Oxygen Plasma Technology-Assisted Preparation of Three-Dimensional Reduced Graphene Oxide/Polypyrrole/Strontium Composite Scaffold for Repair of Bone Defects Caused by Osteoporosis
Abstract
1. Introduction
2. Results and Discussion
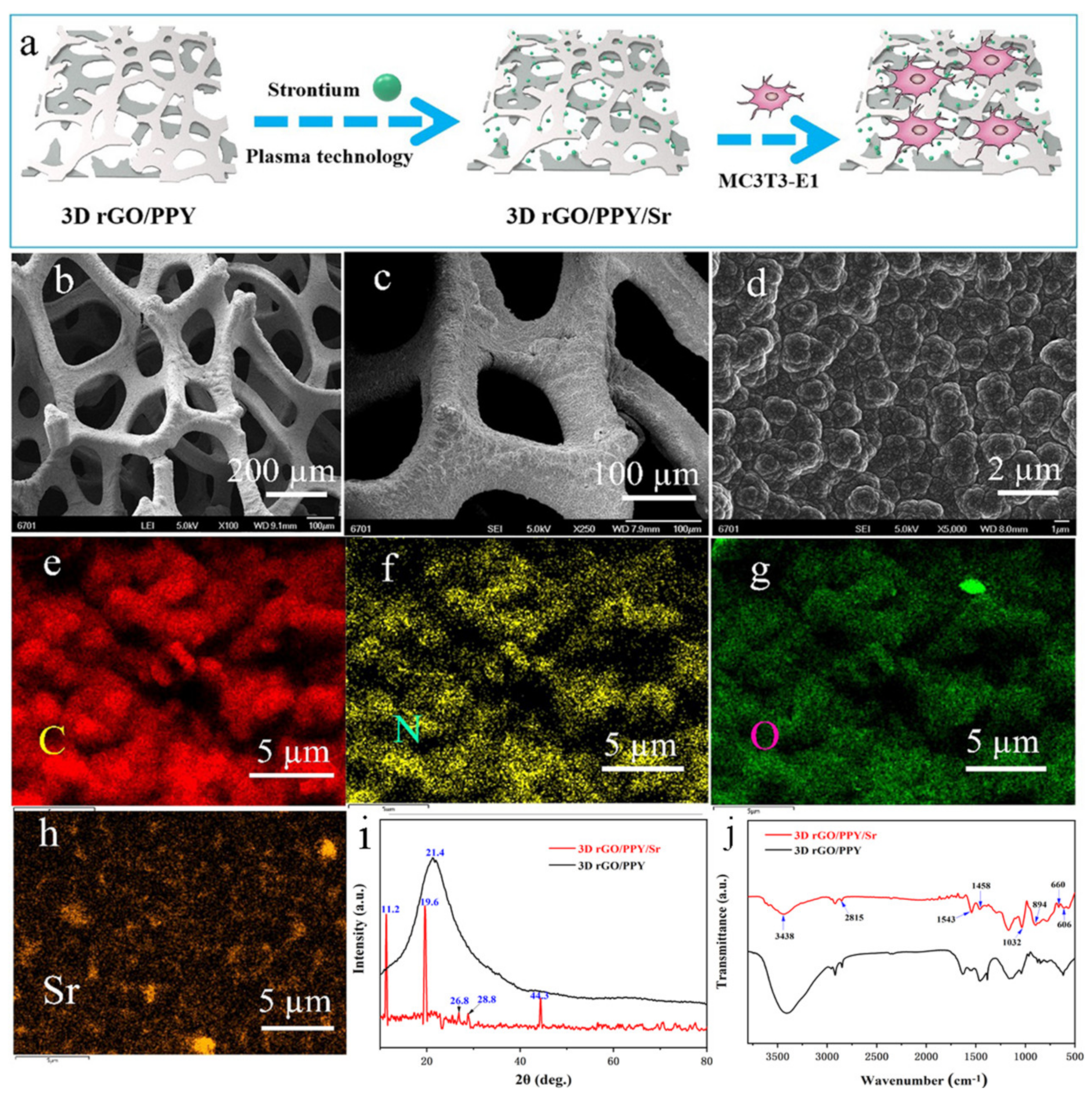
2.1. Preparation and Characterization
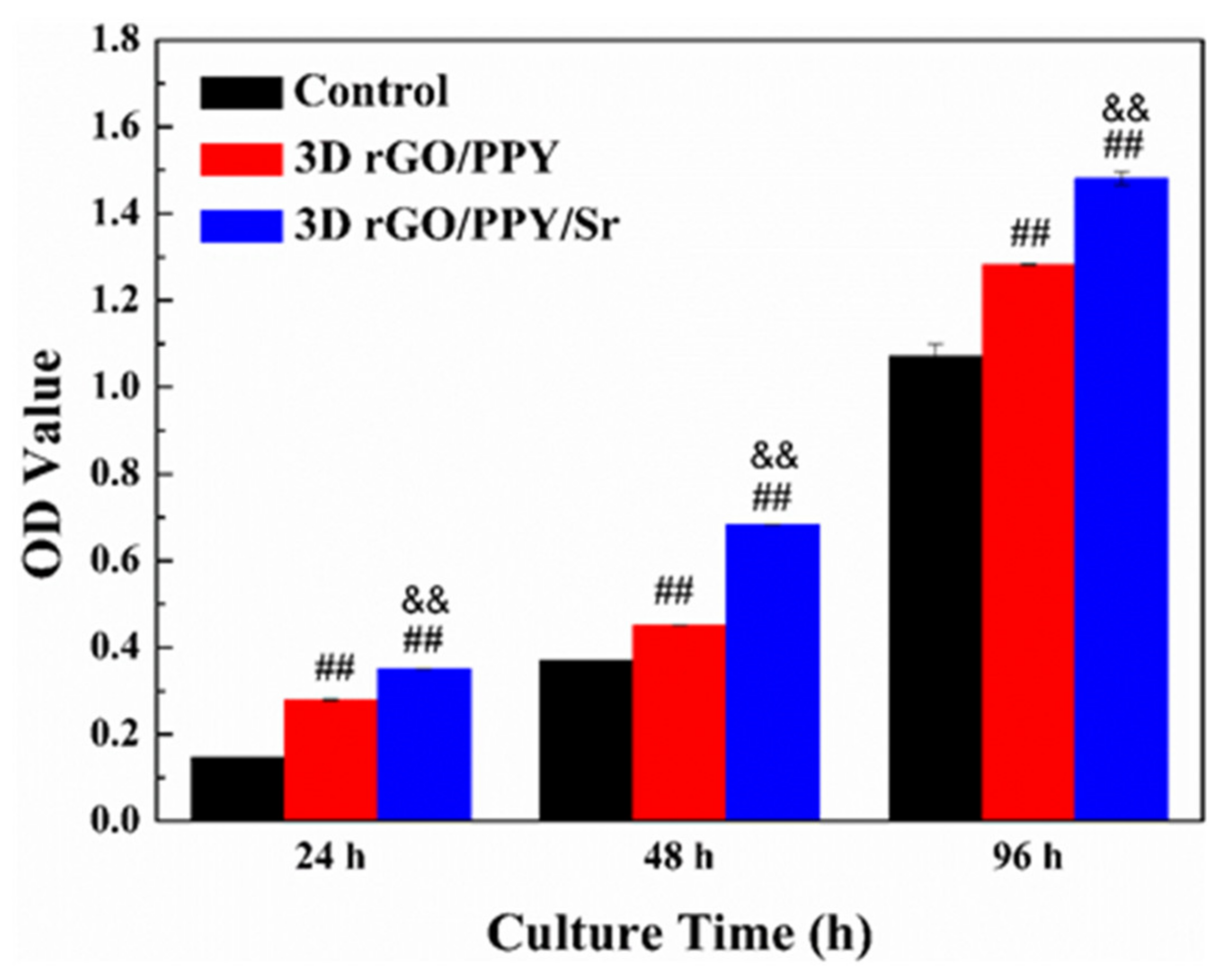
2.2. MTT Assay
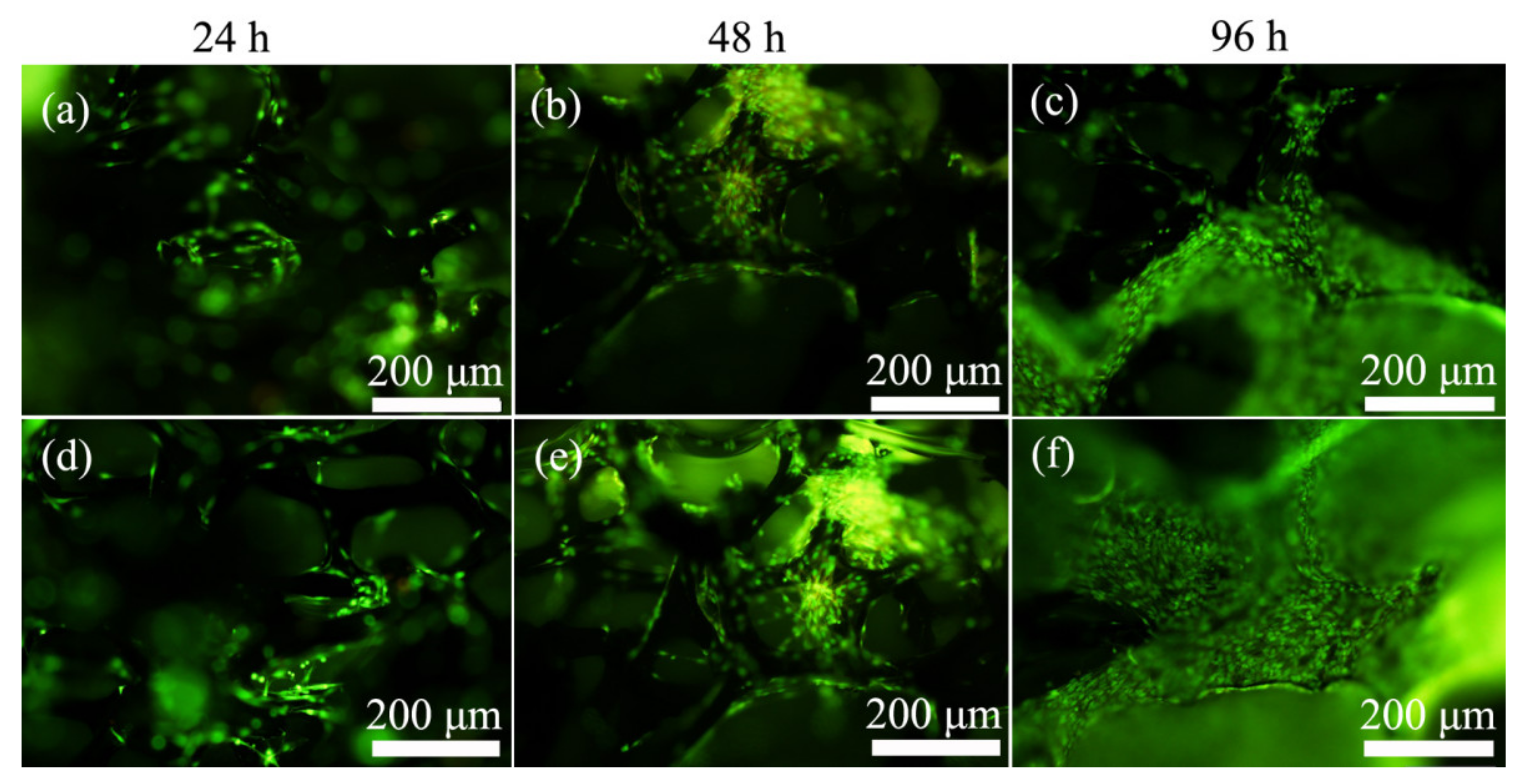
2.3. Acridine Orange/Ethidium Bromide (AO/EB) Double Staining Assay
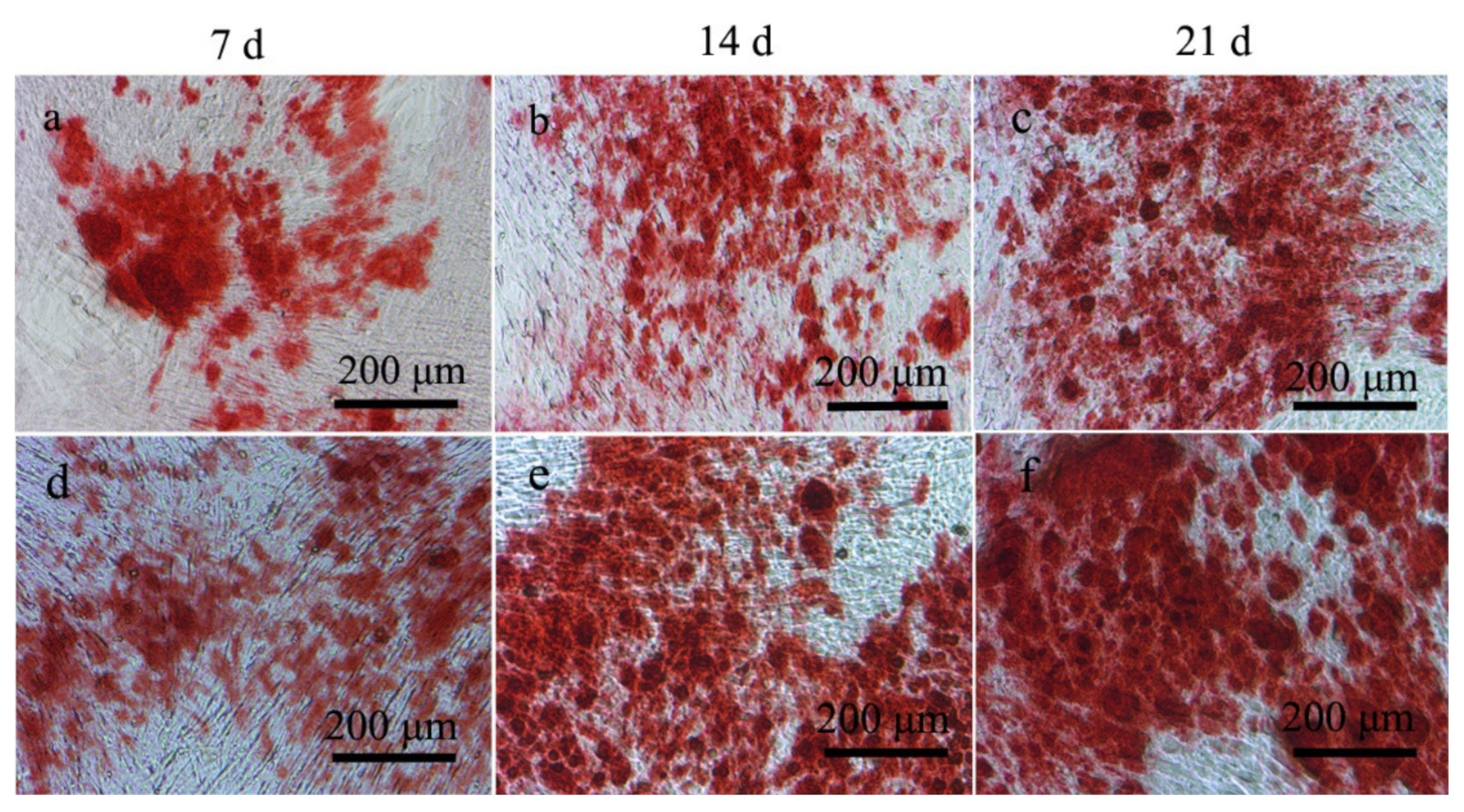
2.4. ALP and Alizarin Red Staining Experimental
2.5. Morphological Observation
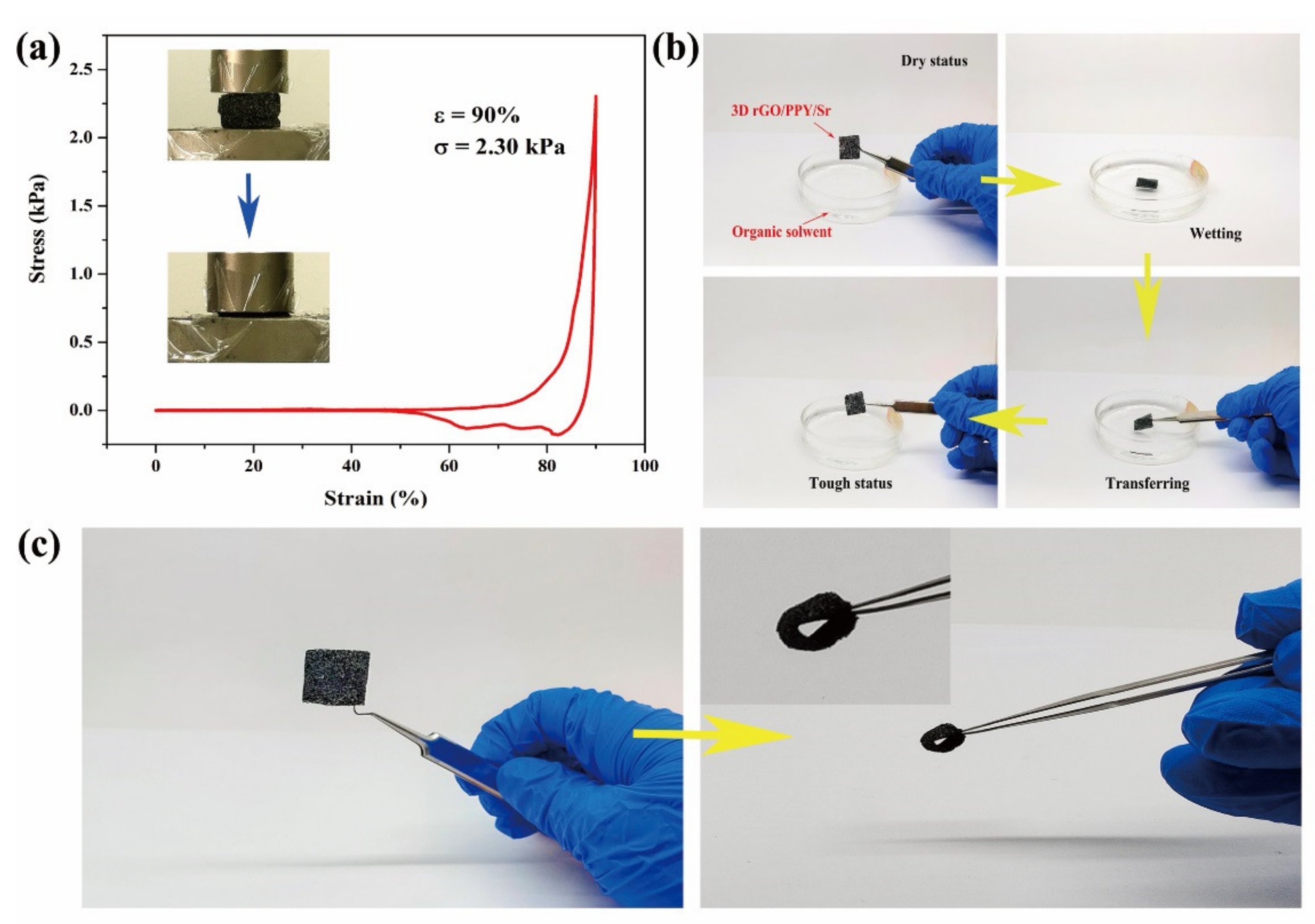
2.6. Mechanically Robust Performance
2.7. Sr Release Determination
3. Materials and Methods
3.1. Fabrication and Characterization of the 3D rGO/PPY/Sr
3.2. MTT Assay
3.3. Acridine Orange/Ethidium Bromide (AO/EB) Double Staining
3.4. Alkaline Phosphatase (ALP) Activity
3.5. Alizarin Red Staining
3.6. Morphological Observation
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cooper, C.; Campion, C.; Melton, L.J. Hip Fractures in the Elderly: A World-Wide Projection. Osteoporos. Int. 1992, 2, 285–289. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Stem Cells and Osteoporosis Therapy. Cell Stem Cell 2010, 7, 553–554. [Google Scholar] [CrossRef]
