Neuroprotective and Anti-Inflammatory Effects of Pinus densiflora Bark Extract in Gerbil Hippocampus Following Transient Forebrain Ischemia
Abstract
:1. Introduction
2. Results
2.1. Total Polyphenols, Flavonoids, and Proanthocyanidins of Pinus densiflora Bark Extract (PBE)
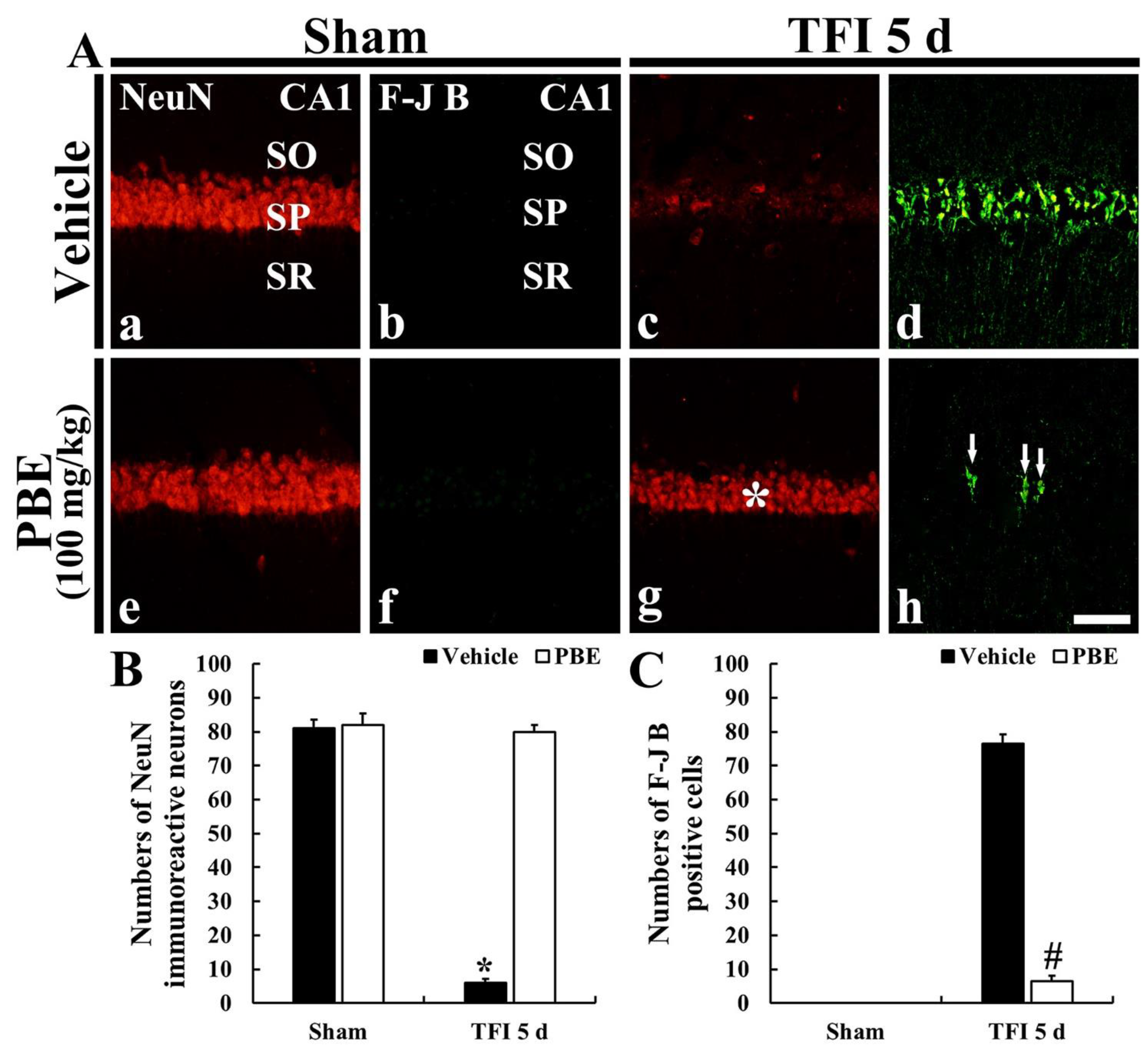
2.2. PBE Protected Hippocampal Neurons from Ischemic Injury
2.2.1. Finding by Hematoxylin and Eosin (H&E) Staining
2.2.2. Finding by Neuron-Specific Soluble Nuclear Antigen (NeuN) Immunohistofluorescence
2.2.3. Finding by Fluoro-Jade B (F-J B) Histofluorescence
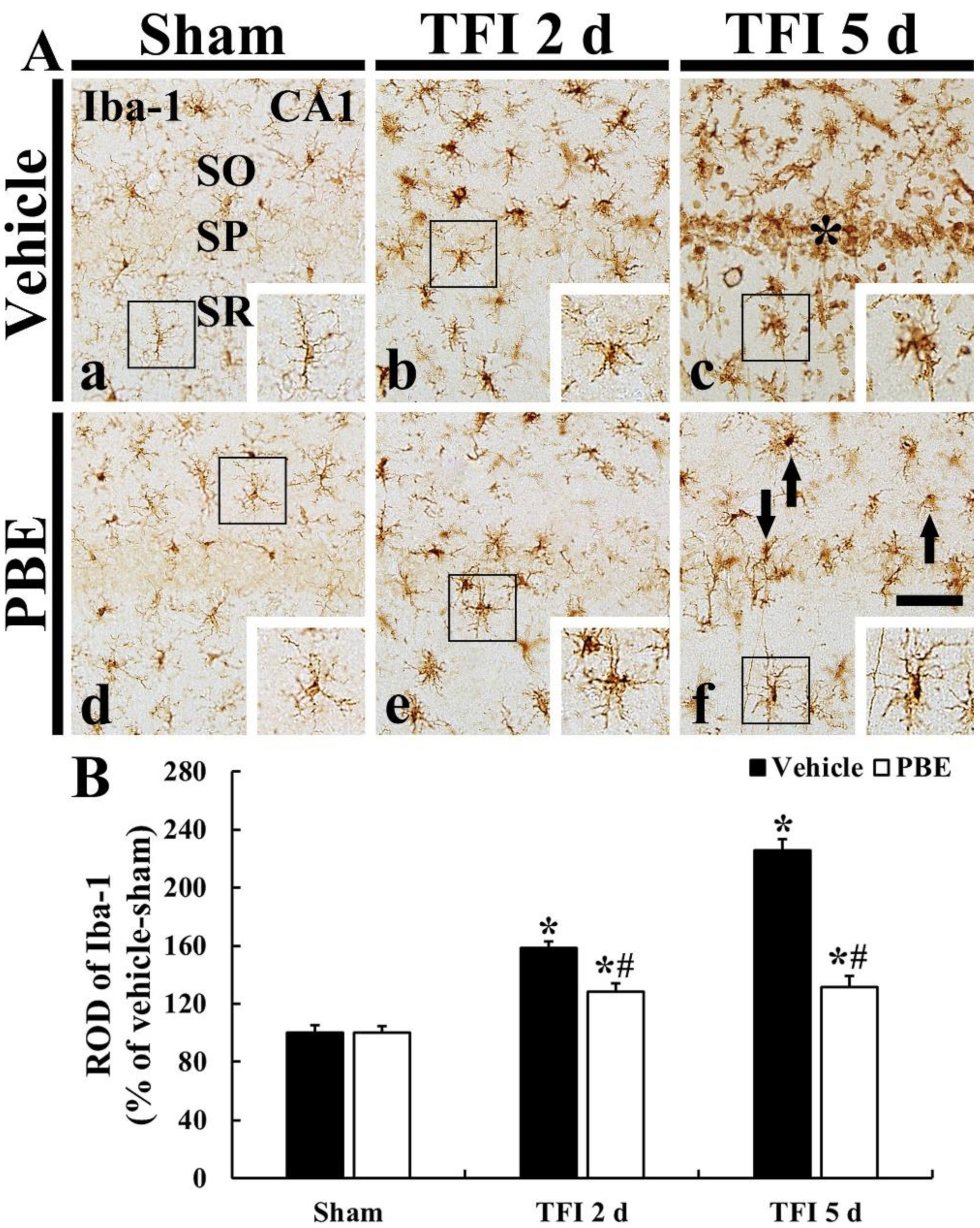
2.3. PBE Attenuated Microglia Activation (Microgliosis) in Ischemic CA1
2.4. PBE Decreased Pro-Inflammatory Cytokines in Ischemic CA1 Pyramidal Neurons
2.4.1. Tumor Necrosis Factor α (TNF- α) Immunoreactivity
2.4.2. Interleukin 1β (IL-1β) Immunoreactivity
2.5. PBE Increased Anti-Inflammatory Cytokines in Ischemic CA1 Pyramidal Neurons
2.5.1. IL-4 Immunoreactivity
2.5.2. IL-13 Immunoreactivity
3. Discussion
4. Materials and Methods
4.1. Preparation of PBE Administration
4.2. Qualitative Analysis of PBE
4.2.1. Total Phenol Content
4.2.2. Total Flavonoid Content
4.2.3. Total Proanthocyanidin Content
4.3. Experimental Animals
4.4. Experimental Groups and PBE Administration
4.5. Induction of TFI
4.6. Preparation of Brain Tissue Sections
4.7. H&E Staining
4.8. NeuN Immunofluorescence and F-J B Histofluorescence Staining
4.9. Immunohistochemistry
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability Statement
Abbreviations
| CA | cornu ammonis |
| F-J B | fluoro-Jade B |
| H&E | hematoxylin and eosin |
| IL | interleukin |
| NeuN | neuron-specific soluble nuclear antigen |
| PBE | Pinus densiflora bark extract |
| RI | relative immunoreactivity |
| ROD | relative optical density |
| SO | stratum oriens |
| SP | stratum pyramidale |
| SR | stratum radiatum |
| TFI | transient forebrain ischemia |
| TNF-α | tumor necrosis factor α |
References
- Khodanovich, M.; Kisel, A.; Kudabaeva, M.; Chernysheva, G.; Smolyakova, V.; Krutenkova, E.; Wasserlauf, I.; Plotnikov, M.; Yarnykh, V. Effects of fluoxetine on hippocampal neurogenesis and neuroprotection in the model of global cerebral ischemia in rats. Int. J. Mol. Sci. 2018, 19, 162. [Google Scholar] [CrossRef] [Green Version]
- Globus, M.Y.; Busto, R.; Martinez, E.; Valdes, I.; Dietrich, W.D.; Ginsberg, M.D. Comparative effect of transient global ischemia on extracellular levels of glutamate, glycine, and gamma-aminobutyric acid in vulnerable and nonvulnerable brain regions in the rat. J. Neurochem. 1991, 57, 470–478. [Google Scholar] [CrossRef]
