Reductive Methylation of Homogeneous Primary β-Lauryl/myristyl 7/3 Polyethyleneoxy n = 3–18 Ethylamines under Phase-Transfer Catalysis Conditions
Abstract
:1. Introduction
2. Results and Discussions
3. Materials and Methods
3.1. Chemicals
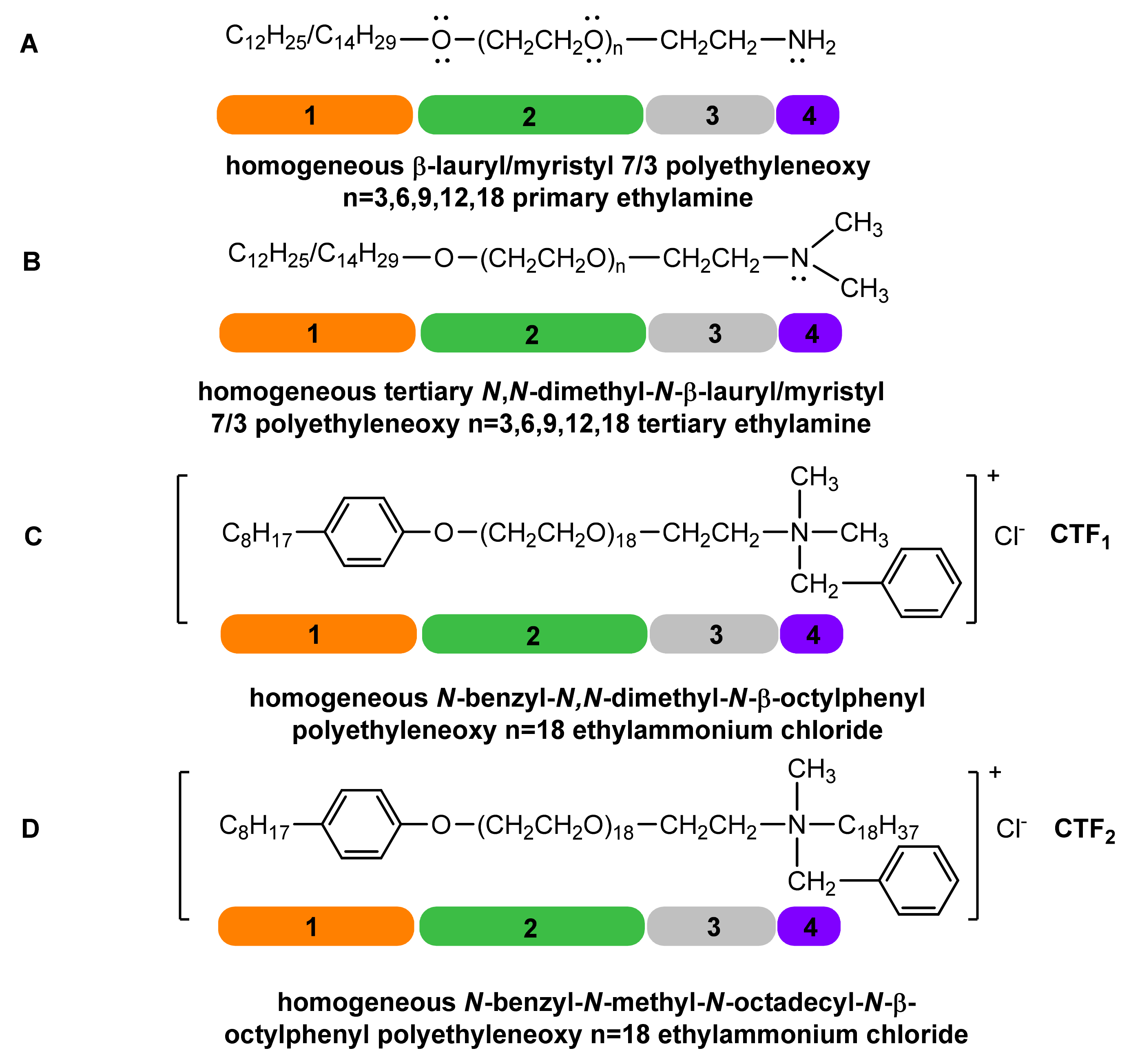
3.2. Synthesis and Characterization of Reference Chemicals
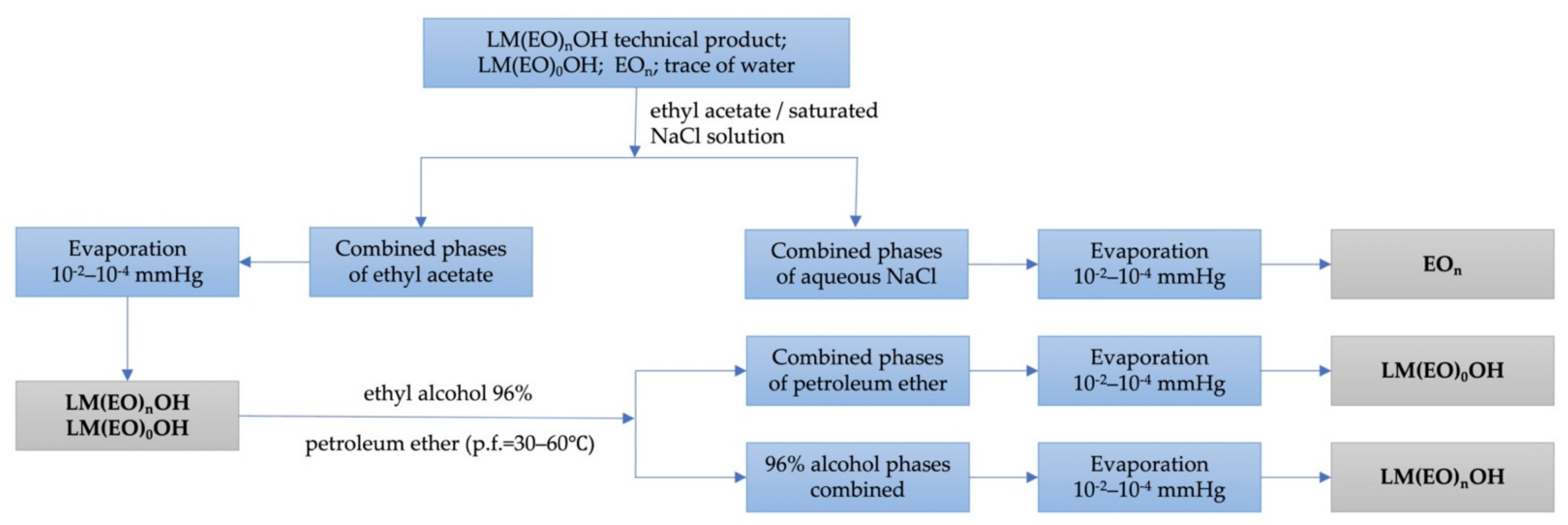
3.3. Chemical and Physico-Chemical Characterization of Homogeneous Lauryl/myristyl Alcohols 7/3 as such and Polyethoxylated n = 3–18
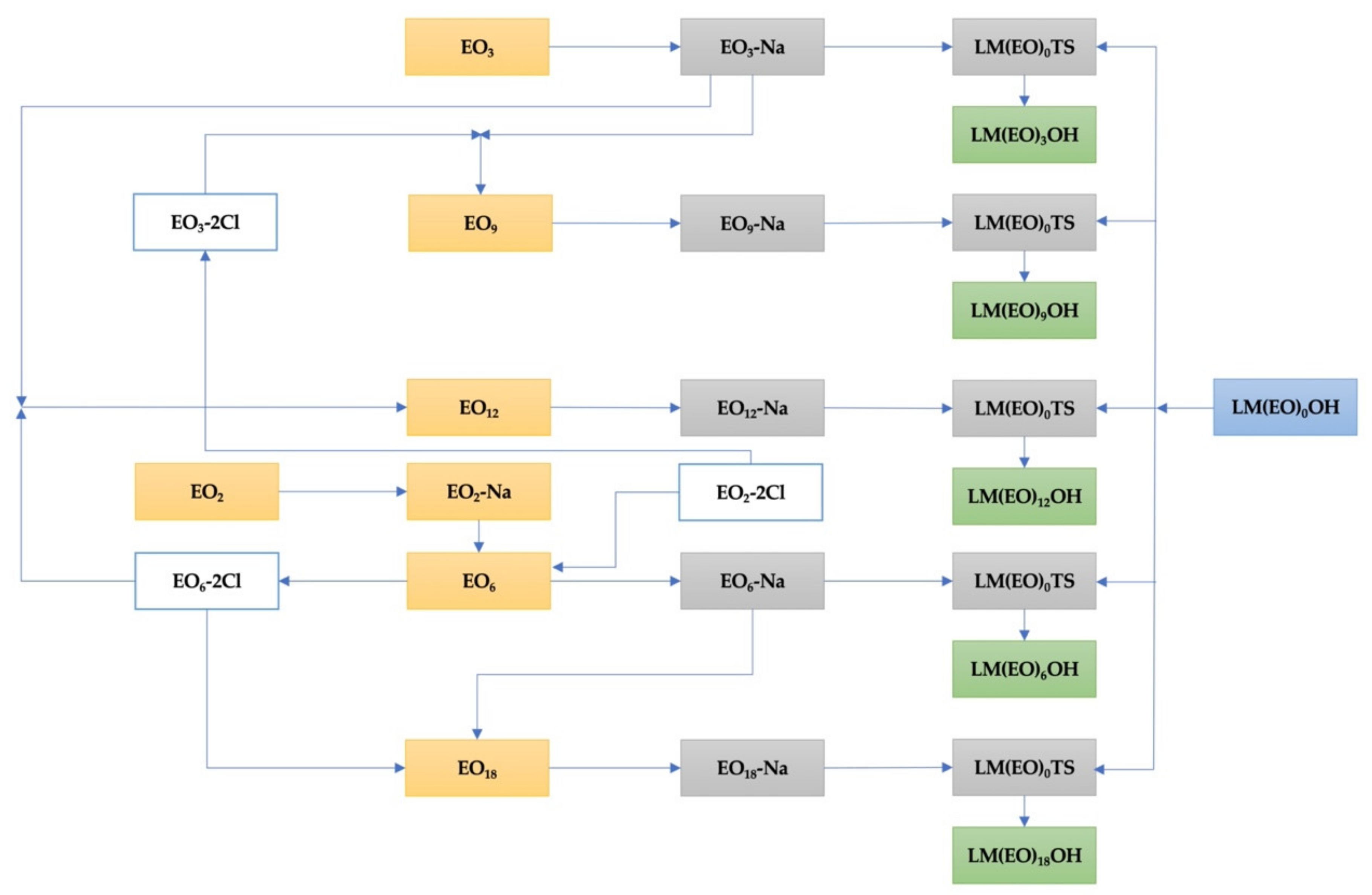
3.4. The Overall Scheme of Synthesis Reactions of Homogeneous LM(EO)nAT from Homogeneous LM(EO)nOH
3.5. Synthesis and Characterization of Homogeneous LM(EO)nAT under Conditions of Phase-Transfer Catalysis
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wallach, O. Über Menthylamin. Ber. Dtsch. Chem. Ges. 1891, 24, 3992–3993. [Google Scholar] [CrossRef]
- Leuckart, R.; Bach, E. Ueber die Einwirkung von Ammoniumformiat auf Benzaldehyd und Benzophenon. Ber. Dtsch. Chem. Ges. 1886, 19, 2128–2131. [Google Scholar] [CrossRef]
- Becker, H.; Berger, W.; Domschke, G. Organicum: Practical Handbook of Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- O’Connor, D.; Lauria, A.; Bondi, S.P.; Saba, S. Direct Leuckart-type reductive amination of aldehydes and ketones: A facile one-pot protocol for the preparation of secondary and tertiary amines. Tetrahedron Lett. 2011, 52, 129–132. [Google Scholar] [CrossRef]
- Lee, O.-Y.; Law, K.-L.; Ho, C.-Y.; Yang, D. Highly chemoselective reductive amination of carbonyl compounds promoted by InCl3/Et3SiH/MeOH system. J. Org. Chem. 2008, 73, 8829–8837. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Magid, A.F.; Carson, K.G.; Harris, B.D.; Maryanoff, C.A.; Shah, R.D. Reductive amination of aldehydes and ketones with sodium triacetoxyborohydride. Studies on direct and indirect reductive amination procedures1. J. Org. Chem. 1996, 61, 3849–3862. