High-Throughput Griess Assay of Nitrite and Nitrate in Plasma and Red Blood Cells for Human Physiology Studies under Extreme Conditions
Abstract
:1. Introduction
2. Results
2.1. Optimization of Reaction Parameters
2.1.1. Color Development and Nitrite Concentration Measurement
2.1.2. Nitrate to Nitrite Reduction and Total Nitrogen Oxides Measurement
2.1.3. Linearity, Detection Limit, Accuracy, and Precision
2.2. Measurement in Plasma
2.2.1. Pre-Analytical Treatment of Plasma
2.2.2. Nitrite and Nitrate Recovery in Plasma
2.2.3. Nitrite and Nitrate Levels in Blood Plasma
2.3. Measurement in Red Blood Cells
2.3.1. Pre-Analytical Treatment and Characterization of Red Blood Cells
2.3.2. Nitrite and Nitrate Recovery in Red Blood Cell Hemolysate
2.3.3. Nitrite and Nitrate Levels in Red Blood Cells
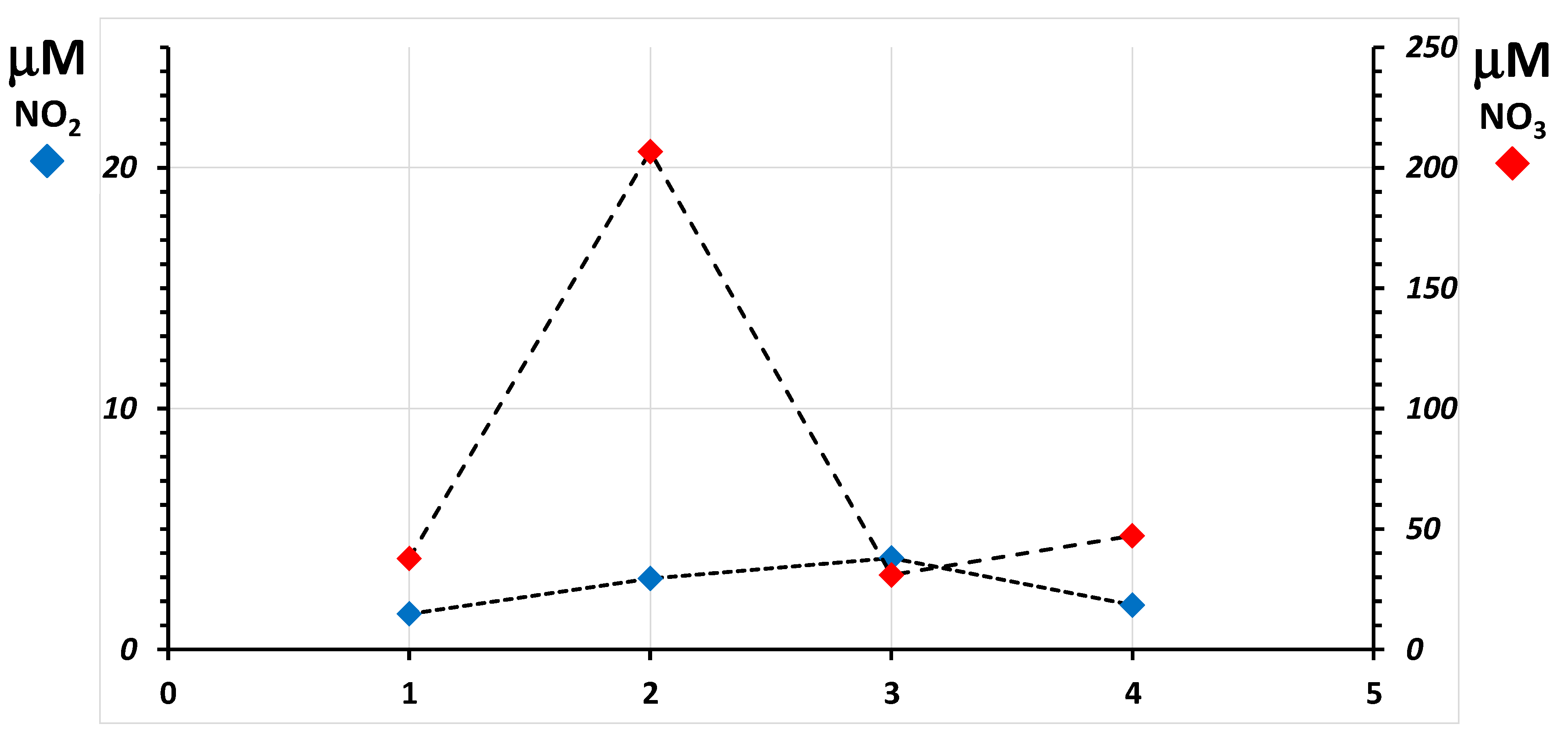
2.4. Pilot Study for the Fitness-for-Purpose Assessment
3. Discussion
4. Materials and Methods
4.1. Equipment
4.1.1. Reagents
4.1.2. Glass and Plasticware
4.1.3. Measurement Equipment
4.1.4. Reagent and Standard Solutions
4.2. Sample Preparation and Measurement
4.2.1. Protein Precipitation
4.2.2. Sample Plate Preparation and Measurement
- (a)
- In total, 100 μL of the sample (from the protein precipitation steps above) or of the water-based calibration solution is pipetted.
