Astatine Facing Janus: Halogen Bonding vs. Charge-Shift Bonding
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Topological Analyses of the C6X6 XB Donors
3.2. C6X6…NMe3 Complexes
3.3. C6X6…C6Y6 Complexes
3.4. C2BX3…C2BY3 Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Politzer, P.; Murray, J.S.; Clark, T. Halogen Bonding and Other σ-Hole Interactions: A Perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef]
- Kolář, M.H.; Hobza, P. Computer Modeling of Halogen Bonds and Other σ-Hole Interactions. Chem. Rev. 2016, 116, 5155–5187. [Google Scholar] [CrossRef] [Green Version]
- Parisini, E.; Metrangolo, P.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen Bonding in Halocarbon–Protein Complexes: A Structural Survey. Chem. Soc. Rev. 2011, 40, 2267–2278. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Xu, Z.; Li, H.; Liu, H.; Zhu, W. Halogen Bonding for Rational Drug Design and New Drug Discovery. Expert Opin. Drug Discov. 2012, 7, 375–383. [Google Scholar] [CrossRef]
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.C.; Boeckler, F.M. Principles and Applications of Halogen Bonding in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2013, 56, 1363–1388. [Google Scholar] [CrossRef]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen Bonds in Crystal Engineering: Like Hydrogen Bonds yet Different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef]
- Berger, G.; Soubhye, J.; Meyer, F. Halogen Bonding in Polymer Science: From Crystal Engineering to Functional Supramolecular Polymers and Materials. Polym. Chem. 2015, 6, 3559–3580. [Google Scholar] [CrossRef]
- Bulfield, D.; Huber, S.M. Halogen Bonding in Organic Synthesis and Organocatalysis. Chem. Eur. J. 2016, 22, 14434–14450. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zang, S.-Q.; Wang, L.-Y.; Mak, T.C.W. Halogen Bonding: A Powerful, Emerging Tool for Constructing High-Dimensional Metal-Containing Supramolecular Networks. Coord. Chem. Rev. 2016, 308, 1–21. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, K.E.; Merz, K.M. Insights into the Strength and Origin of Halogen Bonding: The Halobenzene-Formaldehyde Dimer. J. Phys. Chem. A 2007, 111, 1688–1694. [Google Scholar] [CrossRef]
- Lu, Y.-X.; Zou, J.-W.; Wang, Y.-H.; Jiang, Y.-J.; Yu, Q.-S. Ab Initio Investigation of the Complexes between Bromobenzene and Several Electron Donors: Some Insights into the Magnitude and Nature of Halogen Bonding Interactions. J. Phys. Chem. A 2007, 111, 10781–10788. [Google Scholar] [CrossRef]
- Riley, K.E.; Murray, J.S.; Fanfrlík, J.; Řezáč, J.; Solá, R.J.; Concha, M.C.; Ramos, F.M.; Politzer, P. Halogen Bond Tunability I: The Effects of Aromatic Fluorine Substitution on the Strengths of Halogen-Bonding Interactions Involving Chlorine, Bromine, and Iodine. J. Mol. Model. 2011, 17, 3309–3318. [Google Scholar] [CrossRef] [PubMed]
- Adasme-Carreño, F.; Muñoz-Gutierrez, C.; Alzate-Morales, J.H. Halogen Bonding in Drug-like Molecules: A Computational and Systematic Study of the Substituent Effect. RSC Adv. 2016, 6, 61837–61847. [Google Scholar] [CrossRef] [Green Version]
- Tsuzuki, S.; Uchimaru, T.; Wakisaka, A.; Ono, T. Magnitude and Directionality of Halogen Bond of Benzene with C6F5X, C6H5X, and CF3X (X = I, Br, Cl, and F). J. Phys. Chem. A 2016, 120, 7020–7029. [Google Scholar] [CrossRef] [PubMed]
- Chernysheva, M.V.; Bulatova, M.; Ding, X.; Haukka, M. Influence of Substituents in the Aromatic Ring on the Strength of Halogen Bonding in Iodobenzene Derivatives. Cryst. Growth Des. 2020, 20, 7197–7210. [Google Scholar] [CrossRef]
- Otte, F.; Kleinheider, J.; Hiller, W.; Wang, R.; Englert, U.; Strohmann, C. Weak yet Decisive: Molecular Halogen Bond and Competing Weak Interactions of Iodobenzene and Quinuclidine. J. Am. Chem. Soc. 2021, 143, 4133–4137. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen Bonding: The σ-Hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef]