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, P.N.; Yagafarov, N.Z.; Usanov, D.L.; Maleev, V.I.; Chusov, D. Ruthenium-catalyzed reductive amination without an external hydrogen source. Org. Lett. 2015, 17, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Bougie, F.; Iliuta, M.C. Sterically hindered amine-based absorbents for the removal of CO2 from gas streams. J. Chem. Eng. Data 2012, 57, 635–669. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Chen, Y.-C.; Hsieh, J.-C. Pyridine-catalyzed double C―N coupling reaction of an isocyanate with two benzynes. Eur. J. Org. Chem. 2012, 2, 247–250. [Google Scholar] [CrossRef]
- De, A.; Ghosal, N.C.; Mahato, S.; Santra, S.; Zyryanov, G.V.; Majee, A. Scope and Limitations of Leuckart-Wallach-Type Reductive Amination: Chemoselective Synthesis of Tertiary Amines from Aldehydes under Neat Conditions. Chem. Sel. 2018, 3, 4058–4066. [Google Scholar] [CrossRef]
- Ebbing, D.; Gammon, S.D. General Chemistry; Cengage Learning: Boston, MA, USA, 2016. [Google Scholar]
- Ouellette, R.J.; Rawn, J.D. 19 Aldehydes and Ketones: Nucleophilic Addition Reactions. In Organic Chemistry Study Guide; Ouellette, R.J., Rawn, J.D., Eds.; Elsevier: Boston, MA, USA, 2015; pp. 335–360. [Google Scholar]
- Vlassa, M.; Kezdi, M.; Strajescu, M.; Teodor, F. Cataliza Prin Transfer Interfazic—Aplicatii in Chimia Organica; Editura Dacia: Cluj-Napoca, Romania, 1983. [Google Scholar]
- Starks, C.M.; Liotta, C.L.; Halpern, M.E. Phase-transfer catalysis: Fundamentals I. In Phase-Transfer Catalysis; Springer: Berlin/Heidelberg, Germany, 1994; pp. 23–47. [Google Scholar]
- Weber, W.P.; Gokel, G.W. Phase Transfer Catalysis in Organic Synthesis; Springer: Berlin/Heidelberg, Germany, 2012; Volume 4. [Google Scholar]
- Moller, F.; Schröter, R. Amine durch reduktion. In Methoden der Organischen Chemie; Thieme: Stuttgart, Germany, 1957; Volume 11, pp. 648–664. [Google Scholar]
- Bruson, H.A. Cyanoethylation. Org. React. 2004, 5, 79–135. [Google Scholar]
- Emerson, W.S. The Preparation of Amines by Reductive Alkylation. In Organic Reactions; Wiley: New York, NY, USA, 2011; pp. 174–255. [Google Scholar]
- Jianu, C.; Jianu, I. Colloidal competences of new tailor-made lipids. J. Food Agric. Environ. 2010, 8, 148–155. [Google Scholar]
