, A.; Szabo, C. Therapeutic effects of xanthine oxidase inhibitors: Renaissance half a century after the discovery of allopurinol. Pharmacol. Rev. 2006, 58, 87–114. [Google Scholar] [CrossRef] [PubMed]
- Poon, S.H.; Hall, H.A.; Zimmermann, B. Approach to the treatment of hyperuricemia. R. I. Med. J. 2009, 92, 359–362. [Google Scholar]
- Liu, K.; Wang, W.; Guo, B.H.; Gao, H.; Liu, Y.; Liu, X.H.; Yao, H.L.; Cheng, K. Chemical Evidence for Potent Xanthine Oxidase Inhibitory Activity of Ethyl Acetate Extract of Citrus aurantium L. Dried Immature Fruits. Molecules 2016, 21, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onoue, S.; Nakamura, T.; Uchida, A.; Ogawa, K.; Yuminoki, K.; Hashimoto, N.; Hiza, A.; Tsukaguchi, Y.; Asakawa, T.; Kan, T.; et al. Physicochemical and biopharmaceutical characterization of amorphous solid dispersion of nobiletin, a citrus polymethoxylated flavone, with improved hepatoprotective effects. Eur. J. Pharm. Sci. 2013, 49, 453–460. [Google Scholar] [CrossRef]
- Onoue, S.; Uchida, A.; Takahashi, H.; Seto, Y.; Kawabata, Y.; Ogawa, K.; Yuminoki, K.; Hashimoto, N.; Yamada, S. Development of high-energy amorphous solid dispersion of nanosized nobiletin, a citrus polymethoxylated flavone, with improved oral bioavailability. J. Pharm. Sci. 2011, 100, 3793–3801. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Lu, Y.; Zhou, J.P. Preparation of nobiletin in self-microemulsifying systems and its intestinal permeability in rats. J. Pharm. Pharm. Sci. 2008, 11, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Yao, J.; Zhou, J.P. Preparation of self-assemble nobiletin proliposomes and its pharmacokinetics in rats. Yao Xue Xue Bao 2009, 44, 192–196. [Google Scholar]
- Bhatnagar, P.; Dhote, V.; Mahajan, S.C.; Mishra, P.K.; Mishra, D.K. Solid dispersion in pharmaceutical drug development: From basics to clinical applications. Curr. Drug Deliv. 2014, 11, 155–171. [Google Scholar] [CrossRef]
- Janssens, S.; Van den Mooter, G. Review: Physical chemistry of solid dispersions. J. Pharm. Pharmacol. 2009, 61, 1571–1586. [Google Scholar] [CrossRef]
