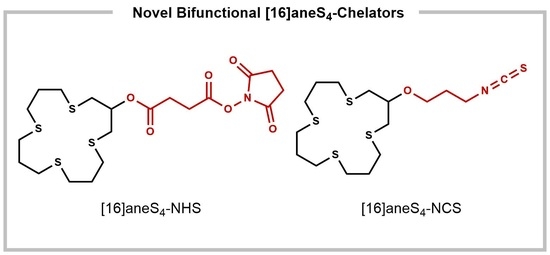
Novel Bifunctional [16]aneS4-Derived Chelators for Soft Radiometals
Abstract
1. Introduction
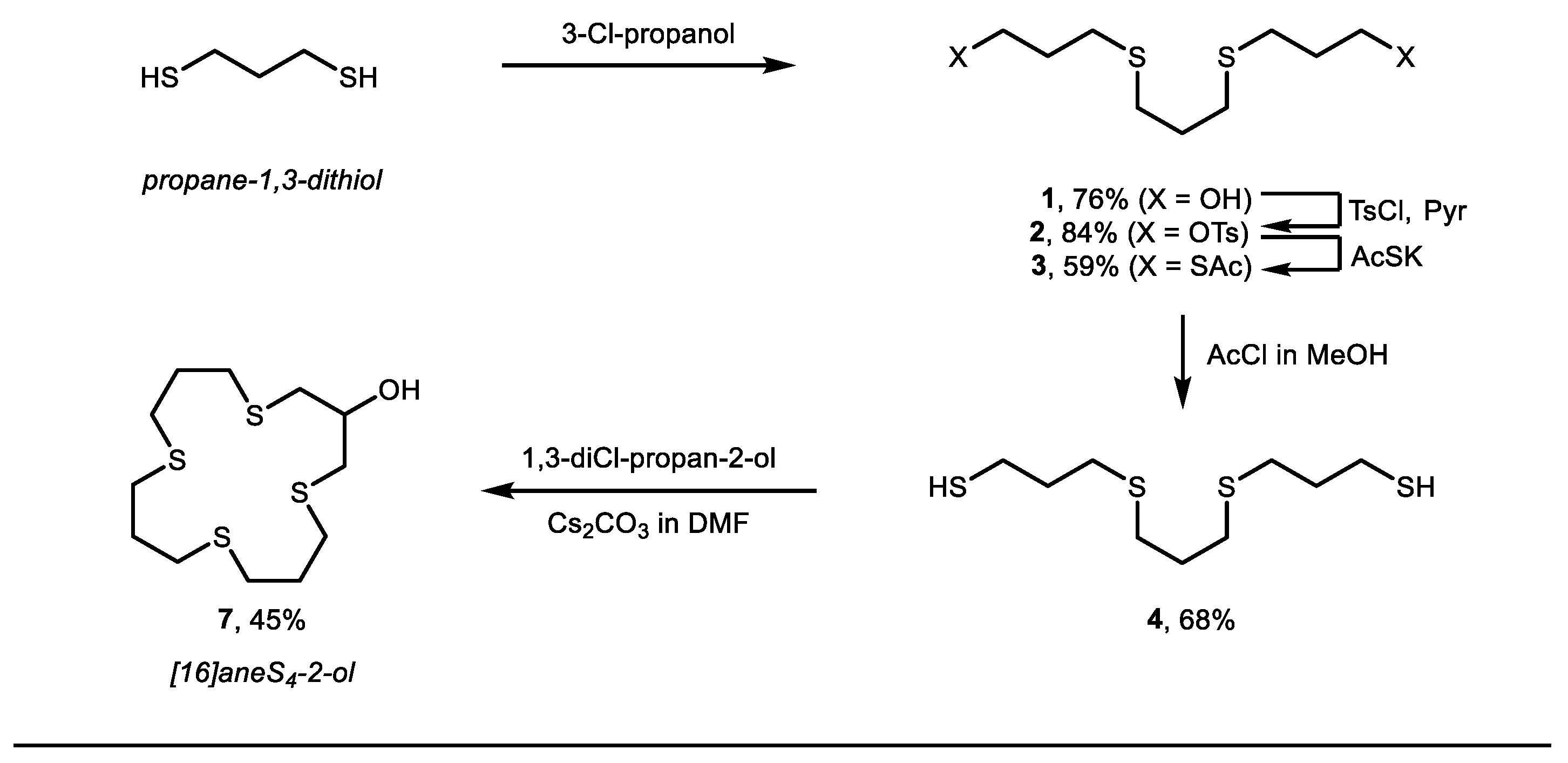
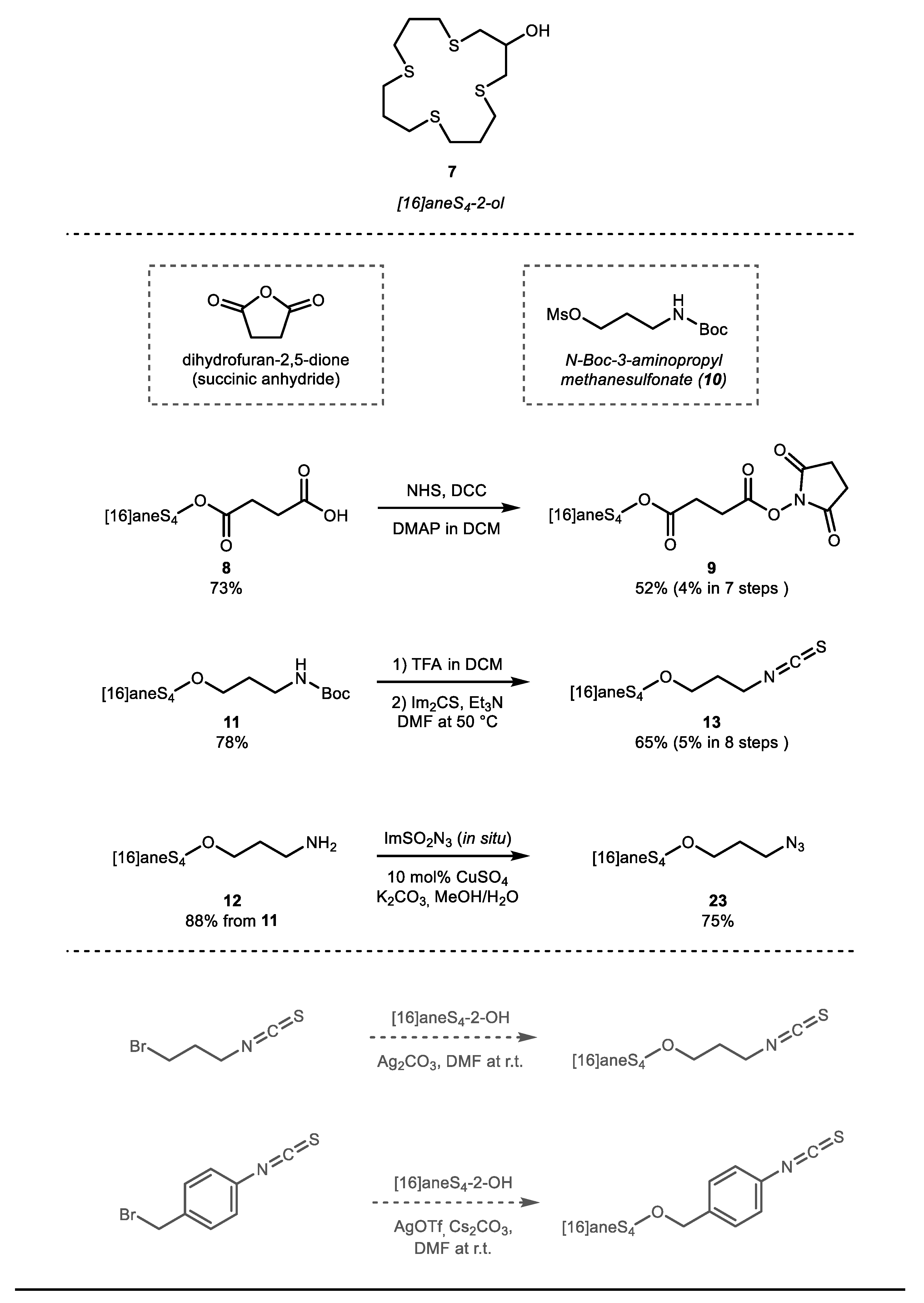
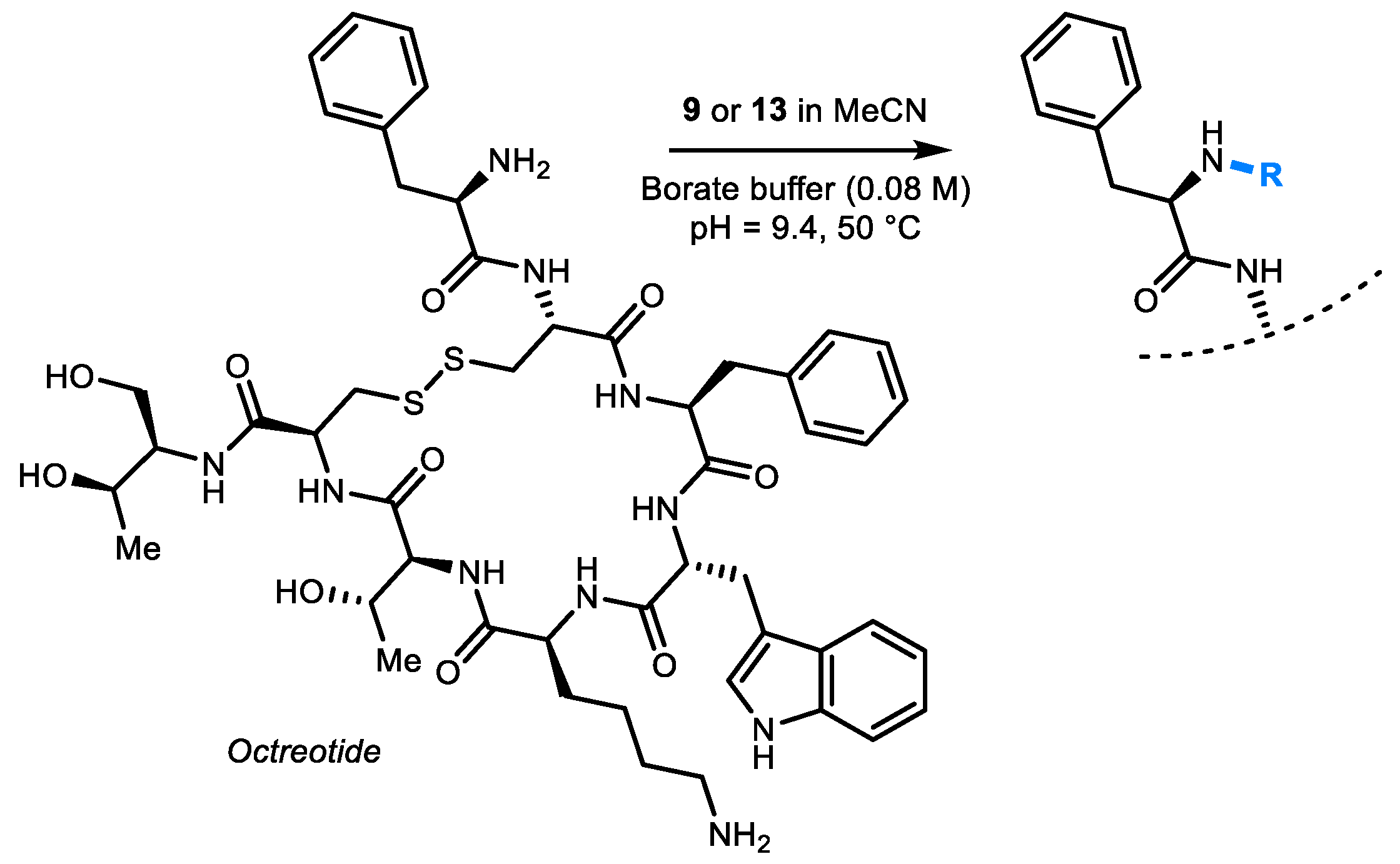
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Filippi, L.; Chiaravalloti, A.; Schillaci, O.; Cianni, R.; Bagni, O. Theranostic approaches in nuclear medicine: Current status and future prospects. Exp. Rev. Med. Devic. 2020, 17, 331–343. [Google Scholar] [CrossRef]
- Haberkorn, U.; Eder, M.; Kopka, K.; Babich, J.W.; Eisenhut, M. New Strategies in Prostate Cancer: Prostate-Specific Membrane Antigen (PSMA) Ligands for Diagnosis and Therapy. Clin. Cancer Res. 2016, 22, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Novartis.com. Available online: https://www.novartis.com/news/media-releases/novartis-announces-positive-result-phase-iii-study-radioligand-therapy-177lu-psma-617-patients-advanced-prostate-cancer (accessed on 25 June 2021).
- Knapp, F.F.; Dash, A. Radiopharmaceuticals for Therapy, 1st ed.; Springer: New Delhi, India, 2016. [Google Scholar]
- Stapleton, S.; Jaffray, D.; Milosevic, M. Radiation effects on the tumor microenvironment: Implications for nanomedicine delivery. Adv. Drug Deliv. Rev. 2017, 109, 119–130. [Google Scholar] [CrossRef]
- Steeg, P.S. Targeting metastasis. Nat. Rev. Cancer 2016, 16, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Guan, X. Cancer metastases: Challenges and opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Howell, R.W. Auger processes in the 21st century. Int. J. Radiat. Biol. 2008, 84, 959–975. [Google Scholar] [CrossRef]