- Alkorta, I.; Blanco, F.; Solimannejad, M.; Elguero, J. Competition of Hydrogen Bonds and Halogen Bonds in Complexes of Hypohalous Acids with Nitrogenated Bases. J. Phys. Chem. A 2008, 112, 10856–10863. [Google Scholar] [CrossRef]
- Hill, J.G.; Hu, X. Theoretical Insights into the Nature of Halogen Bonding in Prereactive Complexes. Chem. Eur. J. 2013, 19, 3620–3628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilbur, D.S. Enigmatic Astatine. Nat. Chem. 2013, 5, 246. [Google Scholar] [CrossRef]
- Guo, N.; Maurice, R.; Teze, D.; Graton, J.; Champion, J.; Montavon, G.; Galland, N. Experimental and Computational Evidence of Halogen Bonds Involving Astatine. Nat. Chem. 2018, 10, 428–434. [Google Scholar] [CrossRef]
- Liu, L.; Guo, N.; Champion, J.; Graton, J.; Montavon, G.; Galland, N.; Maurice, R. Towards a Stronger Halogen Bond Involving Astatine: Unexpected Adduct with Bu3PO Stabilized by Hydrogen Bonding. Chem. Eur. J. 2020, 26, 3713–3717. [Google Scholar] [CrossRef]
- Graton, J.; Rahali, S.; Questel, J.-Y.L.; Montavon, G.; Pilmé, J.; Galland, N. Spin–Orbit Coupling as a Probe to Decipher Halogen Bonding. Phys. Chem. Chem. Phys. 2018, 20, 29616–29624. [Google Scholar] [CrossRef]
- Sarr, S.; Graton, J.; Rahali, S.; Montavon, G.; Galland, N. Delocalized Relativistic Effects, from the Viewpoint of Halogen Bonding. Phys. Chem. Chem. Phys. 2021, 23, 4064–4074. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Tagle, R.; Alvarado-Soto, L.; Villavicencio-Wastavino, A.; Alvarez-Thon, L. Relativistic Effects on the Aromaticity of the Halogenated Benzenes: C6X6, X = H, F, Cl, Br, I, At. Phys. Chem. Chem. Phys. 2016, 18, 25751–25755. [Google Scholar] [CrossRef]
- Mitin, A.V.; van Wüllen, C. Two-Component Relativistic Density-Functional Calculations of the Dimers of the Halogens from Bromine through Element 117 Using Effective Core Potential and All-Electron Methods. J. Chem. Phys. 2006, 124, 064305. [Google Scholar] [CrossRef]
- Yang, D.-D.; Wang, F. Structures and Stabilities of Group 17 Fluorides EF3 (E = I, At, and Element 117) with Spin-Orbit Coupling. Phys. Chem. Chem. Phys. 2012, 14, 15816–15825. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Sergentu, D.-C.; David, G.; Montavon, G.; Maurice, R.; Galland, N. Scrutinizing “Invisible” Astatine: A Challenge for Modern Density Functionals. J. Comput. Chem. 2016, 37, 1345–1354. [Google Scholar] [CrossRef]
- Galland, N.; Montavon, G.; Questel, J.-Y.L.; Graton, J. Quantum Calculations of At-Mediated Halogen Bonds: On the Influence of Relativistic Effects. New J. Chem. 2018, 42, 10510–10517. [Google Scholar] [CrossRef]
- Sarr, S.; Graton, J.; Montavon, G.; Pilmé, J.; Galland, N. On the Interplay between Charge-Shift Bonding and Halogen Bonding. ChemPhysChem 2020, 21, 240–250. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.A. Systematically Convergent Basis Sets with Relativistic Pseudopotentials. I. Correlation Consistent Basis Sets for the Post-d Group 13–15 Elements. J. Chem. Phys. 2003, 119, 11099–11112. [Google Scholar] [CrossRef] [Green Version]
- Peterson, K.A.; Shepler, B.C.; Figgen, D.; Stoll, H. On the Spectroscopic and Thermochemical Properties of ClO, BrO, IO, and Their Anions. J. Phys. Chem. A 2006, 110, 13877–13883. [Google Scholar] [CrossRef]
- Weigend, F.; Baldes, A. Segmented contracted basis sets for one- and two-component Dirac–Fock effective core potentials. J. Chem. Phys. 2010, 133, 174102. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Rappoport, D.; Furche, F. Property-optimized Gaussian basis sets for molecular response calculations. J. Chem. Phys. 2010, 133, 134105. [Google Scholar] [CrossRef] [PubMed]
- Boys, S.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- TURBOMOLE. A Development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH, Since 2007. Available online: http://www.turbomole.com (accessed on 30 June 2021).