- Cheung, W.H.; Miclau, T.; Chow, K.H.; Yang, F.F.; Alt, V. Fracture healing in osteoporotic bone. Injury 2016, 47, S21–S26. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Z.X.; Zhang, Y.; Wang, C.R.; Xie, Q.Y.; Gong, K.; Zhang, B.; Quan, Y.; Pan, X.M. Local Treatment of Osteoporotic Sheep Vertebral Body with Calcium Sulfate for Decreasing the Potential Fracture Risk: Microstructural and Biomechanical Evaluations. Clin. Spine Surg. 2016, 29, E358–E364. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, L.; Feng, S.; Yang, Q.; Yu, B.; Tu, M. Enhanced bone formation by strontium modified calcium sulfate hemihydrate in ovariectomized rat critical-size calvarial defects. Biomed. Mater. 2017, 12, 035004. [Google Scholar] [CrossRef]
- Moreau, J.L.; Xu, H. Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate—Chitosan composite scaffold. Biomaterials 2009, 30, 2675–2682. [Google Scholar] [CrossRef]
- Lee, G.S.; Park, J.H.; Shin, U.S.; Kim, H.W. Direct deposited porous scaffolds of calcium phosphate cement with alginate for drug delivery and bone tissue engineering. Acta Biomater. 2011, 7, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Dua, R.; Centeno, J.; Ramaswamy, S. Augmentation of engineered cartilage to bone integration using hydroxyapatite. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 922–932. [Google Scholar] [CrossRef]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef]
- Marie, P.J. Strontium ranelate: New insights into its dual mode of action. Bone 2007, 40, S5–S8. [Google Scholar] [CrossRef]