- Kassis, A.I. The Amazing World of Auger Electrons. Int. J. Radiat. Biol. 2004, 80, 789–803. [Google Scholar] [CrossRef]
- Reissig, F.; Mamat, C.; Steinbach, J.; Pietzsch, H.-J.; Freudenberg, R.; Navarro-Retamal, C.; Caballero, J.; Kotzerke, J.; Wunderlich, G. Direct and Auger Electron-Induced, Single- and Double-Strand Breaks on Plasmid DNA Caused by 99mTc-Labeled Pyrene Derivatives and the Effect of Bonding Distance. PLoS ONE 2016, 11, e0161973. [Google Scholar] [CrossRef]
- Cornelissen, B.; Vallis, K.A. Targeting the nucleus: An overview of Auger-electron radionuclide therapy. Curr. Drug Discov. Technol. 2010, 7, 263–279. [Google Scholar] [CrossRef]
- Buchegger, F.; Antonescu, C.; Delaloye, A.B.; Helg, C.; Kovacsovics, T.; Kosinski, M.; Mach, J.-P.; Ketterer, N. Long-term complete responses after 131I-tositumomab therapy for relapsed or refractory indolent non-Hodgkin’s lymphoma. Br. J. Cancer 2006, 94, 1770–1776. [Google Scholar] [CrossRef]
- Morgenroth, A.; Dinger, C.; Zlatopolskiy, B.D.; Al-Momani, E.; Glatting, G.; Mottaghy, F.M.; Reske, S.N. Auger electron emitter against multiple myeloma—Targeted endo-radio-therapy with 125I-labeled thymidine analogue 5-iodo-4′-thio-2′-deoxyuridine. Nucl. Med. Biol. 2008, 38, 1067–1077. [Google Scholar] [CrossRef]