- Jianu, C. Colloidal competences of some food cleaning agents based on alkaline and ammonium nonionic soaps. J. Food Agric. Environ. 2012, 10, 10–15. [Google Scholar]
- Jianu, C.; Rinovetz, A.; Cazacu, M.; Ilie, A.C. Nonionic-anionic Colloidal Systems Based on Alkaline and Ammonium 2-ethyl-hexyl Polyethyleneoxy (n = 3–18) Propionates/di-butyl-naphthalenesulphonates. Rev. Chim. 2013, 64, 1072–1077. [Google Scholar]
- Jianu, C.; Cocan, I.; Rujescu, C.; Jianu, I. Colloidal competences of any polyethyleneglycol conjugates. Commun. Agric. Appl. Biol. Sci. 2008, 73, 731–738. [Google Scholar] [PubMed]
- Jianu, C. Effect of Certain Ethylene Oxide Heterogeneous Heterobifunctional Acyclic Oligomers (HEHAO) on Wetting. In Surface Energy; IntechOpen: London, UK, 2015. [Google Scholar]
- Jianu, C. Ethylene Oxide Homogeneous Heterobifunctional Acyclic Oligomers. In Oligomerization of Chemical and Biological Compounds; IntechOpen: London, UK, 2014. [Google Scholar]
- Christensen, J.J.; Izatt, R.M. Progress in Macrocyclic Chemistry; Wiley: Hoboken, NJ, USA, 1979. [Google Scholar]
- Harvey, R.D.; Barlow, D.J.; Drake, A.F.; Kudsiova, L.; Lawrence, M.J.; Brain, A.P.; Heenan, R.K. The effect of electrolyte on the encapsulation efficiency of vesicles formed by the nonionic surfactant, 2C18E12. J. Colloid Interface Sci. 2007, 315, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Yokogama, Y.; Hirajima, R.; Morigaki, K.; Yamaguchi, Y.; Ueda, K. Alkali-cation affinities of polyoxyethylene dodecylethers and helical conformation of their cationized molecules studied by electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 1914–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, A.T.; Scrivens, J.H.; Williams, J.P.; Baker, E.S.; Gidden, J.; Bowers, M.T. Microstructural and conformational studies of polyether copolymers. Int. J. Mass Spectrom. 2004, 238, 287–297. [Google Scholar] [CrossRef]
- Yanagida, S.; Takahashi, K.; Okahara, M. Metal-ion complexation of noncyclic poly (oxyethylene) derivatives. I. Solvent extraction of alkali and alkaline earth metal thiocyanates and iodides. Bull. Chem. Soc. Jpn. 1977, 50, 1386–1390. [Google Scholar] [CrossRef] [Green Version]