- Alves, T.F.R.; das Neves Lopes, F.C.C.; Rebelo, M.A.; Souza, J.F.; da Silva Pontes, K.; Santos, C.; Severino, P.; Junior, J.M.O.; Komatsu, D.; Chaud, M.V. Crystalline Ethylene Oxide and Propylene Oxide Triblock Copolymer Solid Dispersion Enhance Solubility, Stability and Promoting Time-Controllable Release of Curcumin. Recent Pat. Drug Deliv. Formul. 2018, 12, 65–74. [Google Scholar] [CrossRef]
- Chiou, W.L.; Riegelman, S. Pharmaceutical applications of solid dispersion systems. J. Pharm. Sci. 1971, 60, 1281–1302. [Google Scholar] [CrossRef]
- Sato, H.; Kawabata, Y.; Yuminoki, K.; Hashimoto, N.; Yamauchi, Y.; Ogawa, K.; Mizumoto, T.; Yamada, S.; Onoue, S. Comparative studies on physicochemical stability of cyclosporine A-loaded amorphous solid dispersions. Int. J. Pharm. 2012, 426, 302–306. [Google Scholar] [CrossRef]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hens, B.; Tsume, Y.; Bermejo, M.; Paixao, P.; Koenigsknecht, M.J.; Baker, J.R.; Hasler, W.L.; Lionberger, R.; Fan, J.; Dickens, J.; et al. Low Buffer Capacity and Alternating Motility along the Human Gastrointestinal Tract: Implications for in Vivo Dissolution and Absorption of Ionizable Drugs. Mol. Pharm. 2017, 14, 4281–4294. [Google Scholar] [CrossRef]
- Sarisuta, N.; Parrott, E.L. Relationship of dissolution rate to viscosity of polymeric solutions. J. Pharm. Sci. 1982, 71, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Einbu, A.; Naess, S.N.; Elgsaeter, A.; Varum, K.M. Solution properties of chitin in alkali. Biomacromolecules 2004, 5, 2048–2054. [Google Scholar] [CrossRef] [PubMed]
- Onoue, S.; Kojo, Y.; Aoki, Y.; Kawabata, Y.; Yamauchi, Y.; Yamada, S. Physicochemical and pharmacokinetic characterization of amorphous solid dispersion of tranilast with enhanced solubility in gastric fluid and improved oral bioavailability. Drug Metab. Pharmacokinet. 2012, 27, 379–387. [Google Scholar] [CrossRef]
- Ohtani, H.; Ikegawa, T.; Honda, Y.; Kohyama, N.; Morimoto, S.; Shoyama, Y.; Juichi, M.; Naito, M.; Tsuruo, T.; Sawada, Y. Effects of various methoxyflavones on vincristine uptake and multidrug resistance to vincristine in P-gp-overexpressing K562/ADM cells. Pharm. Res. 2007, 24, 1936–1943. [Google Scholar] [CrossRef]