- Othman, M.F.B.; Mitry, N.R.; Lewington, V.J.; Blower, P.J.; Terry, S.Y.A. Re-assessing gallium-67 as a therapeutic radionuclide. Nucl. Med. Biol. 2017, 46, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Imstepf, S.; Pierroz, V.; Raposinho, P.; Bauwens, M.; Felber, M.; Fox, T.; Shapiro, A.B.; Freudenberg, R.; Fernandes, C.; Gama, S.; et al. Nuclear Targeting with an Auger Electron Emitter Potentiates the Action of a Widely Used Antineoplastic Drug. Bioconjug. Chem. 2015, 26, 2397–2407. [Google Scholar] [CrossRef]
- Thisgaard, H.; Jensen, M.; Elema, D.R. Medium to large scale radioisotope production for targeted radiotherapy using a small PET cyclotron. Appl. Rad. Isot. 2011, 69, 1–7. [Google Scholar] [CrossRef]
- McQuade, P.; McCarthy, D.W.; Welch, M.J. Positron Emission Tomography: Metal Radionuclides for PET Imaging; Springer: London, UK, 2003; pp. 237–250. [Google Scholar]
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger electrons for cancer therapy—A review. EJNMMI Radiopharm. Chem. 2019, 4, 1–36. [Google Scholar] [CrossRef]
- AJensen, I.; Zhuravlev, F.; Severin, G.; Magnus, C.B.; Fonslet, J.; Köster, U.; Jensen, M. A solid support generator of the Auger electron emitter rhodium-103m from [103Pd]palladium. Appl. Rad. Isot. 2020, 156, 108985. [Google Scholar] [CrossRef] [PubMed]
- Filosfov, D.; Kurakina, E.; Radchenko, V. Potent candidates for Targeted Auger Therapy: Production and radiochemical considerations. Nucl. Med. Biol. 2021, 94, 1–19. [Google Scholar]
- Gokel, G.W.; Leevy, W.M.; Weber, M.W. Crown Ethers: Sensors for Ions and Molecular Scaffolds for Materials and Biological Models. Chem. Rev. 2004, 104, 2723–2750. [Google Scholar] [CrossRef] [PubMed]