- Chattaraj, P.K.; Sarkar, A.U.; Roy, D.R. Electrophilicity Index. Chem. Rev. 2006, 106, 2065–2091. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, E.K. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Silvi, B.; Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nat. Cell Biol. 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Gillespie, R.J.; Robinson, E.A. Gilbert N. Lewis and the chemical bond: The electron pair and the octet rule from 1916 to the present day. J. Comput. Chem. 2006, 28, 87–97. [Google Scholar] [CrossRef]
- Llusar, R.; Beltrán, A.; Andrés, J.; Noury, S.; Silvi, B. Topological analysis of electron density in depleted homopolar chemical bonds. J. Comput. Chem. 1999, 20, 1517–1526. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; International Series of Monographs on Chemistry; Oxford University Press: New York, NY, USA, 1994; ISBN 978-0-19-855865-1. [Google Scholar]
- Koritsanszky, T.S.; Coppens, P. Chemical Applications of X-ray Charge-Density Analysis. Chem. Rev. 2001, 101, 1583–1628. [Google Scholar] [CrossRef]
- Matta, C.F.; Boyd, R.J. The Quantum Theory of Atoms in Molecules: From Solid State to DNA and Drug Design; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 978-3-527-61069-3. [Google Scholar]
- Bader, R.F.W.; Essen, H. The characterization of atomic interactions. J. Chem. Phys. 1984, 80, 1943–1960. [Google Scholar] [CrossRef]
- Matta, C.F.; Boyd, R.J. An Introduction to the Quantum Theory of Atoms in Molecules. In The Quantum Theory of Atoms in Molecules; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 1–34. ISBN 978-3-527-61070-9. [Google Scholar]
- Fradera, X.; Poater, J.; Simon, S.; Duran, M.; Solà, M. Electron-pairing analysis from localization and delocalization indices in the framework of the atoms-in-molecules theory. Theor. Chem. Accounts 2002, 108, 214–224. [Google Scholar] [CrossRef]
- Pilmé, J.; Renault, E.; Bassal, F.; Amaouch, M.; Montavon, G.; Galland, N. QTAIM Analysis in the Context of Quasirelativistic Quantum Calculations. J. Chem. Theory Comput. 2014, 10, 4830–4841. [Google Scholar] [CrossRef]