- (b)
- A total of 50 μL of the combined Griess reagent mixture is added with the 8-channel multiple pipettes.
- (c)
- Then, for the measurement of nitrite + nitrate, 100 μL of Vanadium(III) chloride (25 mM in 1 M HCl) solution is added with the 8-channel multiple pipettes. For the measurement of nitrite alone, 100 μL of 1 M HCl is added in place of VCl3 solution with the 8-channel multiple pipettes.
4.2.3. Standard Curves Preparation
4.2.4. Sample Plate Reading
4.3. Calculation
4.3.1. Data Input
4.3.2. Calculation of Analyte Concentration
4.3.3. Calculation of the Extended Error of Determination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
Appendix A.1. Effect of Hemolysate Dilution on Detection of Nitrite and Nitrate in RBC

Appendix A.2. Assessment of the Extended Uncertainty for Measurement in Red Blood Cells
| Parameter | HGB 1 | HT 2 | Hb 3 | NO2− 4 | NOx 5 | Notes |
|---|---|---|---|---|---|---|
| interval | 12.7–17.2 | 35.3–48.4 | 1.4–1.9 | 2–20 | 4–90 | |
| StdErr 6 | 0.8 | 2.7 | 0.05 | 0.6 | 2.3 | Employed to calculate uncertainty in sums and differences |
| RSE 7 | 5.2 | 5.9 | 2.9 | 11 | 8 | Employed to calculate uncertainty in products and ratios |
References
- Ignarro, L.J. Nitric Oxide: A Unique Endogenous Signaling Molecule in Vascular Biology. Biosci. Rep. 1999, 19, 51–71. [Google Scholar] [CrossRef]
- Levett, D.Z.; Radford, E.J.; Menassa, D.A.; Graber, E.F.; Morash, A.J.; Hoppeler, H.; Clarke, K.; Martin, D.S.; Ferguson-Smith, A.; Montgomery, H.E.; et al. Acclimatization of skeletal muscle mitochondria to high-altitude hypoxia during an ascent of Everest. FASEB J. 2012, 26, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, S.; Marzorati, M.; Healey, B.; Terraneo, L.; Vezzoli, A.; Della Bella, S.; Dicasillati, R.; Samaja, M. Lack of acclimatization to chronic hypoxia in humans in the Antarctica. Sci. Rep. 2017, 7, 1–6:18090. [Google Scholar] [CrossRef] [Green Version]
- Theunissen, S.; Guerrero, F.; Sponsiello, N.; Cialoni, D.; Pieri, M.; Germonpré, P.; Obeid, G.; Tillmans, F.; Papadopoulou, V.; Hemelryck, W.; et al. Nitric oxide-related endothelial changes in breath-hold and scuba divers. Undersea Hyperb. Med. 2013, 40, 135–144. [Google Scholar]
- Cialoni, D.; Brizzolari, A.; Samaja, M.; Pieri, M.; Marroni, A. Altered Venous Blood Nitric Oxide Levels at Depth and Related Bubble Formation during Scuba Diving. Front. Physiol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Cialoni, D.; Brizzolari, A.; Samaja, M.; Bosco, G.; Paganini, M.; Pieri, M.; Lancellotti, V.; Marroni, A. Nitric Oxide and Oxidative Stress Changes at Depth in Breath-Hold Diving. Front. Physiol. 2021, 11, 609642. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, I.; Boso, A.E.; O’Neill, T.J.; Bennett, P.B.; Piantadosi, C.A. Nitric oxide and cerebral blood flow responses to hyperbaric oxygen. J. Appl. Physiol. 2000, 88, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, S.; Sponsiello, N.; Rozloznik, M.; Germonpré, P.; Guerrero, F.; Cialoni, D.; Balestra, C. Oxidative stress in breath-hold divers after repetitive dives. Diving Hyperb. Med. J. 2013, 43, 63–66. [Google Scholar]