- Martell, A.E.; Hancock, R.D. Metal Complexes in Aqueous Solutions, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Skarnemark, G.; Ödegaard-Jensen, A.; Nilsson, J.; Bartos, B. Production of 103mRh for cancer therapy. J. Rad. Nucl. Chem. 2009, 280, 371–373. [Google Scholar] [CrossRef]
- Wolford, T.L.; Musker, W.K. Cobalt(II) and rhodium(III) complexes of 1,5-dithiacyclooctane (1,5-DTCO) and 1,5,9,13-tetrathiacyclohexadecane (TTH). Inorg. Chim. Acta 1991, 182, 19–23. [Google Scholar] [CrossRef]
- Blake, A.J.; Reid, G.; Schröder, M. Platinum metal thioether macrocyclic complexes: Synthesis, electrochemistry, and single-crystal X-ray structures of cis-[RhCl2L2]PF6 and trans-[RhCl2L3]PF6 (L2 = 1,4,8,11-tetrathiacyclotetradecane, L3 = 1,5,9,13-tetrathiacyciohexadecane). J. Chem. Soc. Dalton Trans. 1989, 1, 1675–1680. [Google Scholar] [CrossRef]
- Lyczko, M.; Pruszynski, M.; Majkowska-Pilip, A.; Lyczko, K.; Was, B.; Meczynska-Wielgosz, S.; Kruszewski, M.; Szkliniarz, K.; Jastrzebski, J.; Stolarz, A.; et al. 211At labeled substance P (5–11) as potential radiopharmaceutical for glioma treatment. Nucl. Med. Biol. 2017, 53, 1–8. [Google Scholar] [CrossRef]
- Li, N.; Struttmnan, M.; Higginbotham, C.; Grall, J.; Skerlj, J.F.; Vollano, J.F.; Bridger, S.A.; Ochrymowycz, L.A.; Ketring, A.R.; Abram, M.J.; et al. Biodistribution of model 105Rh-labeled tetradentate thiamacrocycles in rats. Nucl. Med. Biol. 1997, 24, 85–92. [Google Scholar] [CrossRef]
- Jurisson, S.S.; Ketring, A.R.; Volkert, W.A. Rhodium-105 complexes as potential radiotherapeutic agents. Trans. Met. Chem. 1997, 22, 315–317. [Google Scholar] [CrossRef]