- Panahifar, A.; Cooper, D.; Doschak, M.R. 3-D localization of non-radioactive strontium in osteoarthritic bone: Role in the dynamic labeling of bone pathological changes. J. Orthop. Res. 2015, 33, 1655–1662. [Google Scholar] [CrossRef]
- Yang, F.; Yang, D.; Tu, J.; Zheng, Q.; Cai, L.; Wang, L. Strontium Enhances Osteogenic Differentiation of Mesenchymal Stem Cells and In Vivo Bone Formation by Activating Wnt/Catenin Signaling. Stem Cells 2011, 29, 981–991. [Google Scholar] [CrossRef]
- Bayhan, I.; Dogan, N.U.; Ozaksit, G.; Uygur, D.; Ugurlu, N.; Ugur, M. Effect of strontium ranelate on serum leptin and bone turnover markers in women with established postmenopausal osteoporosis. J. Reprod. Med. 2013, 58, 319–323. [Google Scholar]
- Rizzoli, R.; Chapurlat, R.D.; Laroche, J.M.; Krieg, M.A.; Thomas, T.; Frieling, I.; Boutroy, S.; Laib, A.; Bock, O.; Felsenberg, D. Effects of strontium ranelate and alendronate on bone microstructure in women with osteoporosis. Osteoporos. Int. 2011, 23, 305–315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, L.; Pathak, J.L.; Liang, D.; Zhong, N.; Guan, H.; Wan, M.; Miao, G.; Li, Z.; Ge, L. Fabrication and characterization of strontium-hydroxyapatite/silk fibroin biocomposite nanospheres for bone-tissue engineering applications. Int. J. Biol. Macromol. 