- Petito, C.K.; Feldmann, E.; Pulsinelli, W.A.; Plum, F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology 1987, 37, 1281–1286. [Google Scholar] [CrossRef]
- Hou, S.T.; MacManus, J.P. Molecular mechanisms of cerebral ischemia-induced neuronal death. Int. Rev. Cytol. 2002, 221, 93–148. [Google Scholar] [PubMed]
- Won, S.J.; Kim, D.Y.; Gwag, B.J. Cellular and molecular pathways of ischemic neuronal death. J. Biochem. Mol. Biol. 2002, 35, 67–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Hawkins, K.E.; Dore, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef]
- Kamdem, J.P.; Abolaji, A.O.; Elekofehinti, O.O.; Omotuyi, I.O.; Ibrahim, M.; Hassan, W.; Vargas Barbosa, N.; Onofre Souza, D.; Batista Teixeira da Rocha, J.J.T.N.P.J. Therapeutic potential of plant extracts and phytochemicals against brain ischemia-reperfusion injury: A review. Pharmacol. Exp. Ther. 2016, 6, 250–284. [Google Scholar] [CrossRef]
- Pohl, F.; Lin, P.K.T. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: In vitro, in vivo and clinical trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Y.; Feng, J.; Zhang, X.L.; Cui, Y.Y. Pine bark extracts: Nutraceutical, pharmacological, and toxicological evaluation. J. Pharmacol. Exp. Ther. 2015, 353, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Maimoona, A.; Naeem, I.; Saddiqe, Z.; Jameel, K. A review on biological, nutraceutical and clinical aspects of french maritime pine bark extract. J. Ethnopharmacol. 2011, 133, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Dridi, W.; Bordenave, N. Pine bark phenolic extracts, current uses, and potential food applications: A review. Curr. Pharm. Des. 2020, 26, 1866–1879. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.X.J.; Chepulis, L.; von Hurst, P.; Gammon, C.S.; Page, R.A. An acute, placebo-controlled, single-blind, crossover, dose-response, exploratory study to assess the effects of new zealand pine bark extract (enzogenol((r))) on glycaemic responses in healthy participants. Nutrients 2020, 12, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.; Lee, T.K.; Park, C.W.; Kim, D.W.; Ahn, J.H.; Sim, H.; Lee, J.C.; Yang, G.E.; Kim, J.D.; Shin, M.C.; et al. Pycnogenol((r)) supplementation attenuates memory deficits and protects hippocampal ca1 pyramidal neurons via antioxidative role in a gerbil model of transient forebrain ischemia. Nutrients 2020, 12, 2477. [Google Scholar] [CrossRef]