- ISO. TR 896: 1995 Surface Active Agents. Scientific Classification (Identical with the International ISO/TR 893: 1977); Romanian Standards Association (ASRO): Bucharest, Romania, 1995. [Google Scholar]
- ASRO. STAS, 13139–13193 Auxiliary Chemicals for the Textile Industry. Determination of the Ionogenicity Class of Surfactants; Romanian Standards Association (ASRO): Bucharest, Romania, 1993. [Google Scholar]
- ISO. 2131:1995 Surface Active Agents. Simplified Classification; Romanian Standards Association (ASRO): Bucharest, Romania, 1995. [Google Scholar]
- Jianu, I.C.; Jianu, A.M.; Stana, L.G.; Folescu, R.; Ursoniu, S.; Selaru, M.; Valceanu, A.; Misca, C.D.; Ciuca, I.; Bujanca, G.; et al. RO132484-A0; RO132484-B1. Homogenous Superficially-Active Non-Ionic-Cationic Structures and Process for Obtaining the Same; State Office for Inventions and Trademarks of Romania (OSIM): Bucharest, Romania, 2017. [Google Scholar]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. [Google Scholar] [CrossRef]
- Fendler, J.H.; Fendler, E.J. Chapter 10—Interactions in and Catalysis by Micelles in Nonaqueous Solvents and in Liquid Crystalline Phases. In Catalysis in Micellar and Macromoleular Systems; Fendler, J.H., Fendler, E.J., Eds.; Academic Press: Cambridge, MA, USA, 1975; pp. 314–385. [Google Scholar]
- ISO. 1890:2006 Surface Active Agents. Determination of Cloud Point of Non-Ionic Surface Active Agents Obtained by Condensation of Ethylene Oxide; Romanian Standards Association (ASRO): Bucharest, Romania, 2006. [Google Scholar]
- ISO. 13955:2002 Surface Active Agents—Determination of Krafft Point and Solubility of Ionic Surface Active Agents; Romanian Standards Association (ASRO): Bucharest, Romania, 2002. [Google Scholar]
- ISO. SR EN 13926:2003 Surface Active Agents—Alkoxylated Derivatives—Determination of Hydroxyl Value—N-methyl Imidazole Method; Romanian Standards Association (ASRO): Bucharest, Romania, 2003. [Google Scholar]