2020, 142, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.; Shenoy, S.J.; Babu, S.S.; Nair, R.P.; Varma, H.K.; John, A. Strontium Hydroxyapatite scaffolds engineered with stem cells aid osteointegration and osteogenesis in osteoporotic sheep model. Colloids Surf. B Biointerfaces 2018, 163, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Jie, W.; Zhang, T.; Li, W.; Jiang, Y.; Wan, L.; Liu, W.; Li, X.; Liu, B. Room-temperature fabrication of a three-dimensional reduced-graphene oxide/polypyrrole/hydroxyapatite composite scaffold for bone tissue engineering. RSC Adv. 2016, 6, 92804–92812. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Geim, A.K.; Kim, P. Carbon Wonderland. Sci. Am. 2008, 298, 90–97. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhang, W.H. Application of graphene derivatives in cancer therapy: A review. Carbon 2014, 67, 795. [Google Scholar] [CrossRef]
- Feng, L.; Li, W.; Qu, X. New Horizons for Diagnostics and Therapeutic Applications of Graphene and Graphene Oxide. Adv. Mater. 2013, 25, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Lázaro, I.D.; Kostarelos, K. Graphene materials as 2D non-viral gene transfer vector platforms. Gene Ther. 2017, 24, 123–132. [Google Scholar] [CrossRef]
- Lin, S.; Chen, J.; Teng, L.; Lin, W.; Li, R. The Antibacterial Applications of Graphene and Its Derivatives. Small 2016, 12, 4165–4184. [Google Scholar]