- Kim, J.W.; Im, S.; Jeong, H.R.; Jung, Y.S.; Lee, I.; Kim, K.J.; Park, S.K.; Kim, D.O. Neuroprotective effects of korean red pine (pinus densiflora) bark extract and its phenolics. J. Microbiol. Biotechnol. 2018, 28, 679–687. [Google Scholar] [CrossRef]
- Clifton, G.L.; Taft, W.C.; Blair, R.E.; Choi, S.C.; DeLorenzo, R.J. Conditions for pharmacologic evaluation in the gerbil model of forebrain ischemia. Stroke 1989, 20, 1545–1552. [Google Scholar] [CrossRef] [Green Version]
- Park, C.W.; Ahn, J.H.; Lee, T.K.; Park, Y.E.; Kim, B.; Lee, J.C.; Kim, D.W.; Shin, M.C.; Park, Y.; Cho, J.H.; et al. Post-treatment with oxcarbazepine confers potent neuroprotection against transient global cerebral ischemic injury by activating nrf2 defense pathway. Biomed. Pharmacother. 2020, 124, 109850. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, I.H.; Ahn, J.H.; Noh, Y.H.; Kim, S.S.; Lee, T.K.; Lee, J.C.; Shin, B.N.; Sim, T.H.; Lee, H.S.; et al. Pretreated oenanthe javanica extract increases anti-inflammatory cytokines, attenuates gliosis, and protects hippocampal neurons following transient global cerebral ischemia in gerbils. Neural Regen. Res. 2019, 14, 1536–1543. [Google Scholar]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, eugenia caryophyllata (syzigium aromaticum l. Myrtaceae): A short review. Phytother. Res. PTR 2007, 21, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Jo, G.H.; Kim, S.N.; Kim, M.J.; Heo, Y. Protective effect of paeoniae radix alba root extract on immune alterations in mice with atopic dermatitis. J. Toxicol. Environ. Health Part A 2018, 81, 502–511. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Karim, N.; Abdelhalim, H.; Gavande, N.; Khan, I.; Khan, H. Natural products as an emerging therapeutic alternative in the treatment of neurological disorders. Evid. Based Complement. Alternat. Med. 2018, 2018, 3056847. [Google Scholar] [CrossRef] [Green Version]
- Parvez, M.K. Natural or plant products for the treatment of neurological disorders: Current knowledge. Curr. Drug Metab. 2018, 19, 424–428. [Google Scholar] [CrossRef]
- Kobayashi, M.S.; Han, D.; Packer, L. Antioxidants and herbal extracts protect ht-4 neuronal cells against glutamate-induced cytotoxicity. Free Radic. Res. 2000, 32, 115–124. [Google Scholar] [CrossRef]