- Pilmé, J.; Renault, E.; Ayed, T.; Montavon, G.; Galland, N. Introducing the ELF Topological Analysis in the Field of Quasirelativistic Quantum Calculations. J. Chem. Theory Comput. 2012, 8, 2985–2990. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, D.; Pilmé, J. New insights in quantum chemical topology studies using numerical grid-based analyses. J. Comput. Chem. 2011, 32, 3207–3217. [Google Scholar] [CrossRef]
- Amaouch, M.; Montavon, G.; Galland, N.; Pilmé, J. What can tell the quantum chemical topology on carbon—Astatine bonds? Mol. Phys. 2015, 114, 1326–1333. [Google Scholar] [CrossRef] [Green Version]
- Amaouch, M.; Renault, E.; Montavon, G.; Galland, N.; Pilmé, J. Quantum Chemical Topology in the Field of Qua-sirelativistic Quantum Calculations. In Applications of Topological Methods in Molecular Chemistry; Challenges and Advances in Computational Chemistry and Physics; Chauvin, R., Lepetit, C., Silvi, B., Alikhani, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 553–582. ISBN 978-3-319-29022-5. [Google Scholar]
- Ayed, T.; Pilmé, J.; Teze, D.; Bassal, F.; Barbet, J.; Chérel, M.; Champion, J.; Maurice, R.; Montavon, G.; Galland, N. 211 At-labeled agents for alpha-immunotherapy: On the in vivo stability of astatine-agent bonds. Eur. J. Med. Chem. 2016, 116, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Amaouch, M.; Sergentu, D.-C.; Steinmetz, D.; Maurice, R.; Galland, N.; Pilmé, J. The bonding picture in hypervalent XF3(X = Cl, Br, I, At) fluorides revisited with quantum chemical topology. J. Comput. Chem. 2017, 38, 2753–2762. [Google Scholar] [CrossRef] [PubMed]
- Pech, C.G.; Haase, P.A.B.; Sergentu, D.; Borschevsky, A.; Pilmé, J.; Galland, N.; Maurice, R. Quantum chemical topology at the spin–orbit configuration interaction level: Application to astatine compounds. J. Comput. Chem. 2020, 41, 2055–2065. [Google Scholar] [CrossRef]
- Noury, S.; Colonna, F.; Savin, A.; Silvi, B. Analysis of the delocalization in the topological theory of chemical bond. J. Mol. Struct. 1998, 450, 59–68. [Google Scholar] [CrossRef]