- Chronopoulos, D.D.; Bakandritsos, A.; Pykal, M.; Zbo?Il, R.; Otyepka, M. Chemistry, properties, and applications of fluorographene. Appl. Mater. Today 2017, 9, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.; Bentini, R.; Islam, I.; Tong, C.; Rosa, V. Graphene: A Versatile Carbon-Based Material for Bone Tissue Engineering. Stem Cells Int. 2016, 2015, 804213. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, H.; Li, Z.; Shen, Y.; Zhang, Y. Enhanced Osseointegration of Titanium Alloy Implants with Laser Microgrooved Surfaces and Graphene Oxide Coating. ACS Appl. Mater. Interfaces 2019, 11, 39470–39478. [Google Scholar] [CrossRef]
- Wang, X.; Gu, X.; Yuan, C.; Chen, S.; Zhang, P.; Zhang, T.; Yao, J.; Chen, F.; Chen, G. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J. Biomed. Mater. Res. Part A 2004, 68, 411–422. [Google Scholar] [CrossRef]
- Giglio, E.D.; Sabbatini, L.; Colucci, S.; Zambonin, G. Synthesis, analytical characterization, and osteoblast adhesion properties on RGD-grafted polypyrrole coatings on titanium substrates. J. Biomater. Sci. Polym. Ed. 2000, 11, 1073–1083. [Google Scholar] [CrossRef]
- Cardinaud, C.; Peignon, M.C.; Tessier, P.Y. Plasma etching: Principles, mechanisms, application to micro- and nano-technologies. Appl. Surf. Sci. 2000, 164, 72–83. [Google Scholar] [CrossRef]
- Jia, P.; Pan, F.; Chen, T. Effect of oxygen plasma etching on graphene’s mechanical and electrical properties. IOP Conf. Ser. Mater. Sci. Eng. 2017, 182, 012030. [Google Scholar] [CrossRef]