- ISO. SR EN 15168:2007 Surface Active Agents—Determination of Hydroxyl Value—p-Toluensulfonyl Isocyanate (TSI) Method and Potentiometric Titration with Tetrabutylammonium Hydroxide; Romanian Standards Association (ASRO): Bucharest, Romania, 2007. [Google Scholar]
- ISO. SR ISO 2270:1995 Nonionic Surface Active Agents. Polyethoxilated Derivates Iodometrical Determination of the Oxyethylenic Groups (Identical with the International Standard ISO 2270:1989); Romanian Standards Association (ASRO): Bucharest, Romania, 1995. [Google Scholar]
- ISO. SR ISO 13268:2001/AC:2002—Surface Active Agents—Determination of Ethylene Oxide and Propylene Oxide Groups in Ethylene Oxide and Propylene Oxide Adducts; Romanian Standards Association (ASRO): Bucharest, Romania, 2002. [Google Scholar]
- ISO. SR EN 13716:2002 Surface Active Agents—Determination of Total Base Nitrogen—Potentiometric Titration; Romanian Standards Association (ASRO): Bucharest, Romania, 2002. [Google Scholar]
- ISO. SR ISO EN 13560:2002/AC:2003—Surface Active Agents—Determination of Amide Nitrogen Content—Potentiometric Titration; Romanian Standards Association (ASRO): Bucharest, Romania, 2002. [Google Scholar]
- ASRO. SR 13409:1998 Surface Active Agents. Detergents. Determination of Active Chlorine Content. Potentiometric Method; Romanian Standards Association (ASRO): Bucharest, Romania, 1998. [Google Scholar]
- ASRO. SR 13410:1998 Surface Active Agents. Detergents. Determination of Chloride Content. Potentiometric Method; Romanian Standards Association (ASRO): Bucharest, Romania, 1998. [Google Scholar]


| No. | Degree of Oligomerization (n) | Reductive Methylation Yield (%) |
|---|---|---|
| 1 | 3 | 81.32 |
| 2 | 6 | 86.42 |
| 3 | 9 | 90.17 |
| 4 | 12 | 92.36 |
| 5 | 18 | 95.02 |
| No. | Degree of Oligomerization (n) | Reaction Time (Minute) | Reductive Methylation Yield (%) |
|---|---|---|---|
| 1 | 3 | 10 | 31.22 |
| 2 | 30 | 47.15 | |
| 3 | 50 | 62.51 | |
| 4 | 90 | 80.83 | |
| 5 | 18 | 10 | 49.02 |
| 6 | 30 | 60.61 | |
| 7 | 50 | 77.43 | |
| 8 | 90 | 94.61 |
| No. | Degree of Oligomerization (n) | Molar Ratio Formic Aldehyde /LM(EO)3;18AP | Reductive Methylation Yield (%) |