- Savin, A.; Silvi, B.; Colonna, F. Topological analysis of the electron localization function applied to delocalized bonds. Can. J. Chem. 1996, 74, 1088–1096. [Google Scholar] [CrossRef]
- Shaik, S.; Maitre, P.; Sini, G.; Hiberty, P.C. The charge-shift bonding concept. Electron-pair bonds with very large ionic-covalent resonance energies. J. Am. Chem. Soc. 1992, 114, 7861–7866. [Google Scholar] [CrossRef]
- Shaik, S.; Danovich, D.; Wu, W.; Hiberty, P.C. Charge-shift bonding and its manifestations in chemistry. Nat. Chem. 2009, 1, 443–449. [Google Scholar] [CrossRef]
- Shaik, S.; Danovich, D.; Silvi, B.; Lauvergnat, D.L.; Hiberty, P.C. Charge-Shift Bonding—A Class of Electron-Pair Bonds That Emerges from Valence Bond Theory and Is Supported by the Electron Localization Function Approach. Chem. Eur. J. 2005, 11, 6358–6371. [Google Scholar] [CrossRef]
- Outeiral, C.; Vincent, M.A.; Pendás, M.; Popelier, P.L.A. Revitalizing the concept of bond order through delocalization measures in real space. Chem. Sci. 2018, 9, 5517–5529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvi, B.; Gillespie, R.J.; Gatti, C. Electron Density Analysis. In Comprehensive Inorganic Chemistry II, 2nd ed.; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 187–226. ISBN 978-0-08-096529-1. [Google Scholar]
- Zhang, L.; Ying, F.; Wu, W.; Hiberty, P.C.; Shaik, S. Topology of Electron Charge Density for Chemical Bonds from Valence Bond Theory: A Probe of Bonding Types. Chem. Eur. J. 2009, 15, 2979–2989. [Google Scholar] [CrossRef]
- Réal, F.; Gomes, A.S.P.; Martínez, Y.O.G.; Ayed, T.; Galland, N.; Masella, M.; Vallet, V. Structural, dynamical, and transport properties of the hydrated halides: How do At− bulk properties compare with those of the other halides, from F− to I−? J. Chem. Phys. 2016, 144, 124513. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E. Chemical Bonds without Bonding Electron Density ? Does the Difference Electron-Density Analysis Suffice for a Description of the Chemical Bond? Angew. Chem. Int. Ed. 1984, 23, 627–628. [Google Scholar] [CrossRef]