- Nossum, V.; Hjelde, A.; Brubakk, A.O. Small amounts of venous gas embolism cause delayed impairment of endothelial function and increase polymorphonuclear neutrophil infiltration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2001, 86, 209–214. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Rodriguez-Mateos, A.; Kuhnle, G.; Brown, G.; Feelisch, M.; Kelm, M. A multilevel analytical approach for detection and visualization of intracellular NO production and nitrosation events using diaminofluoresceins. Free Radic. Biol. Med. 2012, 53, 2146–2158. [Google Scholar] [CrossRef] [Green Version]
- Tsikas, D. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free. Radic. Res. 2005, 39, 797–815. [Google Scholar] [CrossRef]
- Griess, P. Preliminary notice of the reaction of nitrous acid with picramic acid and aminonitrophenol. Ann. Chem. Pharm. 1858, 106, 123–125. [Google Scholar]
- Pai, S.-C.; Yang, C.-C.; Riley, J.P. Formation kinetics of the pink azo dye in the determination of nitrite in natural waters. Anal. Chim. Acta 1990, 232, 345–349. [Google Scholar] [CrossRef]
- Ehling, S.; Reddy, T.M. Determination of Nitrite in Milk- and Soy-Based Nutritional Ingredients by Derivatization with 2,3-Diaminonaphthalene and Fluorescence Spectrometry. J. AOAC Int. 2017, 100, 1831–1835. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.A.; Horwáth, W.R. Spectrophotometric Determination of Nitrate with a Single Reagent. Anal. Lett. 2003, 36, 2713–2722. [Google Scholar] [CrossRef]
- Schnetger, B.; Lehners, C. Determination of nitrate plus nitrite in small volume marine water samples using vanadium(III)chloride as a reduction agent. Mar. Chem. 2014, 160, 91–98. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Nitrite and Nitrate Measurement by Griess Reagent in Human Plasma: Evaluation of Interferences and Standardization. Methods Enzymol. 2008, 440, 361–380. [Google Scholar] [CrossRef]
- Casanova, J.A.; Gross, L.K.; McMullen, S.E.; Schenck, F.J. Use of Griess Reagent Containing Vanadium (III) for Post-Column Derivatization and Simultaneous Determination of Nitrite and Nitrate in Baby Food. J. AOAC Int. 2006, 89, 447–451. [Google Scholar] [CrossRef] [Green Version]
- Van Ombergen, A.; Rossiter, A.; Ngo-Anh, T.J. ‘White Mars’—Nearly two decades of biomedical research at the Antarctic Concordia station. Exp. Physiol. 2021, 106, 6–17. [Google Scholar] [CrossRef]
- Cialoni, D.; Brizzolari, A.; Samaja, M.; Bosco, G.; Paganini, M.; Sponsiello, N.; Lancellotti, V.; Marroni, A. Endothelial Nitric Oxide production and Antioxidant Response in BH Diving: Genetic predisposition or environment related? Front. Physiol. 2021, 12, 692204. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.C.; Jane, C. Statistics for Analytical Chemistry; Ellis Horwood Ltd: Chichester, UK, 1984. [Google Scholar]