- Al-Mumen, H.; Rao, F.; Wen, L.; Dong, L. Singular Sheet Etching of Graphene with Oxygen Plasma. Nano-Micro Lett. 2014, 6, 116–124. [Google Scholar] [CrossRef]
- Seah, C.M.; Vigolo, B.; Chai, S.P.; Mohamed, A.R. Mechanisms of graphene fabrication through plasma-induced layer-by-layer thinning. Carbon Int. J. Spons. Am. Carbon Soc. 2016, 105, 496–509. [Google Scholar]
- Hutmacher, D.W.; Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues-state of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef]
- Wu, T.; Li, B.; Wang, W.; Chen, L.; Li, Z.; Wang, M.; Zha, Z.G.; Lin, Z.F.; Xia, H.; Zhang, T. Strontium-substituted hydroxyapatite grown on graphene oxide nanosheet-reinforced chitosan scaffold to promote bone regeneration. Biomater. Sci. 2020, 8, 4603–4615. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, V.; Das, P.; Roy, P.K. Synthesis and application of graphene oxide-coated biochar composite for treatment of strontium-containing solution. Int. J. Environ. Sci. Technol. 2020, 18, 1953–1966. [Google Scholar] [CrossRef]
- Jang, J.; Lee, D.S. Enhanced adsorption of cesium on PVA-alginate encapsulated Prussian blue-graphene oxide hydrogel beads in a fixed-bed column system. Bioresour. Technol. 2016, 218, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Hu, X.L.; Li, Y.J.; Zhang, M.; Liu, X.P.; He, Y.Z.; Dong, F.; Fu, M.; Zhang, Z. One-step preparation of a novel SrCO3/g-C3N4 nano-composite and its application in selective adsorption of crystal violet. RSC Adv. 2018, 8, 6315–6325. [Google Scholar] [CrossRef]