- Mantina, M.; Chamberlin, A.C.; Valero, R.; Cramer, C.; Truhlar, D.G. Consistent van der Waals Radii for the Whole Main Group. J. Phys. Chem. A 2009, 113, 5806–5812. [Google Scholar] [CrossRef] [Green Version]
- Meyer, E.A.; Castellano, R.K.; Diederich, F. Interactions with Aromatic Rings in Chemical and Biological Recognition. Angew. Chem. Int. Ed. 2003, 42, 4120. [Google Scholar] [CrossRef]
- Hoeben, F.J.M.; Jonkheijm, P.; Meijer, E.W.; Schenning, A.P.H.J. About Supramolecular Assemblies of π-Conjugated Systems. Chem. Rev. 2005, 105, 1491–1546. [Google Scholar] [CrossRef] [PubMed]
- Černý, J.; Hobza, P. Non-covalent interactions in biomacromolecules. Phys. Chem. Chem. Phys. 2007, 9, 5291–5303. [Google Scholar] [CrossRef] [PubMed]
- Pitoňák, M.; Neogrády, P.; Řezáč, J.; Jurečka, P.; Urban, M.; Hobza, P. Benzene Dimer: High-Level Wave Function and Density Functional Theory Calculations. J. Chem. Theory Comput. 2008, 4, 1829–1834. [Google Scholar] [CrossRef]
- Balucani, N.; Asvany, O.; Lee, A.Y.T.; Kaiser, R.I.; Galland, N.; Hannachi, Y. Observation of Borirene from Crossed Beam Reaction of Boron Atoms with Ethylene. J. Am. Chem. Soc. 2000, 122, 11234–11235. [Google Scholar] [CrossRef]
- Wilbur, D. [211At]Astatine-Labeled Compound Stability: Issues with Released [211At]Astatide and Development of Labeling Reagents to Increase Stability. Curr. Radiopharm. 2008, 1, 144–176. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Zalutsky, M.R. Applications of 211At and 223Ra in Targeted Alpha-Particle Radiotherapy. Curr. Radiopharm. 2011, 4, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Meyer, G.-J. Astatine. J. Label. Compd. Radiopharm. 2018, 61, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
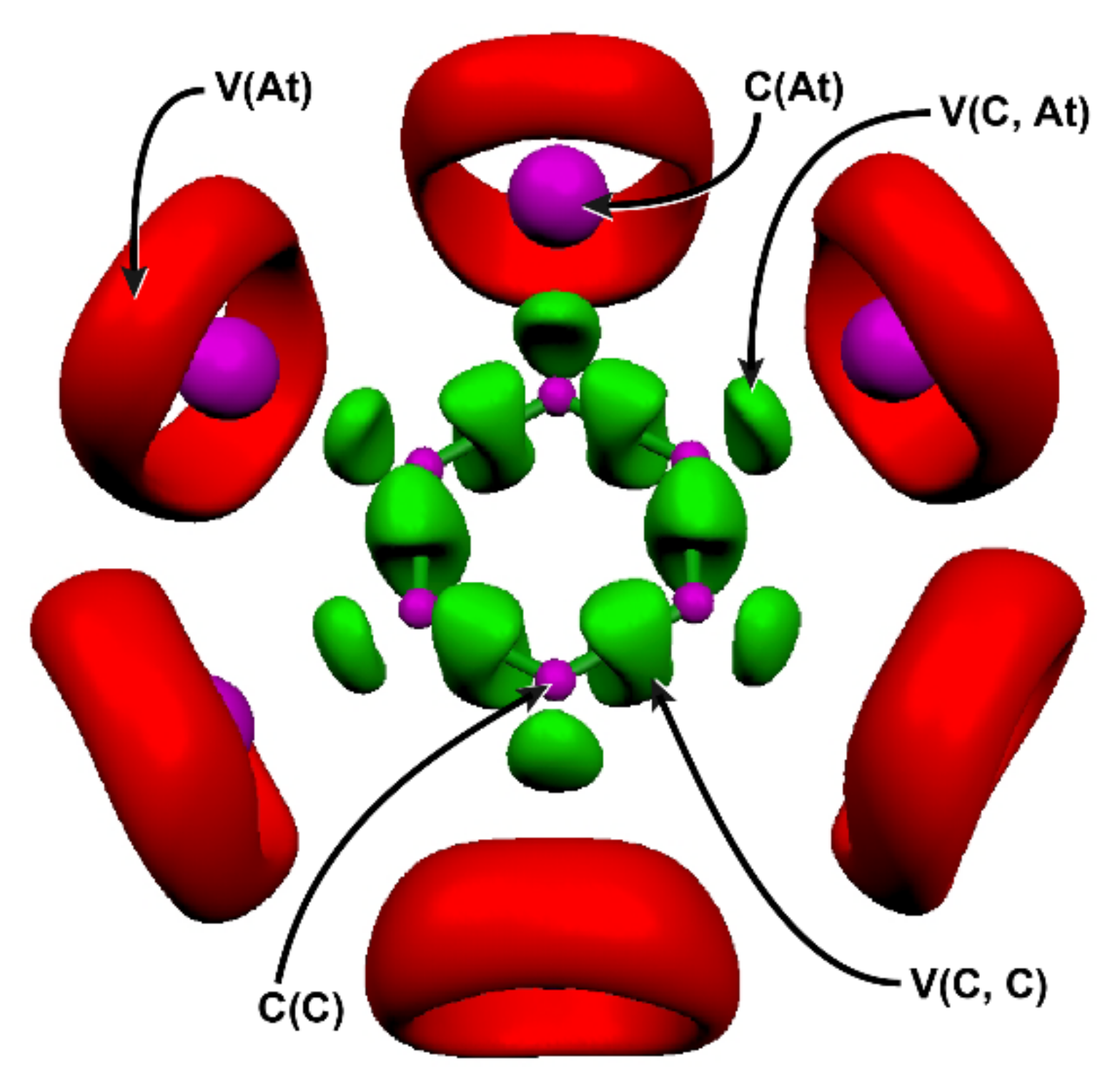

| V(C, C): N | V(C, C): λ | C(X) + V(X): N | V(X): σ2 | V(C, C): N | V(C, X): λ | |
|---|---|---|---|---|---|---|
| X = I | 2.90 | 0.48 | 24.30 | 1.03 | 1.72 | 0.61 |
| ∆SO a | +0.03 | −0.00 | −0.22 | −0.00 | −0.08 | +0.00 |
| X = At | 2.87 | 0.48 | 24.34 | 1.12 | 1.70 | 0.63 |