- JMeadow, R.; Reid, E.E. Ring Compounds and Polymers from Polymethylene Dihalides and Dimercaptans. J. Am. Chem. Soc. 1934, 56, 2177–2180. [Google Scholar]
- Ochrymowycz, L.A.; Mak, C.-P.; Michna, J.D. Synthesis of macrocyclic polythiaethers. J. Org. Chem. 1974, 39, 2079–2085. [Google Scholar] [CrossRef]
- Sokil, L.S.W.L.; Ochrymowycz, L.A.; Rorabacher, D.B. Macrocyclic, ring size, and anion effects as manifested in the equilibrium constants and thermodynamic parameters of copper(II)-cyclic polythia ether complexes. Inorg. Chem. 1981, 20, 3189–3195. [Google Scholar] [CrossRef]
- Bagchi, P.; Morgan, M.T.; Bacsa, J.; Fahrni, C.J. Robust affinity standards for Cu(I) biochemistry. J. Am. Chem. Soc. 2013, 135, 18549–18559. [Google Scholar] [CrossRef]
- Hao, G.; Mastren, T.; Silvers, W.; Hassan, G.; Öz, O.K.; Sun, X. Copper-67 radioimmunotheranostics for simultaneous immunotherapy and immuno-SPECT. Sci. Rep. 2021, 11, 3622–3633. [Google Scholar] [CrossRef] [PubMed]
- George, K.; Jura, M.; Levason, W.; Light, M.E.; Ollivere, L.P.; Reid, G. Unexpected Reactivity and Coordination in Gallium(III) and Indium(III) Chloride Complexes With Geometrically Constrained Thio- and Selenoether Ligands. Inorg. Chem. 2012, 51, 2231–2240. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.J.; Bywater, M.J.; Crofts, R.D.; Gibson, A.M.; Reid, G.; Schröder, M. Synthesis, platinum-195 nuclear magnetic resonance spectroscopic and extended X-ray absorption fine structure studies on platinum-(II) and -(IV) thioether macrocyclic complexes. J. Chem. Soc. Dalton Trans. 1996, 2979–2983. [Google Scholar] [CrossRef]