- Ozoner, B.; Yuceli, S.; Aydin, S.; Yazici, G.N.; Sunar, M.; Arslan, Y.K.; Coban, T.A.; Suleyman, H. Effects of pycnogenol on ischemia/reperfusion-induced inflammatory and oxidative brain injury in rats. Neurosci. Lett. 2019, 704, 169–175. [Google Scholar] [CrossRef]
- Lee, T.K.; Kang, I.J.; Kim, B.; Sim, H.J.; Kim, D.W.; Ahn, J.H.; Lee, J.C.; Ryoo, S.; Shin, M.C.; Cho, J.H.; et al. Experimental pretreatment with chlorogenic acid prevents transient ischemia-induced cognitive decline and neuronal damage in the hippocampus through anti-oxidative and anti-inflammatory effects. Molecules 2020, 25, 3578. [Google Scholar] [CrossRef]
- Lee, T.K.; Ahn, J.H.; Park, C.W.; Kim, B.; Park, Y.E.; Lee, J.C.; Park, J.H.; Yang, G.E.; Shin, M.C.; Cho, J.H.; et al. Pre-treatment with laminarin protects hippocampal ca1 pyramidal neurons and attenuates reactive gliosis following transient forebrain ischemia in gerbils. Mar. Drugs 2020, 18, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Zhu, Q.; Zhang, Y.; Zhao, Y.; Cai, L.; Shields, C.B.; Cai, J. Reciprocal modulation between microglia and astrocyte in reactive gliosis following the cns injury. Mol. Neurobiol. 2013, 48, 690–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.K.; Kim, H.; Song, M.; Lee, J.C.; Park, J.H.; Ahn, J.H.; Yang, G.E.; Kim, H.; Ohk, T.G.; Shin, M.C.; et al. Time-course pattern of neuronal loss and gliosis in gerbil hippocampi following mild, severe, or lethal transient global cerebral ischemia. Neural Regen. Res. 2019, 14, 1394–1403. [Google Scholar]
- Mitchell, D.M.; Sun, C.; Hunter, S.S.; New, D.D.; Stenkamp, D.L. Regeneration associated transcriptional signature of retinal microglia and macrophages. Sci. Rep. 2019, 9, 4768. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.K.; Park, J.H.; Lee, T.K.; Kim, D.W.; Yoo, K.Y.; Ahn, J.H.; Kim, Y.H.; Cho, J.H.; Kim, Y.M.; Won, M.H.; et al. Cd74-immunoreactive activated m1 microglia are shown late in the gerbil hippocampal ca1 region following transient cerebral ischemia. Mol. Med. Rep. 2017, 15, 4148–4154. [Google Scholar] [CrossRef] [PubMed]
- Gaire, B.P.; Song, M.R.; Choi, J.W. Sphingosine 1-phosphate receptor subtype 3 (s1p3) contributes to brain injury after transient focal cerebral ischemia via modulating microglial activation and their m1 polarization. J. Neuroinflammation 2018, 15, 284. [Google Scholar] [CrossRef]