- Ottolenghi, S.; Rubino, F.M.; Sabbatini, G.; Coppola, S.; Veronese, A.; Chiumello, D.; Paroni, R. Oxidative Stress Markers to Investigate the Effects of Hyperoxia in Anesthesia. Int. J. Mol. Sci. 2019, 20, 5492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feuerecker, M.; Crucian, B.E.; Quintens, R.; Buchheim, J.; Salam, A.P.; Rybka, A.; Moreels, M.; Strewe, C.; Stowe, R.; Mehta, S.; et al. Immune sensitization during 1 year in the Antarctic high-altitude Concordia Environment. Allergy 2019, 74, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Strewe, C.; Thieme, D.; Dangoisse, C.; Fiedel, B.; Berg, F.V.D.; Bauer, H.; Salam, A.P.; Gössmann-Lang, P.; Campolongo, P.; Moser, D.; et al. Modulations of Neuroendocrine Stress Responses During Confinement in Antarctica and the Role of Hypobaric Hypoxia. Front. Physiol. 2018, 9, 1647. [Google Scholar] [CrossRef]
- Dei Cas, M.; Paroni, R.; Saccardo, A.; Casagni, E.; Arnoldi, S.; Gambaro, V.; Saresella, M.; Mario, C.; La Rosa, F.; Marventano, I.; et al. A straightforward LC-MS/MS analysis to study serum profile of short and medium chain fatty acids. J. Chromatogr. B 2020, 1154, 121982. [Google Scholar] [CrossRef] [PubMed]






| Nominal (μM) | Recalculated (μM) | SD (μM) | Accuracy ± % | Precision CV% |
|---|---|---|---|---|
| 0 | 0.2 | 0.6 | ||
| 5 | 3.8 | 1.2 | −24% | 31% |
| 10 | 11.3 | 2.1 | 13% | 19% |
| 20 | 21.9 | 3.0 | 10% | 14% |
| 50 | 54.4 | 5.1 | 9% | 9% |
| 100 | 101.5 | 2.1 | 2% | 2% |
| 150 2 | 149.9 | 1.2 | 0% | 1% |
| 200 | 198.3 | 2.4 | −1% | 1% |
| Nominal (μM) | Recalculated (μM) | SD (μM) | Accuracy ± % | Precision CV% |
|---|---|---|---|---|
| 0 | −2.2 | 5.5 | ||
| 5 | 5.5 | 2.6 | −11% | 48% |
| 10 | 9.5 | 1.1 | 3% | 11% |
| 20 | 19.9 | 4.7 | −22.0% | 24% |
| 50 | 51.3 | 2.1 | 8.2% | 4% |
| 100 | 101.2 | 2.8 | 3.4% | 3% |
| 150 2 | 149.2 | 6.2 | −0.5% | 4% |
| 200 | 199.2 | 1.6 | −0.7% | 1% |
| Sample | Nominal (μM) 1 | Recalculated (μM) 1 | Accuracy ± % | Precision CV% | Rec % |
|---|---|---|---|---|---|
| Plasma (not fortified) 1 | 1.3 | 1.1% | |||
| NO2− -fortified. (10.3 μM) 1 | 11.6 | 11.5 | 99.1% | 1.3% | 99.4% |
| NO2− -fortified. (20.6 μM) 1 | 21.9 | 22.0 | 100.5% | 1.4% | 100.5% |
| Plasma (not fortified) 1 | 40.4 | 6% | |||
| NO3− -fortified (21.0 μM) 1 | 61.4 | 61.2 | 99.7% | 2% | 97.3% |
| NO3− -fortified (42.0 μM) 1 | 82.4 | 87.4 | 106.0% | 3% | 104.6% |
| Case Studies | Subj. ID | NO2− 1 | NOx 1 | NO3− 1 |
|---|---|---|---|---|
| Control Subjects (n = 6) | AB79M | 1.9 ± 1.3 | 34.8 ± 2.4 | 32.9 ± 2.7 |
| FP89F | 2.1 ± 1.3 | 44.2 ± 2.4 | 42.1 ± 2.7 | |
| FR60M | 2.1 ± 1.3 | 32.0 ± 2.4 | 29.9 ± 2.7 | |
| GP97F | 1.9 ± 1.3 | 28.1 ± 2.4 | 26.2 ± 2.7 | |
| GS82M | 2.5 ± 1.3 | 29.5 ± 2.4 | 27.0 ± 2.7 | |
| MS51M | 2.3 ± 1.3 | 40.6 ± 2.4 | 38.3 ± 2.7 | |
| Mean | 2.1 | 34.9 | 32.7 | |
| S.D. | 0.3 | 6.4 | 6.4 | |
| CV% | 14.2 | 18.3 | 19.5 |
| Sample | Nominal (μM) 1 | Recalculated (μM) 1 | Accuracy ± % | Precision CV% | Rec % |
|---|---|---|---|---|---|
| RBC hemolysate (not fortified) 1 | 2.7 | 0.1% | |||
| NO2− -fortified (2.0 μM) 1 | 4.7 | 4.0 | 85.2% | 0.1% | 84.5% |
| NO2− -fortified (4.1 μM) 1 | 6.8 | 6.7 | 98.1% | 2.3% | 99.3% |
| RBC hemolysate (not fortified) 1 | 51.3 | 0.4% | |||
| NO3− -fortified (20.8 μM) 1 | 72.1 | 70.2 | 99.2% | 1.4% | 97.3% |