- Blake, A.J.; Holder, A.J.; Reid, G.; Schröder, M. Platinum thioether macrocyclic chemistry: Synthesis and electrochemistry of [PtL][PF6]2 (L = [12]-, [14]- or [16]-aneS4) and [Pt2([28]aneS8)][PF6]4. Crystal structure of [Pt([12]aneS4)][PF6]2·MeCN. J. Chem. Soc. Dalton Trans. 1994, 627–631. [Google Scholar] [CrossRef]
- Barton, A.J.; Hill, N.J.; Levason, W.; Reid, G. Synthesis and structural studies on polymeric assemblies derived from antimony(III) halide complexes with bi- and tri-dentate and macrocyclic thio- and seleno-ether ligands. J. Chem. Soc. Dalton Trans. 2001, 1621–1627. [Google Scholar] [CrossRef]
- Pietzsch, H.-J.; Spies, H.; Leibnitz, P.; Reck, G. Technetium complexes with thioether ligands—III. Synthesis and structural characterization of cationic nitridotechnetium(V) complexes with thiacrown ethers. Polyhedron 1993, 12, 2995–3002. [Google Scholar] [CrossRef]
- Antczak, C.; Jaggi, J.S.; LeFave, C.V.; Curcio, M.J.; McDevitt, M.R.; Scheinberg, D.A. Influence of the Linker on the Biodistribution and Catabolism of Actinium-225 Self-Immolative Tumor-Targeted Isotope Generators. Bioconjug. Chem. 2006, 17, 1551–1560. [Google Scholar] [CrossRef][Green Version]
- Pickens, C.J.; Johnson, S.N.; Pressnall, M.M.; Leon, M.A.; Berkland, C.J. Practical Considerations, Challenges, and Limitations of Bioconjugation via Azide-Alkyne Cycloaddition. Bioconjug. Chem. 2018, 29, 686–701. [Google Scholar] [CrossRef]
- Compound Name—Catalogue Number: 3-Bromopropyl Isothiocyanate—008751 (22 £/gram), 2-Bromoethyl Isothiocyanate—002516 (16 £/gram). Available online: Fluorochem.co.uk (accessed on 25 June 2021).