- Kim, H.; Ahn, J.H.; Song, M.; Kim, D.W.; Lee, T.K.; Lee, J.C.; Kim, Y.M.; Kim, J.D.; Cho, J.H.; Hwang, I.K.; et al. Pretreated fucoidan confers neuroprotection against transient global cerebral ischemic injury in the gerbil hippocampal ca1 area via reducing of glial cell activation and oxidative stress. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 109, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Vollmer, M.K.; Ahmad, A.S.; Fernandez, V.M.; Kim, H.; Dore, S. Pretreatment with korean red ginseng or dimethyl fumarate attenuates reactive gliosis and confers sustained neuroprotection against cerebral hypoxic-ischemic damage by an nrf2-dependent mechanism. Free. Radic. Biol. Med. 2019, 131, 98–114. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. 2), 136–153. [Google Scholar] [CrossRef] [Green Version]
- Dugue, R.; Nath, M.; Dugue, A.; Barone, F.C. Roles of pro-and anti-inflammatory cytokines in traumatic brain injury and acute ischemic stroke. Mech. Neuroinflamm. 2017, 211, 4901. [Google Scholar]
- Hamilton, T.A.; Ohmori, Y.; Tebo, J.M.; Kishore, R. Regulation of macrophage gene expression by pro- and anti-inflammatory cytokines. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 1999, 67, 241–244. [Google Scholar] [CrossRef]
- Pawluk, H.; Wozniak, A.; Grzesk, G.; Kolodziejska, R.; Kozakiewicz, M.; Kopkowska, E.; Grzechowiak, E.; Kozera, G. The role of selected pro-inflammatory cytokines in pathogenesis of ischemic stroke. Clin. Interv. Aging 2020, 15, 469–484. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using folin-ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, X.; Zhang, J.; Zou, W.; Zhang, Y.; Li, L.; Liu, J. Screening of bacillus strains from sun vinegar for efficient production of flavonoid and phenol. Indian J. Microbiol. 2016, 56, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Romero, S.; Hereu, M.; Molinar-Toribio, E.; Almajano, M.P.; Mendez, L.; Medina, I.; Taltavull, N.; Romeu, M.; Nogues, M.R.; Torres, J.L. Effects of the combination of omega-3 pufas and proanthocyanidins on the gut microbiota of healthy rats. Food Res. Int. 2017, 97, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Ahn, J.H.; Lee, T.K.; Park, C.W.; Kim, B.; Lee, J.C.; Kim, D.W.; Shin, M.C.; Cho, J.H.; Lee, C.H.; et al. Laminarin pretreatment provides neuroprotection against forebrain ischemia/reperfusion injury by reducing oxidative stress and neuroinflammation in aged gerbils. Mar. Drugs 2020, 18, 213. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, J.W. Exotic Animal Formulary; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Park, J.H.; Park, C.W.; Ahn, J.H.; Choi, S.Y.; Shin, M.C.; Cho, J.H.; Lee, T.K.; Kim, I.H.; Cho, J.H.; Lee, J.C.; et al. Neuroprotection and reduced gliosis by pre- and post-treatments of hydroquinone in a gerbil model of transient cerebral ischemia. Chem. Biol. Interact. 