- Murakami, A.; Nakamura, Y.; Ohto, Y.; Yano, M.; Koshiba, T.; Koshimizu, K.; Tokuda, H.; Nishino, H.; Ohigashi, H. Suppressive effects of citrus fruits on free radical generation and nobiletin, an anti-inflammatory polymethoxyflavonoid. Biofactors 2000, 12, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Ishiwa, J.; Sato, T.; Mimaki, Y.; Sashida, Y.; Yano, M.; Ito, A. A citrus flavonoid, nobiletin, suppresses production and gene expression of matrix metalloproteinase 9/gelatinase B in rabbit synovial fibroblasts. J. Rheumatol. 2000, 27, 20–25. [Google Scholar] [PubMed]
- Akachi, T.; Shiina, Y.; Ohishi, Y.; Kawaguchi, T.; Kawagishi, H.; Morita, T.; Mori, M.; Sugiyama, K. Hepatoprotective effects of flavonoids from shekwasha (Citrus depressa) against d-galactosamine-induced liver injury in rats. J. Nutr. Sci. Vitaminol. 2010, 56, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, R.W.; Watts, J.E.; Seegmiller, J.E. Xanthine oxidase activity in human tissues and its inhibition by allopurinol (4-hydroxypyrazolo[3,4-d]pyrimidine). J. Lab. Clin. Med. 1965, 66, 688–697. [Google Scholar]
- Johnson, W.J.; Stavric, B.; Chartrand, A. Uricase inhibition in the rat by s-triazines: An animal model for hyperuricemia and hyperuricosuria. Proc. Soc. Exp. Biol. Med. 1969, 131, 8–12. [Google Scholar] [CrossRef] [PubMed]

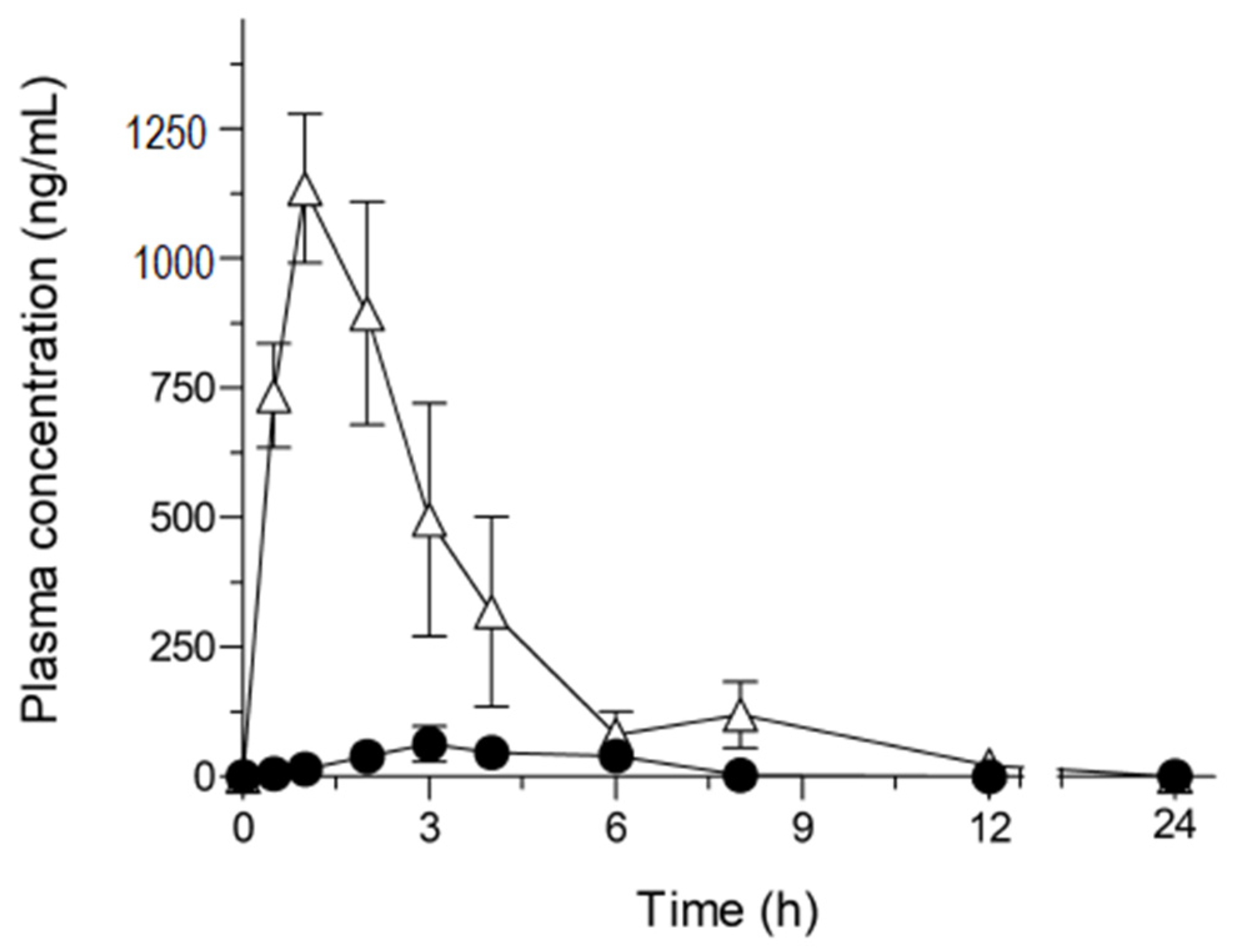
| Cmax (μg/mL) | Tmax (h) | AUC0–24 (μg·h/mL) | BA (%) | |
|---|---|---|---|---|
| Crystalline NOB (50 mg/kg) | 0.087 ± 0.029 | 3.0 ± 0.58 | 0.23 ± 0.086 | 0.20 ± 0.072 |
| ASD/NOB (50 mg-NOB/kg) | 1.2 ± 0.16 | 1.2 ± 0.15 | 4.1 ± 0.80 | 3.5 ± 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nihei, T.; Ushiro, E.; Sato, H.; Onoue, S. Biopharmaceutical Study on Nobiletin-Loaded Amorphous Solid Dispersion with Improved Hypouricemic Effect. Molecules 2021, 26, 4447. https://doi.org/10.3390/molecules26154447
Nihei T, Ushiro E, Sato H, Onoue S. Biopharmaceutical Study on Nobiletin-Loaded Amorphous Solid Dispersion with Improved Hypouricemic Effect. Molecules. 2021; 26(15):4447. https://doi.org/10.3390/molecules26154447
Chicago/Turabian StyleNihei, Takuya, Eri Ushiro, Hideyuki Sato, and Satomi Onoue. 2021. "Biopharmaceutical Study on Nobiletin-Loaded Amorphous Solid Dispersion with Improved Hypouricemic Effect" Molecules 26, no. 15: 4447. https://doi.org/10.3390/molecules26154447