|---|---|---|---|
| 1 | 3 | 1.1/1 | 59.43 |
| 2 | 1.3/1 | 62.54 | |
| 3 | 1.7/1 | 69.61 | |
| 4 | 2.0/1 | 76.32 | |
| 5 | 2.2/1 | 90.52 | |
| 6 | 2.5/1 | 99.14 | |
| 7 | 18 | 1.1/1 | 74.32 |
| 8 | 1.3/1 | 77.13 | |
| 9 | 1.7/1 | 92.20 | |
| 10 | 2.0/1 | 94.03 | |
| 11 | 2.2/1 | 97.74 | |
| 12 | 2.5/1 | 99.25 |
| No. | Homogeneous Degree of Oligomerization, n, of Ethylene Oxide in the Structure a CTF1 | Critical Micellar Concentration (×10–5 mol/L) |
|---|---|---|
| 1 | 3 | 1.31 |
| 2 | 6 | 1.11 |
| 3 | 9 | 0.82 |
| 4 | 12 | 0.53 |
| 5 | 18 | 0.40 |
| No. | Homogeneous Degree of Oligomerization, n, of Ethylene Oxide in the Structure LM(EO)nAT | Critical Micellar Concentration (×10–5 mol/L) |
|---|---|---|
| 1 | 3 | 1.43 |
| 2 | 6 | 1.22 |
| 3 | 9 | 0.94 |
| 4 | 12 | 0.64 |
| 5 | 18 | 0.49 |
| Alcohol | Physico-Chemical Characteristics | ||||
|---|---|---|---|---|---|
| Density (g/cm3)/Temperature (°C) | Solidification Range, (°C) | Clouding Point [36,37], (°C) | Hydroxyl Value [38,39], (mg KOH/g) | Ethylene Oxide Content [40,41], (%) | |
| Lauryl/myristyl alcohol | 0.830/25 | 33–35 | - | - | - |
| Polyethoxylated lauryl/myristyl alcohol (n = 3) | 0.891/70 | - | - | 180.41 | 42.52 |
| Polyethoxylated lauryl/myristyl alcohol (n = 6) | 0.933/70 | 20–23 | 46–50 | 126.58 | 59.67 |
| Polyethoxylated lauryl/myristyl alcohol (n = 9) | 0.943/70 | 26–28 | 58–67 | 97.49 | 68.94 |
| Polyethoxylated lauryl/myristyl alcohol (n = 12) | 0.966/70 | 31–34 | 87–96 | 79.27 | 74.74 |
| Polyethoxylated lauryl/myristyl alcohol (n = 16) | 0.975/70 | 36–38 | >100 | 63.45 | 79.78 |
| Polyethoxylated lauryl/myristyl alcohol (n = 18) | 0.984/70 | 38–40 | >100 | 52.91 | 83.14 |
| No. | Primary Polyether Amine | Nitrogen Content [42,43], (%) | Ethylene Oxide Content [40,41], (%) | ||
|---|---|---|---|---|---|
| Experimental | Theoretical | Experimental | Theoretical | ||
| 1 | β-lauryl/myristyl 7/3 oxy-ethylamine | 5.884 | 3.897 | - | - |
| 2 | β-lauryl/myristyl 7/3 polyethyleneoxy n = 3 ethylamine | 3.770 | 3.790 | 35.545 | 35.733 |
| 3 | β-lauryl/myristyl 7/3 polyethyleneoxy n = 6 ethylamine | 2.781 | 2.792 | 52.449 | 52.652 |