2017, 278, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Radtke-Schuller, S.; Schuller, G.; Angenstein, F.; Grosser, O.S.; Goldschmidt, J.; Budinger, E. Brain atlas of the mongolian gerbil (meriones unguiculatus) in ct/mri-aided stereotaxic coordinates. Brain Struct. Funct. 2016, 221 (Suppl. 1), 1–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Ahn, J.H.; Song, M.; Kim, H.; Park, C.W.; Park, Y.E.; Lee, T.K.; Lee, J.C.; Kim, D.W.; Lee, C.H.; et al. A 2-min transient ischemia confers cerebral ischemic tolerance in non-obese gerbils, but results in neuronal death in obese gerbils by increasing abnormal mtor activation-mediated oxidative stress and neuroinflammation. Cells 2019, 8, 1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Ahn, J.H.; Kim, H.; Kim, D.W.; Lee, T.K.; Lee, J.C.; Kim, Y.M.; Lee, C.H.; Hwang, I.K.; Yan, B.C.; et al. Chronic high-fat diet-induced obesity in gerbils increases pro-inflammatory cytokines and mtor activation, and elicits neuronal death in the striatum following brief transient ischemia. Neurochem. Int. 2018, 121, 75–85. [Google Scholar] [CrossRef]



| Total Polyphenols (mg GAE/g) | Total Flavonoids (mg QE/g) | Total Proanthocyanidins (mg CE/g) |
|---|---|---|
| 92.89 ± 0.66 | 23.57 ± 0.11 | 53.42 ± 6.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H.; Kim, J.D.; Lee, T.-K.; Han, X.; Sim, H.; Kim, B.; Lee, J.-C.; Ahn, J.H.; Lee, C.-H.; Kim, D.W.; et al. Neuroprotective and Anti-Inflammatory Effects of Pinus densiflora Bark Extract in Gerbil Hippocampus Following Transient Forebrain Ischemia. Molecules 2021, 26, 4592. https://doi.org/10.3390/molecules26154592
Park JH, Kim JD, Lee T-K, Han X, Sim H, Kim B, Lee J-C, Ahn JH, Lee C-H, Kim DW, et al. Neuroprotective and Anti-Inflammatory Effects of Pinus densiflora Bark Extract in Gerbil Hippocampus Following Transient Forebrain Ischemia. Molecules. 2021; 26(15):4592. https://doi.org/10.3390/molecules26154592
Chicago/Turabian StylePark, Joon Ha, Jong Dai Kim, Tae-Kyeong Lee, Xionggao Han, Hyejin Sim, Bora Kim, Jae-Chul Lee, Ji Hyeon Ahn, Choong-Hyun Lee, Dae Won Kim, and et al. 2021. "Neuroprotective and Anti-Inflammatory Effects of Pinus densiflora Bark Extract in Gerbil Hippocampus Following Transient Forebrain Ischemia" Molecules 26, no. 15: 4592. https://doi.org/10.3390/molecules26154592