- Zhang, W.J.; Cao, H.L.; Zhang, X.C.; Li, G.L.; Chang, Q.; Zhao, J.; Qiao, Y.Q.; Ding, X.; Yang, G.Z.; Liu, X.L.; et al. A strontium-incorporated nanoporous titanium implant surface for rapid osseointegration. Nanoscale 2016, 8, 5291–5310. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.J.; Long, H.Y. Systematic strontium substitution in hydroxyapatite coatings on titanium via micro-arc treatment and their osteoblast/osteoclast responses. Acta Biomater. 2011, 7, 4081–4087. [Google Scholar] [CrossRef]
- Guo, Y.P.; Guan, J.J.; Yang, J.; Wang, Y.; Zhang, C.Q.; Ke, Q.F. Hybrid nanostructured hydroxyapatite-chitosan composite scaffold: Bioinspired fabrication, mechanical properties and biological properties. J. Mater. Chem. B. Mater. Biol. Med. 2015, 3, 4679–4689. [Google Scholar] [CrossRef] [PubMed]
- Tat, S.K.; Pelletier, J.P.; Mineau, F.O.; Caron, J.; Martel-Pelletier, J. Strontium ranelate inhibits key factors affecting bone remodeling in human osteoarthritic subchondral bone osteoblasts. Bone 2011, 49, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Fielding, G.A.; Smoot, W.; Bose, S. Effects of SiO2, SrO, MgO, and ZnO dopants in tricalcium phosphates on osteoblastic Runx2 expression. J. Biomed. Mater. Res. Part. A 2014, 102, 2417–2426. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, X.; Kang, Z.; Wang, N.; Qin, X.; Xie, W.; Song, F. Oxygen Plasma Technology-Assisted Preparation of Three-Dimensional Reduced Graphene Oxide/Polypyrrole/Strontium Composite Scaffold for Repair of Bone Defects Caused by Osteoporosis. Molecules 2021, 26, 4451. https://doi.org/10.3390/molecules26154451
Mai X, Kang Z, Wang N, Qin X, Xie W, Song F. Oxygen Plasma Technology-Assisted Preparation of Three-Dimensional Reduced Graphene Oxide/Polypyrrole/Strontium Composite Scaffold for Repair of Bone Defects Caused by Osteoporosis. Molecules. 2021; 26(15):4451. https://doi.org/10.3390/molecules26154451
Chicago/Turabian StyleMai, Xiaoxue, Zebiao Kang, Na Wang, Xiaoli Qin, Weibo Xie, and Fuxiang Song. 2021. "Oxygen Plasma Technology-Assisted Preparation of Three-Dimensional Reduced Graphene Oxide/Polypyrrole/Strontium Composite Scaffold for Repair of Bone Defects Caused by Osteoporosis" Molecules 26, no. 15: 4451. https://doi.org/10.3390/molecules26154451
APA StyleMai, X., Kang, Z., Wang, N., Qin, X., Xie, W., & Song, F. (2021). Oxygen Plasma Technology-Assisted Preparation of Three-Dimensional Reduced Graphene Oxide/Polypyrrole/Strontium Composite Scaffold for Repair of Bone Defects Caused by Osteoporosis. Molecules, 26(15), 4451. https://doi.org/10.3390/molecules26154451