| 4 | β- lauryl/myristyl 7/3 polyethyleneoxy n = 9 ethylamine | 2.201 | 2.210 | 62.268 | 62.520 |
| 5 | β-lauryl/myristyl 7/3 polyethyleneoxy n = 12 ethylamine | 1.818 | 1.829 | 68.594 | 68.983 |
| 6 | β lauryl/myristyl 7/3 polyethyleneoxy n = 16 ethylamine | 1.482 | 1.487 | 74.546 | 74.782 |
| 7 | β-lauryl/myristyl 7/3 polyethyleneoxy n = 18 ethylamine | 1.250 | 1.253 | 78.582 | 78.757 |
| No. | Symbol | Ethylene Oxide Content [40,41], (%) | Hydroxyl Value [38,39], (mg KOH/g) | Refractive Index, | |||
|---|---|---|---|---|---|---|---|
| Determined | Calculated | Determined | Calculated | Determined | Calculated | ||
| 1 | PEG—3 | 87.204 | 88.000 | 370.294 | 373.330 | - | - |
| 2 | PEG—6 | 92.480 | 93.617 | 106.168 | 198.501 | 1.4523 | 1.4520 |
| 3 | PEG—9 | 94.772 | 95.650 | 130.024 | 135.256 | 1.4593 | 1.4591 |
| 4 | PEG—12 | 95.886 | 96.703 | 101.698 | 102.564 | 1.4606 | 1.4608 |
| 5 | PEG—18 | 97.080 | 97.770 | 68.639 | 69.135 | 1.4626 | 1.4628 |
| No. | Homogeneous Degree of Oligomerization (n) | EOn—Ac | EOn—2Ac | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethylene Oxide Content [40,41], (%) | Hydroxyl Value [38,39], (mg KOH/g) | Purity (%) | Ethylene Oxide Content [40,41], (%) | Hydroxyl Value [38,39], (mg KOH/g) | Purity (%) | ||||||||
| Detd. | Calcd. | Detd. | Calcd. | Detd. | Calcd. | Detd. | Calcd. | Detd. | Calcd. | Detd. | Calcd. | ||
| 1 | 3 | 68.56 | 68.75 | 290.88 | 291.67 | 99.73 | 55.78 | 56.41 | 1.782 | - | 98.90 | 3 | 68.56 |
| 2 | 6 | 81.11 | 81.48 | 172.05 | 172.84 | 99.54 | 70.32 | 71.93 | 1.803 | - | 97.76 | 6 | 81.11 |
| 3 | 9 | 86.21 | 86.84 | 121.93 | 122.81 | 99.28 | 79.17 | 79.52 | 1.647 | - | 99.56 | 9 | 86.21 |
| 4 | 18 | 89.05 | 89.79 | 94.45 | 95.24 | 99.17 | 83.54 | 83.81 | 1.025 | - | 99.68 | 12 | 89.05 |
| No. | Homogeneous Degree of Oligomerization (n) | (EO)n—Na | (EO)n—2Na | ||
|---|---|---|---|---|---|
| Purity (%) | Purity (%) | ||||
| Determined 1 | Calculated | Determined 1 | Calculated | ||
| 1 | 3 | 99.04 | 99.18 | 99.18 | 99.79 |
| 2 | 6 | 99.37 | 99.86 | 99.03 | 99.77 |