| ∆SO a | +0.00 | +0.00 | +0.01 | +0.00 | −0.01 | +0.01 |
| ρbb | ∇2ρb c | |Vb|/Gb d | δ(C, X) e | q(X) f | |
|---|---|---|---|---|---|
| X = I | 0.13 | −0.03 | 2.13 | 1.11 | 0.19 |
| ∆SO a | −0.00 | +0.00 | −0.01 | −0.01 | −0.00 |
| X = At | 0.10 | 0.04 | 1.82 | 0.99 | 0.19 |
| ∆SO a | −0.01 | +0.01 | −0.07 | −0.09 | −0.00 |
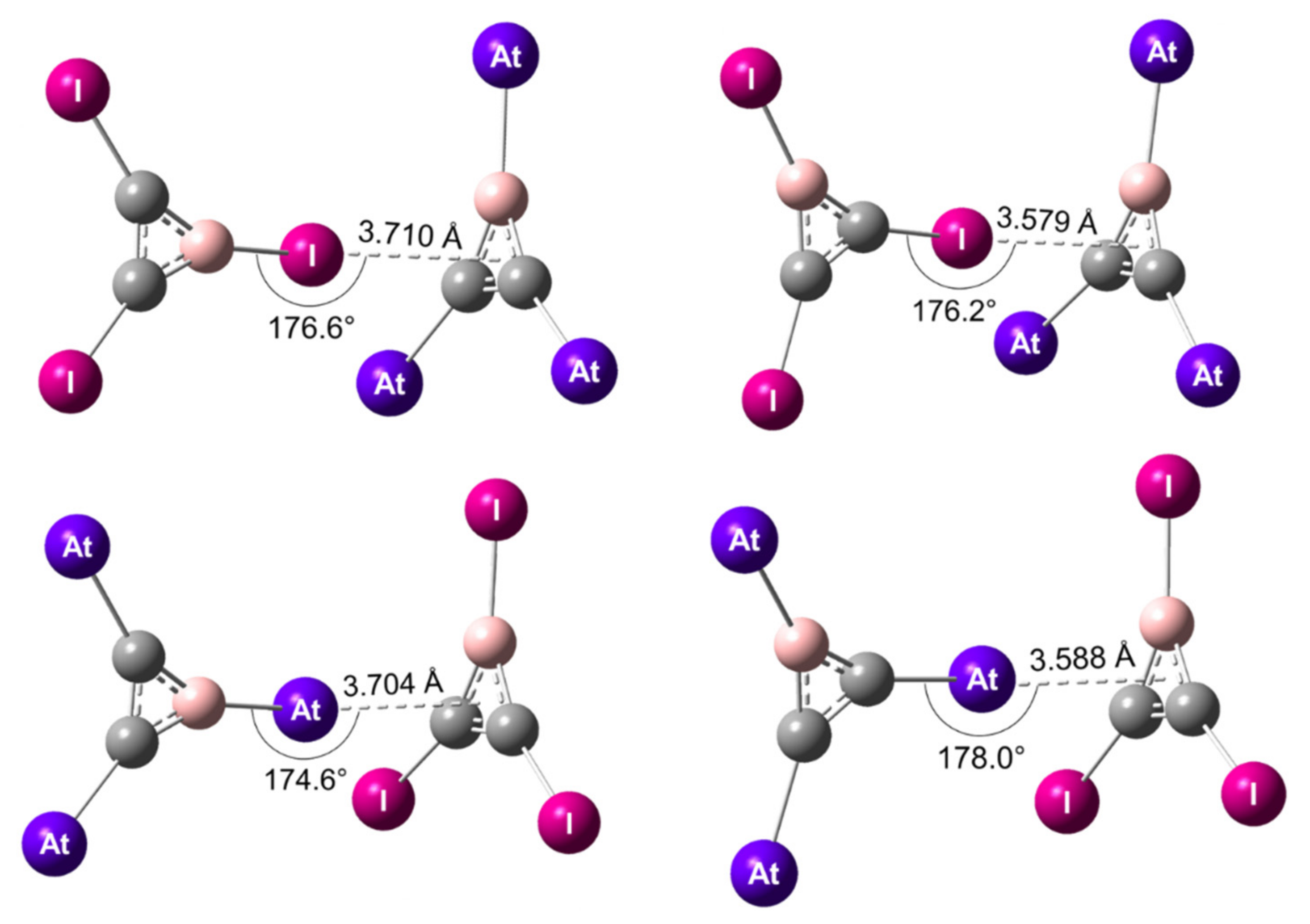
| ΔECP (kJ/mol) | dX…N (Å) | ∆dC–X (Å) | αCXN (°) | |
|---|---|---|---|---|
| I–XB | −32.6 | 2.835 | 0.026 | 179.8 |
| sr-PW6B95-D3 | ||||
| At–XB | −40.9 | 2.826 | 0.036 | 179.7 |
| I–XB | −32.6 | 2.799 a | 0.028 a | 179.7 a |
| sr-MP2 | ||||
| At–XB | −32.6 | 2.800 a | 0.037 a | 179.8 a |
| I–XB | −32.1 | 2.842 | 0.024 | 180.0 |
| ∆SO | 0.5 | 0.007 | −0.002 | 0.2 |
| 2c-PW6B95-D3 | ||||
| At–XB | −36.0 | 2.895 | 0.019 | 179.5 |
| ∆SO | 4.9 | 0.069 | −0.017 | −0.2 |
| I–XB | −35.3 | – | – | – |
| ∆SO b | 0.3 | – | – | – |
| 2c-MP2 | – | – | – | |
| At–XB | −39.9 | – | – | – |
| ∆SO b | 3.6 | – | – | – |
| ΔECP (kJ/mol) | IB–XB | IC–XB | AtB–XB | AtC–XB |
|---|---|---|---|---|
| 2c-PW6B95-D3/TZVPPD | −13.7 | −15.8 | −13.9 | −15.6 |
| 2c-MP2/TZVPPD | −14.2 a | −16.7a | −14.0 a | −15.9 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarr, S.; Pilmé, J.; Montavon, G.; Le Questel, J.-Y.; Galland, N. Astatine Facing Janus: Halogen Bonding vs. Charge-Shift Bonding. Molecules 2021, 26, 4568. https://doi.org/10.3390/molecules26154568
Sarr S, Pilmé J, Montavon G, Le Questel J-Y, Galland N. Astatine Facing Janus: Halogen Bonding vs. Charge-Shift Bonding. Molecules. 2021; 26(15):4568. https://doi.org/10.3390/molecules26154568
Chicago/Turabian StyleSarr, Serigne, Julien Pilmé, Gilles Montavon, Jean-Yves Le Questel, and Nicolas Galland. 2021. "Astatine Facing Janus: Halogen Bonding vs. Charge-Shift Bonding" Molecules 26, no. 15: 4568. https://doi.org/10.3390/molecules26154568
APA StyleSarr, S., Pilmé, J., Montavon, G., Le Questel, J.-Y., & Galland, N. (2021). Astatine Facing Janus: Halogen Bonding vs. Charge-Shift Bonding. Molecules, 26(15), 4568. https://doi.org/10.3390/molecules26154568