- Mignani, S.; Aszodi, J.; Babin, D.; Liutkus, M.; Bedel, O. Synthesis of new macromolecular, functionalized carboxylic-acid–PEG–DHLA surface ligands. Tetrahedron Lett. 2010, 51, 5364–5367. [Google Scholar] [CrossRef]
- Fujihara, H.; Imaoka, K.; Furukawa, N.; Oae, S. Synthesis and phase-transfer property of macrocyclic polythiaether sulphoxides. J. Chem. Soc. Perkin Trans. 1 1986, 465–470. [Google Scholar] [CrossRef]
- Gomez-Caballero, E.; Martines-Álvarez, R.; Sierra, M.A. Unexpected Reaction Pathways Leading to Thiodiglycol During the Degradation of Long-Chain Sulfur Mustards. J. Org. Chem. 2018, 83, 12432–12439. [Google Scholar] [CrossRef] [PubMed]
- Takei, Y.; Ando, H.; Tsutsui, K. (Eds.) Handbook of Hormones; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar]
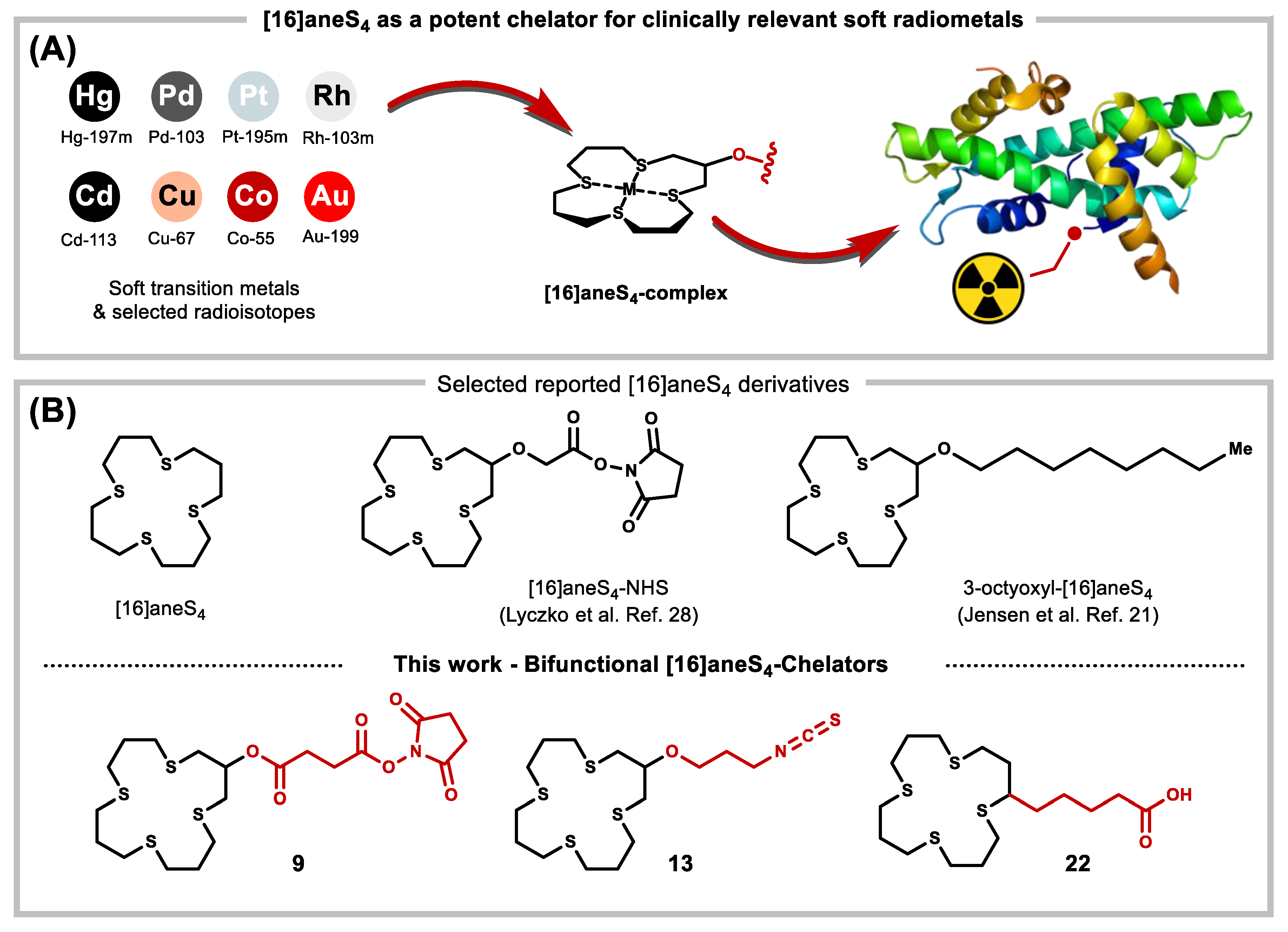

| Entry | Chelator | Metal | Isotopes | (Potential) Application a | Ref. |
|---|---|---|---|---|---|
| 1 | [16]aneS4, [16]aneS4-ol, [16]aneS4-diol | Rh | 103Rh, 103mRh, 105Rh | MLC, TRT | [26,27,29] |
| 2 | [16]aneS4-ol | RhX | X = 211At, 131I, 123I, 124I | MLC, TRT, SPECT, PET | [28] |
| 3 | [16]aneS4 | Cu | 60Cu, 64Cu, 67Cu | MLC, PET, TRT, SPECT | [19,32,34,35] |