| NO3− -fortified (41.6 μM) 1 | 92.9 | 97.2 | 105.1% | 1.4% | 104.6% |
| Case Studies | Subj. ID | μM in Hemol 1 | μM/ mM Hb 2 | μM in RBC 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hb 3 | Ht% 4 | Hb 5 | NO2− | NOx | NO3− | NO2− | NOx | NO3− | NO2− | NOx | NO3− | ||
| Control Subjects (n = 6) | AB79M | 17.2 | 48.4 | 1.77 | 5.3 | 30.4 | 25.1 | 3.0 | 17.2 | 14.2 | 16.6 | 95.0 | 78.4 |
| FP89F | 14.1 | 40.8 | 1.86 | 7.5 | 29.4 | 21.9 | 4.1 | 15.8 | 11.8 | 21.8 | 85.0 | 63.2 | |
| FR60M | 13.9 | 41.7 | 1.74 | 6.7 | 35.5 | 28.8 | 3.9 | 20.8 | 16.9 | 20.1 | 107.6 | 87.5 | |
| GP97F | 12.7 | 35.3 | 1.74 | 4.6 | 20.9 | 16.3 | 2.6 | 12.1 | 9.4 | 14.7 | 67.4 | 52.6 | |
| GS82M | 15.6 | 46.9 | 1.37 | 10.0 | 72.0 | 62.0 | 7.3 | 52.6 | 45.2 | 38.3 | 274.9 | 236.6 | |
| MS51M | 16.6 | 47.6 | 1.65 | 5.1 | 19.0 | 13.9 | 3.1 | 11.5 | 8.4 | 16.7 | 62.5 | 45.8 | |
| Mean | 15.0 | 43.5 | 1.7 | 6.5 | 34.5 | 28.0 | 4.0 | 21.7 | 17.7 | 21.4 | 115.4 | 94.0 | |
| S.D. | 1.7 | 5.1 | 0.1 | 2.0 | 19.4 | 17.5 | 1.7 | 15.5 | 13.9 | 8.7 | 79.9 | 71.6 | |
| RSE 6 | 40.6 | 69.2 | 76.1 | ||||||||||
| Case studies | Subj. ID | NO2− | NOx | NO2− | NO3− |
|---|---|---|---|---|---|
| Plasma (µM) | RBC (µM) | ||||
| Scuba divers at a training facility (Y-40, Italy) (n = 8; range of 3 samples) | S12 | <LoD–2.3 | 37.5–45.6 | 13.1–42.4 | 41.3–107.1 |
| S13 | <LoD | 36.4–47.5 | 15.2–25.4 | 55.7–77.5 | |
| S14 | <LoD | 35.4–53.4 | 11.2–64.5 | 58.1–116.1 | |
| S15 | 1.9–2.3 | 74.5–77.3 | 16.1–29.8 | 73.5–96.4 | |
| S16 | <LoD–1.9 | 34.8–36.7 | 17.5–40.4 | 80.7–106.7 | |
| S17 | <LoD–4.2 | 47.5–58.4 | 32.7–50.3 | 46.3–68.2 | |
| S18 | <LoD | 45.4–55.5 | 25.2–47.8 | 69.5–123.8 | |
| S19 | <LoD–2.3 | 48.3–58.1 | 31.9–58.5 | 80.6–102.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brizzolari, A.; Dei Cas, M.; Cialoni, D.; Marroni, A.; Morano, C.; Samaja, M.; Paroni, R.; Rubino, F.M. High-Throughput Griess Assay of Nitrite and Nitrate in Plasma and Red Blood Cells for Human Physiology Studies under Extreme Conditions. Molecules 2021, 26, 4569. https://doi.org/10.3390/molecules26154569
Brizzolari A, Dei Cas M, Cialoni D, Marroni A, Morano C, Samaja M, Paroni R, Rubino FM. High-Throughput Griess Assay of Nitrite and Nitrate in Plasma and Red Blood Cells for Human Physiology Studies under Extreme Conditions. Molecules. 2021; 26(15):4569. https://doi.org/10.3390/molecules26154569
Chicago/Turabian StyleBrizzolari, Andrea, Michele Dei Cas, Danilo Cialoni, Alessandro Marroni, Camillo Morano, Michele Samaja, Rita Paroni, and Federico Maria Rubino. 2021. "High-Throughput Griess Assay of Nitrite and Nitrate in Plasma and Red Blood Cells for Human Physiology Studies under Extreme Conditions" Molecules 26, no. 15: 4569. https://doi.org/10.3390/molecules26154569
APA StyleBrizzolari, A., Dei Cas, M., Cialoni, D., Marroni, A., Morano, C., Samaja, M., Paroni, R., & Rubino, F. M. (2021). High-Throughput Griess Assay of Nitrite and Nitrate in Plasma and Red Blood Cells for Human Physiology Studies under Extreme Conditions. Molecules, 26(15), 4569. https://doi.org/10.3390/molecules26154569