| 3 | 9 | 99.43 | 99.88 | 98.99 | 99.83 |
| 4 | 18 | 99.78 | 99.53 | 99.36 | 99.88 |
| No. | Homogeneous Tertiary Polyether Amine | Ethylene Oxide Content [40,41], (%) | Nitrogen Content [42,43], (%) | ||
|---|---|---|---|---|---|
| Experimental | Theoretical | Experimental | Theoretical | ||
| 1 | N,N-dimethyl-N-β-lauryl/myristyl 7/3 oxy ethylamine | - | - | 5.247 | 5.275 |
| 2 | N,N-dimethyl-N-β-lauryl/myristyl 7/3 polyethyleneoxy n = 3 ethylamine | 33.061 | 33.216 | 3.505 | 3.522 |
| 3 | N,N-dimethyl-N-β-lauryl/myristyl 7/3 polyethyleneoxy n = 6 ethylamine | 46.619 | 49.867 | 2.631 | 2.644 |
| 4 | N,N-dimethyl-N-β-lauryl/myristyl 7/3 polyethyleneoxy n = 9 ethylamine | 59.704 | 59.873 | 2.111 | 2.117 |
| 5 | N,N-dimethyl-N-β-lauryl/myristyl 7/3 polyethyleneoxy n = 12 ethylamine | 66.294 | 66.549 | 1.758 | 1.765 |
| 6 | N,N-dimethyl-N-β-lauryl/myristyl 7/3 polyethyleneoxy n = 18 ethylamine | 76.270 | 76.829 | 1.213 | 1.222 |
| No. | Homogeneous Degree of Oligomerization (n) | (EO)n-2Cl | |||
|---|---|---|---|---|---|
| Ethylene Oxide Content [40,41], (%) | Chlorine Content [44,45], (%) | ||||
| Determined | Calculated | Determinated | Calculated | ||
| 1 | 3 | 56.67 | 57.14 | 30.48 | 30.74 |
| 2 | 6 | 72.42 | 72.73 | 19.48 | 19.56 |
| 3 | 9 | 79.59 | 80.00 | 14.27 | 14.34 |
| 4 | 12 | 84.06 | 84.21 | 11.30 | 11.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jianu, C. Reductive Methylation of Homogeneous Primary β-Lauryl/myristyl 7/3 Polyethyleneoxy n = 3–18 Ethylamines under Phase-Transfer Catalysis Conditions. Molecules 2021, 26, 4612. https://doi.org/10.3390/molecules26154612
Jianu C. Reductive Methylation of Homogeneous Primary β-Lauryl/myristyl 7/3 Polyethyleneoxy n = 3–18 Ethylamines under Phase-Transfer Catalysis Conditions. Molecules. 2021; 26(15):4612. https://doi.org/10.3390/molecules26154612
Chicago/Turabian StyleJianu, Călin. 2021. "Reductive Methylation of Homogeneous Primary β-Lauryl/myristyl 7/3 Polyethyleneoxy n = 3–18 Ethylamines under Phase-Transfer Catalysis Conditions" Molecules 26, no. 15: 4612. https://doi.org/10.3390/molecules26154612