| 4 | [16]aneS4 | Pt | 191Pt, 193mPt, 195mPt | TRT | [20,37,38] |
| 5 | [16]aneS4-3-octyoxyl | Pb | 103Pd | MLC, TRT | [21] |
| 6 | [16]aneS4 | Sb | 119Sb | TRT | [20,39] |
| 7 | [16]aneS4 | Tc | 94mTc, 99mTc | TRT, PET, SPECT | [19,20,40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Straathof, N.J.W.; Magnus, C.B.; Zhuravlev, F.; Jensen, A.I. Novel Bifunctional [16]aneS4-Derived Chelators for Soft Radiometals. Molecules 2021, 26, 4603. https://doi.org/10.3390/molecules26154603
Straathof NJW, Magnus CB, Zhuravlev F, Jensen AI. Novel Bifunctional [16]aneS4-Derived Chelators for Soft Radiometals. Molecules. 2021; 26(15):4603. https://doi.org/10.3390/molecules26154603
Chicago/Turabian StyleStraathof, Natan J. W., Charlotte B. Magnus, Fedor Zhuravlev, and Andreas I. Jensen. 2021. "Novel Bifunctional [16]aneS4-Derived Chelators for Soft Radiometals" Molecules 26, no. 15: 4603. https://doi.org/10.3390/molecules26154603
APA StyleStraathof, N. J. W., Magnus, C. B., Zhuravlev, F., & Jensen, A. I. (2021). Novel Bifunctional [16]aneS4-Derived Chelators for Soft Radiometals. Molecules, 26(15), 4603. https://doi.org/10.3390/molecules26154603